Abstract
Studies have reported that a selective deficit in visual motion processing is present in certain developmental disorders, including Williams syndrome and autism. More recent evidence suggests a visual motion impairment is also present in adults with fragile X syndrome (FXS), the most common form of inherited mental retardation. The goal of the current study was to examine low-level cortical visual processing in infants diagnosed with FXS in order to explore the developmental origin of this putative deficit. We measured contrast detection of first-order (luminance-defined) and second-order (contrast-defined) gratings at two levels of temporal frequency, 0 Hz (static) and 4 Hz (moving). Results indicate that infants with FXS display significantly higher detection thresholds only for the second-order, moving stimuli compared to mental age-matched typically-developing controls.
Keywords: fragile X syndrome, contrast detection, second-order, motion, threshold, forced-choice preferential looking
1. Introduction
Fragile X syndrome (FXS) is the most common form of inherited mental retardation, affecting approximately 1 in 3600 individuals in the general population (Crawford et al., 2001; 2002). The genetic cause of FXS is a trinucleotide repeat expansion in the 5′ untranslated region of the fragile X mental retardation-1 (FMR1) gene on the X chromosome, which results in disruption or complete absence of the mRNA-binding protein, fragile X mental retardation protein (FMRP). It is the lack or significant reduction of this protein that causes the physical, cognitive and behavioral phenotype characteristic of FXS. The histological expression of FMRP has been reported to be highest in the brain, testes, and eyes, and is especially critical in the early stages of development in these areas (Bakker et al., 2000). This finding is supported by evidence showing that FMRP is involved in dendridic spine formation, a process known to play a role in synaptic development and plasticity (Jin & Warren, 2000). Such dendritic abnormalities have been found in occipito-parietal areas of FMR1 knock-out mice as well as visual cortices of autopsied tissue from patients with FXS (Comery et al., 1997; Irwin et al., 2002), suggesting that FMRP is an important protein in the development of neural networks in visual areas of the brain.
While the gene for FXS has been identified and DNA testing through routine blood test is available, FXS is often undiagnosed until the age of three or later unless previous family history of the disorder exists, or the physical features of the disorder (e.g., macroorchidism, long, narrow face and prominent ears, and connective tissue problems) are noted by a physician (Bailey et al., 2002; Mirrett et al., 2004). For this reason, a vast literature exists describing the molecular, cognitive and behavioral profiles of children and adults with FXS, but very little research has explored the early sensory or cognitive development of infants with FXS.
Mental retardation is the primary phenotype of children and adults with FXS, although a profile of cognitive strengths and weaknesses has been reported (Bennetto & Pennington, 2002). Strengths have been identified in areas including vocabulary, long-term memory, and face and emotion discrimination (Crowe & Hay, 1990; Freund & Reiss, 1991; Simon & Finucane, 1996), while poorer performance is typically demonstrated on tasks requiring skills in visual memory, visual-spatial and visual-motor coordination, processing of sequential information, numerical processing, and inhibitory control (Cornish et al., 1998, 2001; Mazzocco et al., 2006; Rivera et al., 2002; Scerif et al., 2007). Females are generally less severely affected compared to males because their FMRP levels are commonly higher than those of males as a result of their normal second X chromosome, but both sexes show lower cognitive functioning when compared to mental age-matched (MA) typically- developing (TD) individuals (Loesch et al., 2003).
Research studies suggest that FXS is not associated with a global deficit in visual processing; rather, impairments in individuals with the disorder are specific and primarily observed in abilities subserved by the dorsal stream (Cornish et al., 1998, 1999, 2001; Kogan et al., 2004a; 2004b). It is well established that visual information is processed through two distinct, but interacting, streams; the ventral and dorsal cortical pathways. The ventral stream involves processing of object features such as color and form, as well as face recognition, and projects from primary visual cortex to the inferotemporal cortex, while visually-guided actions and processing of motion information are served by the dorsal stream, projecting from primary visual cortex to the posterior parietal cortex (Milner & Goodale, 1995; Ungerleider & Mishkin, 1982). The identification of a specific dorsal stream deficit in FXS comes from experimental findings that individuals with FXS perform worse on tasks that require visual-spatial and visual-motor coordination, while visual acuity and recognition are normal. For example, visual abilities such as those required to identify partially complete object drawings (Gestalt Closure Task) and discriminate between objects have been reported to be intact in individuals with FXS, but impaired performance is observed on tasks requiring the replication of an abstract block design (Block Design Task) or copying a drawing from a model (Cornish et al., 1998, 1999; Crowe & Hay, 1990). Other developmental disorders including Williams syndrome, autism, and developmental dyslexia have also been found to show characteristic patterns of dorsal stream dysfunction (Atkinson et al., 1997; Braddick et al., 2003; Spencer et al., 2000).
A recent set of studies by Kogan et al. (2004a; 2004b) examined the possible neurobiological factors contributing to visual difficulties in adolescents and adults with FXS. Their study provided immunohistochemical evidence that neurons in the Magnocellular (M) layers of the LGN from an adult male with FXS were abnormally small and displayed no FMRP staining, suggesting that a lack of FMRP protein may result in disruption of function of cells in the M layers. Individuals with FXS showed reduced contrast sensitivity for the detection of low spatial frequency gratings, but no difference from control groups for detecting high spatial frequency gratings. Additionally, they reported no group difference for chromatic contrast thresholds. These findings were interpreted as lending support for the hypothesis that an absence of FMRP in individuals with FXS differentially impacts M pathway functioning while processing of P pathway stimuli remains spared. Kogan et al.(2004b) also found that patients with FXS had significantly higher thresholds for detecting coherent motion, but did not demonstrate a difference in performance on a form coherence task.
Kogan et al. (2004a) further examined sensitivity to discriminating the orientation and the direction of motion of first- and second-order gratings stimuli. They reported that adults with FXS displayed increased orientation discrimination thresholds for second-order form stimuli and increased direction discrimination thresholds for first- and second-order motion stimuli, but near normal perception for discriminating the orientation of first-order form stimuli. While this may support the hypothesis of a pervasive dorsal stream deficit present in individuals FXS, the significantly greater threshold levels in response to second-order form stimuli may be a consequence of processing differences between first- and second-order stimuli. First-order stimuli are luminance-defined, while second-order stimuli have no variation in mean luminance and are defined by other attributes such as contrast, texture, or depth (Cavanagh & Mather, 1989; Chubb & Sperling, 1988; Seiffert & Cavanagh, 1998).
The aim of the current study was to determine whether infants with FXS differ compared to MA-matched TD infants in their ability to detect first- or second-order gratings of varying contrast at two levels of temporal frequency, either static (0 Hz) or moving (4 Hz).
2. Methods
2.1. Participants
Two groups of participants were enrolled in this study, a group of 32 infants with FXS (27 males and 5 females) and a group of 37 TD infants (29 males and 8 females). Infants with FXS were recruited and clinically evaluated at the UC Davis M.I.N.D. Institute Fragile X Research and Treatment Center (FXRTC), and molecular DNA testing was carried out to confirm their diagnosis. TD infants were recruited through letters to families, fliers, and word of mouth in Davis, California.
Data from five infants with FXS were not included in the final analysis because the infant was either fussy or crying during the eye tracking testing session (2) or did not provide gaze data for all trials (3). Four TD infants were not included as a result of fussiness (1), parent verbal interference (1), or insufficient trial number (2). Mean chronological age for the FXS and TD groups was 24.42 months (± 10.52) and 17.86 months (± 9.49), respectively.
2.2. Cognitive Assessment
To control for differences in developmental level, all infants with FXS were assessed using the Mullen Scales of Early Learning (Mullen, 1995) to derive a mental age. The MSEL is a standardized developmental test for children ages 3 to 60 months, consisting of 5 subscales: gross motor, fine motor, visual reception, expressive language, and receptive language. The MSEL was administered to participants with FXS according to standard instructions by a trained researcher. Infants in the FXS group had a mean mental age of 14.31 months (± 6.64), which was matched as closely as possible to infants in the TD group (17.86 months ± 9.49), who were chronologically younger than the FXS participants. An independent samples t-test confirmed that mental age did not differ significantly between the two final groups (t1,58 = 1.716, p = 0.095, two-tailed). The marginal difference in mean mental age between groups is unlikely to have contributed to the experimental findings because the two groups performed equally on some of the detection task conditions.
2.3. Apparatus and stimuli
All stimuli were presented on a Tobii 1750 binocular eye tracker monitor (Tobii Technology, www.tobii.com). This eye tracking system consists of a high-resolution camera embedded in a 17-inch monitor (1280 × 1024 pixels resolution, 50 Hz refresh rate) with infrared light-emitting diodes that generate even lighting to capture images of the subject’s eyes. The fixed wide-angle camera allows data to be recorded from a freely-moving person, with approximately 20 centimeters (cm) of freedom on each side. Gaze signals can be reacquired 100 milliseconds (ms) after blinks or other interruptions; there is no delay caused by reorientation of the camera. Data are captured at a frame rate of 50 Hz and sent in real-time to the ClearView application (version 2.7.0) to be overlaid on the stimuli for analysis purposes. There are several benefits of the Tobii 1750 system that make it conducive to testing infants, including high tolerance to head-motion without requiring any head restraints, reusable calibrations, and an average precision of 0.5 degrees of visual angle. The luminance of the Tobii LCD display was gamma-corrected to minimize luminance non-linearities. Speakers were located directly behind the monitor and allowed the infant to hear the auditory component of the inter-stimuli attention-getter video. All gaze data were collected using ClearView analysis software. Stimuli were generated using The Vision Shell PPC program, controlled by an Apple G4 Power Macintosh with OS9.
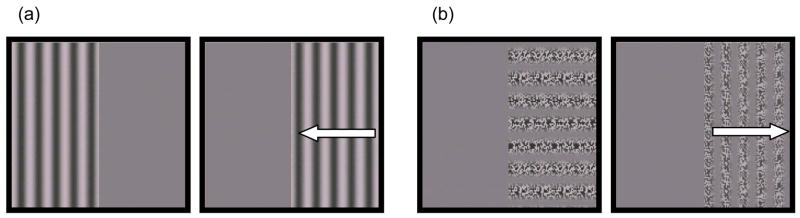
Four types of stimuli were used: first-order (luminance-defined) and second-order (contrast- or texture-defined) sine wave gratings at two levels of temporal frequency, static (0 Hz) or moving (4 Hz) (Fig. 1). The first-order grating was a single luminance-defined sinusoidal carrier with a spatial frequency of 0.35 cycles (cyc)/degree (deg), oriented vertically or horizontally, counterbalanced on either the left or right side of the monitor. First-order stimuli were either 0 Hz, static gratings or 4 Hz gratings drifting in a leftward or rightward direction. These specific spatial and temporal frequencies were chosen because they are near optimal for young infants (Atkinson et al., 1977; Banks, 1982–1983; Dobkins et al., 1999). The Michelson contrast of the first-order grating was varied at 4 levels (7, 14, 21 or 28%) to determine contrast detection thresholds. The second-order grating stimuli consisted of a dynamic (flickering) random-dot carrier pattern (each dot was 0.2 deg by 0.2 deg, updated randomly on each frame) modulated by a contrast-defined sinusoid which was 0.35 cyc/deg, oriented either vertically or horizontally, at a TF of 0 Hz or 4 Hz. The mean luminance of the second-order grating was equal at all points on the grating. The amplitude of the second-order sinusoidal contrast modulation was varied at 4 levels (10, 21, 31, or 42%) by manipulating the carrier contrast from 0 (Nishida, 1993). Although peaks in the carrier contrast could give away the presence of a putatively second-order pattern, this possibility holds for all carrier contrasts, not just those varied from zero (Cropper, 1998), and while randomizing the carrier contrast is ideal (Cropper, 1998), it is not possible with infant subjects. Importantly, because the carrier was identical in both second-order TF conditions, any threshold difference between the two conditions cannot be attributed to the first-order component. Gratings were presented within a 3 second (s) temporal Gaussian window (fading in and out of view), and subtended a 16.0 deg by 24.6 deg region on the screen when viewed from a distance of 60 cm. A schematic of the first- and second-order stimuli used in each detection task is presented in Figure 1.
Figure 1.
A schematic representation of the stimuli presented during each of the four conditions. (a) First-order, luminance-defined static (0 Hz) stimuli (left) and moving (4 Hz) (right). (b) Second-order, contrast-defined static (0 Hz) stimuli (left) and moving (4 Hz) (right). All gratings had a spatial frequency of 0.35 cycles/degree, were randomly oriented vertically or horizontally, and were presented on either the left of right half of the screen. Arrows indicate possible direction of motion (either left or right, randomly). Grating type was presented between infants and temporal frequency condition was presented in counterbalanced order within infants.
2.4. Procedure
The experimental protocol was approved by the Institutional Review Board at the University of California, Davis, and informed consent was obtained from parents of all infants. Infants were tested while seated on a caregiver’s lap. An experimenter monitored the infant’s eye position through the ClearView application real-time track-status meter in addition to a standard VHS camera feed projected to a television monitor in the control room. Eye-tracking began with a five-point calibration routine, and instances in which the infant was not looking at the target or was moving his/her eyes were discarded and the routine was repeated to collect useable calibration data for that quadrant of the screen.
All conditions were presented as a 2-alternative forced-choice preferential looking procedure (Teller, 1979). This paradigm makes use of infants’ preference to look at a patterned stimulus rather than a uniform field of equal luminance to the mean luminance of the stimulus. During each trial, gratings faded in during the first 500 ms either on the left or right half of the screen, determined randomly, and remained at the peak contrast level for 2 s before fading out for the last 500 ms. Between trials, attention was drawn to the middle of the screen with a centrally-located flashing colorful circle accompanied by a single tone, lasting for 2 s. Each detection task consisted of 10 trials at each contrast level, presented in random order. The level of temporal frequency (0 Hz or 4 Hz) was a within-subject factor. The grating type (luminance- or contrast-defined) was a between-subject factor. This was necessary to keep the duration of the experiment manageable for the infant subjects. Temporal frequency conditions were presented in counterbalanced order, among other tasks related to a different experiment.
3. Analyses
3.1 Coding
Infants who provided gaze data on all trials within a given detection task condition were included in the final analysis. For each infant, a video recording of the stimuli overlaid with the eye tracking gaze data was imported into Noldus Observer 5.0 software for coding. Eye tracking data were coded using the Area-Of-Interest (AOI) definition tool within the ClearView application. AOIs were defined by splitting the screen at the midline into two equal areas. For each recording, fixation position (left, right, center, or away) during each frame of each 3 s test trial was coded from the raw data. A fixation was defined as data points within a 30 pixel radius for a minimum duration of 100 ms. Fixations occurring on the midline between the left and right AOI were interpreted as detection of the stimulus edge, and thus included in the looking time toward the stimulus side. Because the locations of the stimulus edges at the center of the screen were identical in all conditions, this did not bias the results in any way. A Visual Preference (VP) score, which indexes the proportion of looking time to the stimulus side of the screen, was calculated using the following formula: (looking time to stimulus side + center)/(looking time left + right + center). VP scores were between 0 and 1, with 0.5 considered performance at the chance level. Trials in which no fixations occurred were considered missing trials and were not given a VP score or included in the final calculation. For each infant, a mean VP score was calculated at each contrast level, and used to determine group VP scores for each of the four detection task conditions.
3.2 Threshold Estimation
To obtain individual contrast detection thresholds, a logistic function was fit to each infant’s average VP scores as a function of contrast using the psignifit toolbox (version 2.5.6) for Matlab, which implements the maximum-likelihood procedure described by Wichmann and Hill (2001). Threshold performance was defined as the contrast value yielding a 75% VP score. To estimate parameters, threshold, slope, and error, a bootstrapping technique was used which included 5000 replications for each fitted function. The distribution of these thresholds was used to generate 95% confidence intervals for the threshold estimate. Individual infant threshold values were used to calculate group detection thresholds for each task condition.
4. Results
Of the fifteen infants with FXS who were presented the first-order stimulus condition, twelve successfully completed at least one of the temporal frequency conditions and were included in the final analysis (0 Hz = 7, 4 Hz = 9, 4 infants completed both), and of the seventeen who were presented the second-order stimulus condition, fifteen were included in the final analysis (0 Hz = 9, 4 Hz = 9, 3 infants completed both). Seventeen of the TD infants were presented the first-order stimulus condition, of which, fifteen were included in the final analysis (0 Hz = 11, 4 Hz = 8, 4 infants completed both), and of the twenty who were shown the second-order stimulus condition eighteen were able to complete at least one temporal frequency condition and were included in the analysis (0 Hz = 13, 4 Hz = 14, 9 infants completed both). While the majority of infants completed all trials of at least one temporal frequency level, it was challenging to maintain the interest of all infants across both stimulus conditions.
A repeated measures ANOVA using VP scores, with one within-group factor (contrast level: 1-4), and one between-group factor (group: TD, FXS), was conducted for each stimulus condition to confirm a main effect of contrast on preferential looking. Because grating type (luminance- or contrast-defined) was used as a between-subject factor in the study, first- and second-order conditions were analyzed separately. A significant main effect of contrast (F3,48 = 3.255, p = 0.030, η2 = 0.169), but no effect of group (F1,16 = 0.004, p = 0.948) or contrast × group interaction (F3,48 = 0.366, p = 0.778) was found for the first-order 0 HZ gratings. The detection of first-order, 4 HZ stimuli revealed a significant main effect of contrast (F3,45 = 6.629, p = 0.001, η2 = 0.306), but no effect of group (F1,15 = 1.684, p = 0.214) or contrast × group interaction (F3,45 = 0.424, p = 0.736). For the second-order, 0 HZ detection task there was a significant main effect of contrast (F3,60 = 20.692, p = 0.001, η2 = 0.509), but no effect of group (F1,20 = 0.166, p = 0.688) or contrast × group interaction (F3,60 = 3.126, p = 0.092). Finally, a significant main effect of contrast (F3,63 = 22.918, p = 0.001, η2 = 0.522) and group (F1,21 = 4.414, p = 0.048, η2 = 0.174), but no contrast × group interaction (F3,63 = 2.075, p = 0.112) was found for the second-order, 4 HZ stimulus condition. These results demonstrate that VP scores increased as a function of increasing contrast for each stimulus condition, confirming that the manipulation of contrast was effective. A significant group difference was found only for the second-order higher temporal frequency stimulus condition, driven by the lower VP scores in the FXS group.
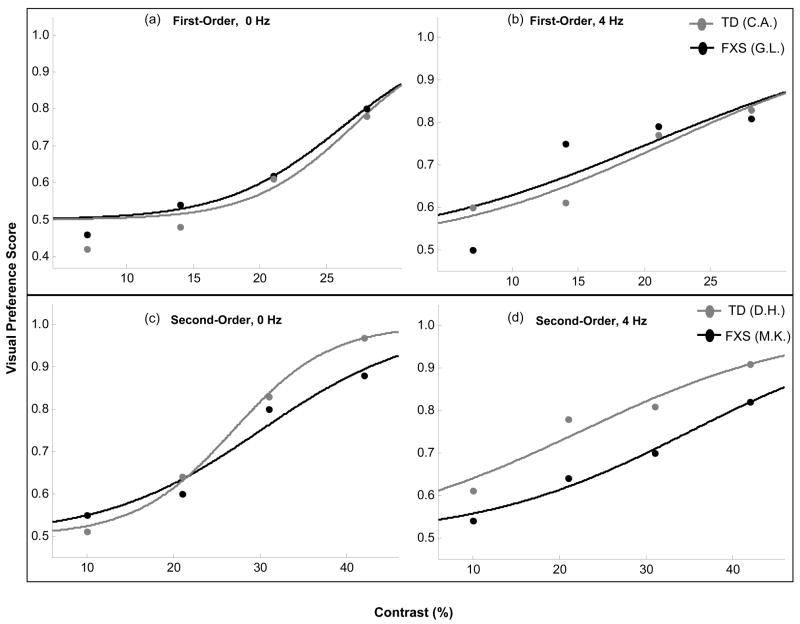
Given that there was a significant effect of stimulus contrast on VP scores for each condition, we examined group differences by calculating individual detection thresholds. Representative psychometric functions from one infant in each group who completed both temporal frequency levels of either the first- or second-order task condition are shown in Figure 2. The stimulus contrast required for a 75% VP score was taken as the threshold of stimulus detection. For each grating type, a two-factor ANOVA was performed to compare mean threshold contrasts at each of the temporal frequency levels by group. The results for first-order stimuli yielded no significant difference in thresholds between groups for either the 0 HZ (F1,16 = 0.008, p = 0.981) or 4 HZ (F1,15 = 0.841, p = 0.984) conditions. For the second-order condition, a comparison of threshold values revealed no difference for detection of the 0 HZ stimuli (F1,20 = 0.814, p = 0.378), but a significant difference for detection of the 4 HZ stimuli (F1,21 = 4.897, p = 0.038, η2 = 0.189). These results indicate that infants with FXS had significantly elevated contrast detection thresholds only for the second-order stimuli of higher temporal frequency (mean threshold = 34.4% ± 0.174) compared to the TD control group (mean threshold = 21.8% ± 0.100), as graphed in Figure 3.
Figure 2.
Example of psychometric functions from one subject in each group who completed both temporal frequency conditions of either (a) and (b), luminance-defined, or (c) and (d), contrast-defined stimuli. The contrast required for a 75% Visual Preference (VP) score was used as a measure of the threshold of stimulus detection, and individual threshold values were used to compare group thresholds for each task condition.
Figure 3.
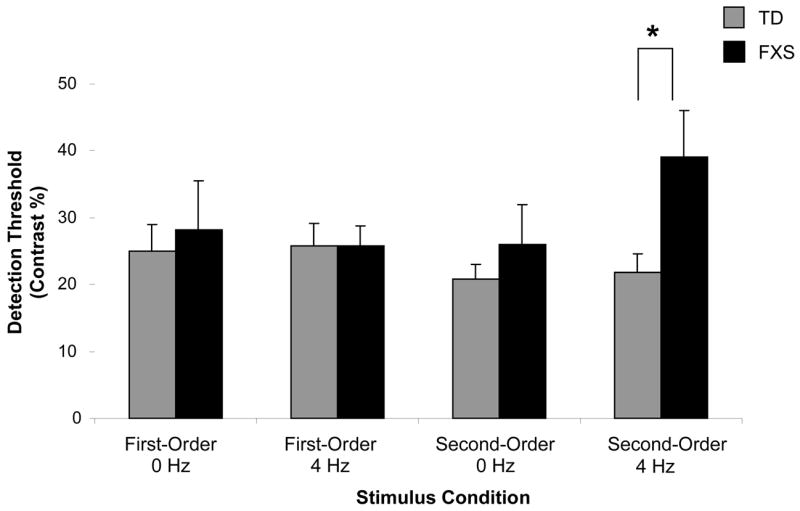
Mean contrast detection thresholds for each group of infants tested in each task condition. No significant difference in thresholds between groups was found for either the static (0 Hz) (F1,16 = 0.008, p = 0.981) or moving (4 Hz) (F1,15 = 0.841, p = 0.984) first-order stimuli. For the second-order stimuli, a comparison of threshold values revealed no significant difference for detection of the static stimuli (F1,20 = 0.814, p = 0.378), but a significant difference for detecting the moving stimuli (F1,21 = 4.897, p = 0.038, η2 = 0.189). Infants with FXS had significantly elevated thresholds only for the second-order higher temporal frequency gratings (mean threshold = 34.4% ± 0.174), compared to the TD control group (mean threshold = 21.8% ± 0.100). Asterisk indicates significance at the p < 0.05 level. Error bars represent + SEM.
5. Discussion
In this study we tested whether infants with FXS differ from MA-matched TD infants in their contrast detection thresholds for first- or second-order gratings at two temporal frequencies. The results reveal that there is a selective deficit in detection of dynamic second-order stimuli in infants with FXS. This finding can be explained by an impairment in either temporal or motion processing. These explanations need not be mutually exclusive. Active, or attention-based, motion perception has been shown to critically depend on the temporal discrimination of events in space, such that deficits in temporal processing cause deficits in differentiating the onsets and offsets present in apparent motion displays (Battelli et al., 2001; Battelli et al., 2007; Cavanagh, 1992; Cavanagh & Mather, 1989; Verstraten et al., 2000). Therefore, fine temporal resolution and motion perception may be closely linked. Indeed, both are subserved by higher stages of processing associated with parietal areas of the brain (Battelli et al., 2001; Battelli et al., 2003; Battelli et al., 2007; Culham et al., 1998). It may be that neural networks mediating temporal tracking are impaired from infancy in individuals with FXS. This interpretation is consistent with the finding that attentional deficits are characteristic of FXS (Cornish et al., 2001; Munir et al., 2000; Scerif et al., 2007; Scerif et al., 2004), and supported by evidence from children and adults with FXS demonstrating lower performance on other parietally-mediated tasks such as coherent motion processing, visual-motor coordination, and basic numerical computation (Cornish et al., 1999; Kogan et al., 2004b; Rivera et al., 2002). Our findings are discussed in terms of a possible attention-based temporal deficit in the tracking of second-order dynamic information, and with respect to previous findings of typical and atypical development of second-order processing.
5.1 Second-Order Motion Processing
There is ample evidence for a dissociation between first-and second-order motion processing. Evidence from psychophysical (Ashida et al., 2001; Cavanagh & Mather, 1989; Chubb & Sperling, 1988; Derrington & Badcock, 1985; Ledegeway & Smith, 1994; Nishida & Sato, 1995; Seiffert & Cavanagh, 1998), neuropsychological (Greenlee & Smith, 1997; Vaina & Cowey, 1996; Vaina et al., 1998) and visual evoked potential (Ellemberg et al., 2003a) data suggest that first- and second-order motion are processed by different neuronal mechanisms. For example, sensitivity to first- or second-order motion is not affected by adaptation to motion of the other order (Nishida et al., 1997), and humans do not integrate alternating frames containing first- and second-order motion into an unambiguous motion percept (Ledegeway & Smith, 1994). It has also been observed that, unlike first-order motion, second-order motion stimuli do not readily induce optokinetic nystagmus (Harris & Smith, 1992). Finally, Ellemberg et al. (2003a) have shown that the psychophysical reaction time and the latency of the visual evoked potential are longer for the onset of second-order compared to first-order motion. This dissociation is supported by evidence of order-specific clinical disorders of motion perception found in some brain damaged patients (Greenlee & Smith, 1997; Vaina & Cowey, 1996; Vaina et al., 2000).
Evidence for both a low-level motion-energy or gradient mechanism and a higher-level, feature-tracking and attention-based mechanism exists for the detection of second-order, contrast-defined, motion (Cavanagh, 1992; Johnston et al., 1992; Nishida & Sato, 1995; Seiffert & Cavanagh, 1998; Sperling, 1989; Whitney & Bressler, 2007). It is likely that both of these systems operate together to detect second-order motion. Given that infants with FXS were not impaired when detecting first-order motion, which is processed by a passive, velocity sensitive mechanism (Seiffert & Cavanagh, 1998), their impairment may be in the higher-level attentive or feature-tracking mechanism.
The existence of higher-level feature or “attentive” tracking of second-order motion (Ashida et al., 2001; Cavanagh, 1992; Derrington et al., 2004; Seiffert & Cavanagh, 1998) is supported by studies showing that dividing attention between different second-order moving stimuli reduces sensitivity to the motion (Allen & Ledgeway, 2003; Ho, 1998; Lu & Liu, 2000), and that visual search rate for low contrast second-order targets is slower than for first-order targets and operates in a serial manner (Ashida et al., 2001). A network of several cortical areas, including parietal and frontal regions, is believed to be engaged in attentive tracking (Culham et al., 1998) and may mediate the perception of second-order motion, although the neuroimaging results are mixed (Dumoulin et al., 2003; Nishida et al., 2003; Seiffert et al., 2003; Smith et al., 1998).
5.2 Typical and Atypical Development of Second-Order Motion Processing
Few studies have examined the development of first- versus second-order motion perception during infancy and early childhood. It has been reported that infants as young as 2 months of age can detect both first- and second-order moving stimuli, as demonstrated by a significant preference to look at a stimulus containing either a first- or second-order dynamic pattern when paired with a control stimulus of similar spatiotemporal properties but no motion. Infants’ preferential looking at the moving stimuli was slightly stronger for first- than second-order, but because this difference was equally present in an older group of 4–5-month-old infants the authors conclude that both age groups are sensitive to second-order dynamic stimuli and a differential developmental time course likely does not exist for sensitivity to the two kinds of motion (Atkinson et al., 1993; Braddick et al., 1996). It is important to consider that since infants were not required to discriminate motion direction, they may have simply preferred dynamic over static stimuli and were not necessarily detecting motion.
Ellemberg et al. (2003b) compared discrimination thresholds for first- versus second-order motion in 5-year-olds and adults at temporal frequencies of 1.5 Hz and 6 Hz and found that 5-year-olds were significantly less sensitive than adults when discriminating the upward or downward motion of both orders of motion at both frequencies, and that the developmental difference in sensitivity for first-order was much less than for second-order at the higher temporal frequency. These findings indicate that at five years of age, sensitivity to first-order motion is more mature than sensitivity to second-order motion, specifically at the faster velocity. A recent study by Bertone et al. (2008) measured orientation- and direction-identification thresholds for first- and second-order stimuli across the ages of 5 to 10 years and found that perception of second-order moving stimuli (2 Hz) is significantly less developed in the youngest age group, and reaches adult levels earlier than first-order. Taken together, this pattern of results suggests that development of second-order motion discrimination may develop at a different rate than that of first-order motion, particularly during the childhood years. With respect to our study, typically developing infants showed no difference in thresholds for first-versus second-order static or moving stimuli, but because comparing first- and second-order raw thresholds within-group is not valid unless the salience or effective contrast is psychophysically equated, we are unable to draw any conclusion about the developmental time course of sensitivity. We also cannot directly compare thresholds obtained from typically developing infants in our study to thresholds found in the study by Ellemberg et al.(2003b) because of differences in stimuli (second-order stimuli are defined differently), tasks (detection versus discrimination), and age groups. Since our preferential looking task assessed thresholds for detection rather than discrimination, our data may be most comparable to, and in agreement with, results from other studies of infants showing parallel maturation for the detection of first- and second-order dynamic stimuli relative to older infants or adults (Braddick et al., 1996; Thibault et al., 2007).
A comparison between the present study and the few other studies that have measured first- and second-order visual processing in individuals with developmental delay indicates that similar patterns of performance exist among certain conditions, possibly as a result of related etiologies. Thibault et al. (2007) examined the development of sensitivity to first- and second-order dynamic stimuli (2Hz) in a group of children with strabismus, ranging in age from 10 months to 7 years, compared to prematurely born infants (8–24-months-old) and infants without any visual disorders. Thresholds for detecting both stimulus types decreased with age in all groups. In the group of infants with strabismus, both first- and second-order detection thresholds were significantly higher than in the control group, while premature infants corrected for gestational age perceived first-order stimuli as well as controls, but did show a significant delay in their development of sensitivity to second-order dynamic stimuli. Whether these results are restricted to dynamic stimuli or whether they extend to the detection of second-order static stimuli was not tested.
With regard to individuals with high-functioning autism, the only study to directly examine first- versus second-order motion processing found that direction discrimination sensitivities for first-order stimuli were comparable for control and autism groups, while significantly reduced sensitivity to second-order stimuli was observed in the autism group (Bertone et al., 2003). In addition, individuals with autism were found to demonstrate superior performance, relative to controls, in orientation discrimination of first-order stimuli, but had significantly higher thresholds for second-order static gratings (Bertone et al., 2005). This led to the “complexity-specific hypothesis” in autism since the processing of second-order information, whether static or dynamic, is deficient (Bertone & Faubert, 2006; Bertone et al., 2003; Bertone et al., 2005).
The results reported here reveal the first infant patient population with a selective deficit in detecting second-order moving (temporally varying) gratings and confirm the dissociation between first- and second-order processing of dynamic stimuli. Our data extend the results of Kogan et al. (2004a; 2004b) by uncovering the developmental trajectory of the putative dorsal stream deficit in both male and female infants diagnosed with FXS.
6. Conclusions
This study demonstrates that infants with FXS can be tested using psychophysical tasks, and that precise thresholds can be estimated. These results are important in that they do not support the interpretation of a low-level motion processing or strictly subcortical deficit in infants with FXS. Indeed, only detection thresholds for the second-order, dynamic stimuli were significantly elevated in the FXS group, suggesting, instead, that abnormalities may exist in higher-level cortical areas responsible for attention-based temporal processing involved in position tracking.
Our psychophysical methods can easily be extended to other visual stimuli, older age groups, and other developmental disorders. It is not clear precisely when in development this visual processing impairment may arise or if there is a critical period for the involvement of FMRP in the early development of these visual areas. Future psychophysical experiments are needed to further understand the nature of this temporal processing deficit, particularly as it relates to mechanisms of direction discrimination. We will explore the relationship between this visual processing difference and downstream early cognitive abilities such as object tracking in future studies.
Acknowledgments
Thanks to Drs. Emilio Ferrer and Jeremy Hill for providing advice in the statistical analysis of the data, and to Dr. Karen Dobkins for her discussion of the experimental results at the VSS meeting in May 2007. We are grateful to the families who participated in this study. This research was partially supported by NICHD grants HD02274 and HD36071 to RJH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen HA, Ledgeway T. Attentional modulation of threshold sensitivity to first-order motion and second-order motion patterns. Vision Research. 2003;43:2927–2936. doi: 10.1016/j.visres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Ashida H, Seiffert AE, Osaka N. Inefficient visual search for second-order motion. Journal of the Optical Society of America A. 2001;18:2255–2266. doi: 10.1364/josaa.18.002255. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Moar K. Development of contrast sensitivity over the first 3 months of life in the human infant. Vision Research. 1977;17:1037–1044. doi: 10.1016/0042-6989(77)90007-4. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Wattam-Bell J. Infant cortical mechanisms controlling OKN, saccadic shifts, and motion processing. Investigative Ophthalmology. 1993;34:1357. [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Roberts JE, Mirrett P, Hatton DD. Identifying infants and toddlers with fragile X syndrome: Issues and recommendations. Infants and Young Children. 2002;14:24–33. [Google Scholar]
- Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, et al. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Experimental Cell Research. 2000;10:162–170. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- Banks MS. The development of spatial and temporal contrast sensitivity. Current Eye Research. 1982–1983;2:191–198. doi: 10.3109/02713688208997694. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, et al. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–995. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Martini P, Barton JJ. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;12:2164–2174. doi: 10.1093/brain/awg221. [DOI] [PubMed] [Google Scholar]
- Battelli L, Pascual-Leone A, Cavanagh P. The ‘when’ pathway of the right parietal lobe. Trends in Cognitive Sciences. 2007;11:204–210. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Pennington B. The neuropsychology of fragile X syndrome. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. Vol. 3. Baltimore, MD: Johns Hopkins University Press; 2002. pp. 206–248. [Google Scholar]
- Bertone A, Faubert J. Demonstrations of decreased sensitivity to complex motion information not enough to propose an autism-specific neural etiology. Journal of Autism and Developmental Disorders. 2006;36:55–64. doi: 10.1007/s10803-005-0042-5. [DOI] [PubMed] [Google Scholar]
- Bertone A, Hanck J, Cornish KM, Faubert J. Development of static and dynamic perception for luminance-defined and texture-defined information. Neuroreport. 2008;19:225–228. doi: 10.1097/WNR.0b013e3282f48401. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: A “complex” issue. Journal of Cognitive Neuroscience. 2003;15:218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Hood B. Striate cortex, extrastriate cortex, and colliculus: some new approaches. In: Vital-Durand F, Braddick O, Atkinson J, editors. Infant Vision. Oxford: Oxford University Press; 1996. pp. 203–220. [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41:1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Attention-based motion perception. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: the long and short of it. Spatial Vision. 1989;42:103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. Journal of the Optical Society of America A. 1988;5:1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(10):5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. The nature of the spatial deficit in young females with Fragile-X syndrome: a neuropsychological and molecular perspective. Neuropsychologia. 1998;36(11):1239–1246. doi: 10.1016/s0028-3932(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with Fragile-X syndrome: evidence for a neuropsychological phenotype. Cortex. 1999;35(2):263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Differential impact of the FMR-1 full mutation on memory and attention functioning: a neuropsychological perspective. Journal of Cognitive Neuroscience. 2001;13(1):144–150. doi: 10.1162/089892901564126. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, et al. Prevalence of the fragile X syndrome in African-Americans. American Journal of Medical Genetics. 2002;110(3):226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Cropper SJ. The detection of chromatic and luminance contrast-modulation by the visual system. Journal of the Optical Society of America A. 1998;15:1969–1986. doi: 10.1364/josaa.15.001969. [DOI] [PubMed] [Google Scholar]
- Crowe SF, Hay DA. Neuropsychological dimensions of the fragile X syndrome: support for a non-dominant hemisphere dysfunction hypothesis. Neuropsychologia. 1990;28(1):9–16. doi: 10.1016/0028-3932(90)90082-y. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell B. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Allen HA, Delicato LS. Visual mechanisms of motion analysis and motion perception. Annual Review of Psychology. 2004;55:181–205. doi: 10.1146/annurev.psych.55.090902.141903. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Badcock DR. Separate detectors for simple and complex grating patterns? Vision Research. 1985;25:1869–1878. doi: 10.1016/0042-6989(85)90010-0. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: evidence for precocious magnocellular development? Vision Research. 1999;39:3223–3229. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Baker CL, Jr, Hess RF, Evans AC. Cortical specialization for processing first- and second-order motion. Cerebral Cortex. 2003;13:1375–1385. doi: 10.1093/cercor/bhg085. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lavoie K, Lewis TL, Maurer D, Lepore F, Guillemot JP. Longer VEP latencies and slower reaction times to the onset of second order motion than to the onset of first order motion. Vision Research. 2003a;43:651–658. doi: 10.1016/s0042-6989(03)00006-3. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Meghji KS, Maurer D, Guillemot JP, Lepore F. Comparison of sensitivity to first- and second-order local motion in 5-year-olds and adults. Spatial Vision. 2003b;16(5):419–428. doi: 10.1163/156856803322552748. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL. Cognitive profiles associated with the fra(X) syndrome in males and females. American Journal of Medical Genetics. 1991;38:542–547. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- Greenlee MW, Smith AT. Detection and discrimination of first- and second-order motion in patients with unilateral brain damage. Journal of Neuroscience. 1997;17:804–818. doi: 10.1523/JNEUROSCI.17-02-00804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LR, Smith AT. Motion defined exclusively by second-order characteristics does not evoke optokinetic nystagmus. Visual Neuroscience. 1992;9:565–570. doi: 10.1017/s0952523800001802. [DOI] [PubMed] [Google Scholar]
- Ho CE. Letter recognition reveals pathways of second order and third order motion. Proceedings of the National Academy of Sciences, USA. 1998;95:400–404. doi: 10.1073/pnas.95.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Weiler IJ, Beckel-Mitchener A, Greenough WT. Brain Structure and Functions of FMR1 Protein. In: Hagerman RJ, Hagerman PJ, editors. Fragile X Syndrome: Diagnosis, Treatment and Research. 3. Baltimore: The Johns Hopkins University Press; 2002. pp. 191–205. [Google Scholar]
- Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Human Molecular Genetics. 2000;9:901–908. doi: 10.1093/hmg/9.6.901. [DOI] [PubMed] [Google Scholar]
- Johnston A, McOwan PW, Buxton H. A computational model of the analysis of some first-order and second-order motion patterns by simple and complex cells. Proceedings of the Royal Society of London B. 1992;250:297–306. doi: 10.1098/rspb.1992.0162. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, et al. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004a;63(9):1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Zangenehpour S, Mullen KT, Holden JJ, et al. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004b;127(Pt 3):591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- Ledegeway T, Smith AT. Evidence for separate motion-detecting mechanisms for first and second order motion in human vision. Vision Research. 1994;34:2727–2740. doi: 10.1016/0042-6989(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Bui QM, Taylor AK, Hagerman RJ. Relationship of deficits of FMR1 gene specific protein with physical phenotype of fragile X males and females in pedigrees: a new perspective. American Journal of Medical Genetics. 2003;118(2):127–134. doi: 10.1002/ajmg.a.10099. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu C. Attention mechanisms for multi-location first- and second-order motion perception. Vision Research. 2000;40:173–186. doi: 10.1016/s0042-6989(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Mazzocco MMM, Bhatia NS, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner Syndrome. Child Neuropsychology. 2006;12:87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The Visual Brain in Action. Oxford: Oxford University Press; 1995. [Google Scholar]
- Mirrett PL, Bailey DB, Jr, Roberts JE, Hatton DD. Developmental screening and detection of developmental delays in infants and toddlers with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2004;25(1):21–27. doi: 10.1097/00004703-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service Inc; 1995. [Google Scholar]
- Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38:1261–1270. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Nishida S. Spatiotemporal properties of motion perception for random-check contrast modulations. Vision Research. 1993;33:633–645. doi: 10.1016/0042-6989(93)90184-x. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ledgeway T, Edwards M. Dual multiple-scale processing for motion in the human visual system. Vision Research. 1997;37:2685–2698. doi: 10.1016/s0042-6989(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sasaki Y, Murakami I, Watanabe T, Tootell RB. Neuroimaging of direction-selective mechanisms for second-order motion. Journal of Neurophysiology. 2003;90:3242–3254. doi: 10.1152/jn.00693.2003. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sato T. Motion aftereffect with flickering test patterns reveal higher stages of motion processing. Vision Research. 1995;35:477–490. doi: 10.1016/0042-6989(94)00144-b. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X Syndrome is related to FMR1 protein expression. Human Brain Mapping. 2002;16(4):206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J. Delineation of early attentional control difficulties in fragile X syndrome: Focus on neurocomputational mechanisms. Neuropsychologia. 2007;45:1889–1898. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Developmental Science. 2004;7(1):116–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position displacement, not velocity, is the cue to motion detection of second-order stimuli. Vision Research. 1998;38:3569–3582. doi: 10.1016/s0042-6989(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Somers DC, Dale AM, Tootell RB. Functional MRI studies of human visual motion perception: texture, luminance, attention and after-effects. Cerebral Cortex. 2003;13:340–349. doi: 10.1093/cercor/13.4.340. [DOI] [PubMed] [Google Scholar]
- Simon EW, Finucane BM. Facial emotion identification in males with fragile X syndrome. American Journal of Medical Genetics. 1996;67(1):77–80. doi: 10.1002/(SICI)1096-8628(19960216)67:1<77::AID-AJMG13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Smith AT, Greenlee MW, Singh KD, Kraemer FM, Hennig J. The processing of first- and second-order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI) Journal of Neuroscience. 1998;15:3816–3830. doi: 10.1523/JNEUROSCI.18-10-03816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11:2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Sperling G. Three stages and two systems of visual processing. Spatial Vision. 1989;4:183–207. doi: 10.1163/156856889x00112. [DOI] [PubMed] [Google Scholar]
- Teller DY. The forced-choice preferential looking procedure: A psychophysical technique for use with human infants. Infant Behavior and Development. 1979;2:135–153. [Google Scholar]
- Thibault D, Brosseau-Lachaine O, Faubert J, Vital-Durand F. Maturation of the sensitivity for luminance and contrast modulated patterns during development of normal and pathological human children. Vision Research. 2007;47:1561–1569. doi: 10.1016/j.visres.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge: MIT Press; 1982. [Google Scholar]
- Vaina LM, Cowey A. Impairment of the perception of second-order motion but not first-order motion in a patient with unilateral focal brain damage. Proceedings of the Royal Society London. 1996;263:1225–1232. doi: 10.1098/rspb.1996.0180. [DOI] [PubMed] [Google Scholar]
- Vaina LM, Makris N, Kennedy D, Cowey A. The selective impairment of the perception of first-order motion by unilateral cortical brain damage. Visual Neuroscience. 1998;15:333–348. doi: 10.1017/s0952523898152082. [DOI] [PubMed] [Google Scholar]
- Vaina LM, Soloviev S, Bienfang DC, Cowey A. A lesion of cortical area V2 selectively impairs the perception of the direction of first-order visual motion. Neuroreport. 2000;7:1039–1044. doi: 10.1097/00001756-200004070-00028. [DOI] [PubMed] [Google Scholar]
- Verstraten FAJ, Cavanagh P, Labianca A. Limits of attentive tracking reveal temporal properties of attention. Vision Research. 2000;40:3651–3664. doi: 10.1016/s0042-6989(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Whitney D, Bressler DW. Second-order motion without awareness: Passive adaptation to second-order motion produces a motion aftereffect. Vision Research. 2007;47:569–589. doi: 10.1016/j.visres.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Perception and Psychophysics. 2001;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]