Abstract
Older and younger participants learned single-function lists of paired associates with no contextual overlap (e.g., J-K, L-M) and double-function lists of paired associates consisting of chains of pairs (e.g., A-B, B-C). Although younger adults out-performed older adults on both pair types, there was a robust pair-type by age interaction. Evidence from intrusion analyses argues that older adults performed better than would be expected on the contextually overlapping double-function pairs because they were less subject to response competition for the double-function pairs. Younger adults made a larger proportion of backward and remote intrusions to double-function probes than did older adults. Thus, group differences in both correct recall probabilities and intrusion analysis suggest that backward and transitive associations are sensitive to aging. The results are discussed within the theoretical framework of the temporal context model and the hypothesis that older adults are impaired at forming new item-context associations.
Normal aging is correlated with a decline in memory abilities across cognitive tasks. The generalized slowing hypothesis suggests this is because all information processing is similarly affected by age (e.g. Brinley, 1965; Cerella, 1985, 1991, 1994; Salthouse, 1985, 1991, 1996). Rather than a global memory impairment, however, domain specific hypotheses argue that various task domains are differentially affected by age-mediated slowing. For example, episodic memory, specifically associative learning, is particularly vulnerable to the effects of age (Light, 1991). Older adults consistently demonstrate an associative deficit across study material and test paradigms, including word-word tests (Naveh-Benjamin, 2000; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003), word-context tests (Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003), picture-picture tests (Naveh-Benjamin et al., 2003), pattern-location tests (Collie, Myers, Schnirman, Wood, & Maruff, 2002), and name-face tests (Naveh-Benjamin, Guez, Kilb, & Reedy, 2004). An age-related associative deficit has also been observed across testing methodologies, including tests of cued-recall (Naveh-Benjamin, 2000; Kliegl & Lindenberger, 1993), yes-no recognition (Naveh-Benjamin, 2000), forced-choice recognition (Naveh-Benjamin et al., 2004), and associative recognition (Bastin & Van der Linden, 2006; Castel & Craik, 2003; Light, Patterson, Chung, & Healy, 2004; Healy, Light, & Chung, 2005; Prull, Dawes, Martin, Rosenberg, & Light, 2006). Additionally, in delayed and immediate free recall the temporal contiguity of study items exerted a weaker influence on older adults’ recall transitions (Kahana, Howard, Zaromb, & Wingfield, 2002), suggesting that associations formed between nearby list items were weaker for older adults.
The goal of this experiment is to examine age-mediated associative deficits in paired-associate learning (PAL), an episodic memory task in which pairs of unrelated items, e.g. ABSENCE-HOLLOW, are presented. At test the first item is presented as a cue for response of the second item. Importantly, memory for test items individually is insuffcient to support accurate PAL memory performance. Instead, PAL tests whether items have been associated, or bound together in memory. The associative deficit observed in cognitive aging has been hypothesized to arise from age-compromised mechanisms for binding together multiple aspects of a memory episode (Chalfonte & Johnson, 1996; Mitchell, Johnson, Raye, Mather, & D’Esposito, 2000), such as an impaired ability to bind items together with their episodic context into a coherent whole (Hultsch & Dixon, 1983; Tun, 1989; Howard & Kahana, 2002; Kliegl & Lindenberger, 1993; Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003, 2004). Naveh-Benjamin (2000) proposed the associative deficit hypothesis (ADH), which suggests that aging is associated with a deficiency in creating and retrieving links between single units of information, such as content and context, or between stimuli. The ADH focuses on the distinction between memory for single units and memory for the association between these units and predicts that, although item information remains relatively intact with age, the strength of associations between items is reduced.
Recent modeling work on the effect of age on temporal contiguity effects in free recall (Howard, Kahana, & Wingfield, 2006) provides a quantitative implementation of the ADH in the context of the temporal context model (TCM, Howard & Kahana, 2002; Howard, Fotedar, Datey, & Hasselmo, 2005), a formal model of episodic recall. In TCM, the cue for episodic recall is the current state of temporal context. During study, context changes gradually from moment to moment. During retrieval, items are activated for recall to the extent that the probe context overlaps with their encoding context. Associations between items are mediated by the effects those items have on the probe context. This ability for items to retrieve states of temporal context turns out to be crucial in describing properties of declarative memory (Howard et al., 2005).
In fitting the temporal contiguity effects of older adults, consistent with the ADH, Howard et al. (2006) found that the change with age was characterized by a diffculty in binding items to the temporal contexts in which those items were presented. This prevents these items from successfully recovering context during retrieval and leads to specific effects on the shape of temporal contiguity effects—in particular, weakened backward associations for older adults. That is, in TCM, item-context binding is essential for the formation of backward associations between items presented in sequence. For instance, after learning the pair A-B item-context binding is essential for the model to learn the backward association B-A. In addition, Howard et al. (2005) also showed that intact item-context binding allows for the formation of associations between items that were not presented close together in time, but that were presented together in similar temporal contexts. For instance, after studying a list of pairs including A-B and B-C, item A and C were never presented together, but were presented in a similar temporal context—in particular, both were presented in the context of B. We will refer to these A-C associations that bridge across pairs as transitive associations (Howard, Jing, Rao, Provyn, & Datey, Revised).
According to TCM, aging is associated with a deficit in item-context-binding (Howard et al., 2006) and a deficit in item-context binding should be manifest as a disruption of backward and transitive associations. In fact, just these deficits have been observed in rats with damage to the hippocampus (Bunsey & Eichenbaum, 1996), a structure that is known to be compromised in normal aging (Erickson & Barnes, 2003). If TCM is an accurate description of episodic recall, and if older adults are indeed impaired at binding items to temporal contexts, then this makes several specific predictions about older adults’ performance in paired-associate learning. The goal of this manuscript is to determine whether older adults performance in a paired-associates learning task is consistent with a deficit in item-context binding as described by TCM—that is, to determine if older adults show a preferential disruption in backward and transitive associations.
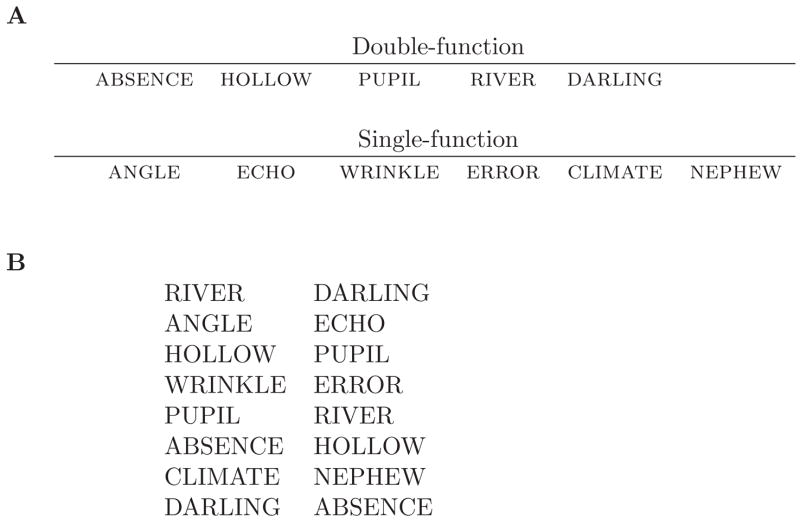
In order to examine age differences between backward and transitive associations in PAL, we turned to the use of double-function lists of paired associates. Double-function pairs are those in which each item serves as both a stimulus in one pair and a response in another pair (Primoff, 1938). Figure 1 illustrates a list composed of both double-function (e.g., ABSENCE-HOLLOW, HOLLOW-PUPIL) and single-function pairs that do not overlap with other pairs (e.g., ANGLE-ECHO, WRINKLE-ERROR). There is a robust effect of pair-type on PAL recall: double-function pairs are markedly more diffcult than single-function pairs (Primoff, 1938; Slamecka, 1976; Umemoto & Hilgard, 1961; Young, 1959, 1961; Young & Jennings, 1964; Howard et al., Revised). This diffculty for double-function pairs has often been attributed to response competition from the backward (B-A, Primoff, 1938; Slamecka, 1976; Umemoto & Hilgard, 1961; Young, 1961; Howard et al., Revised) and transitive associations (A-C, Slamecka, 1976; Howard et al., Revised). For example, consider the pairs ABSENCE-HOLLOW and HOLLOW-PUPIL from Figure 1. When cued with HOLLOW the correct response is PUPIL and the backward associate is ABSENCE. This backward association may compete with the correct response, creating associative response interference and a decrease in correct recalls. Similarly, if ABSENCE is given as a cue, the transitive association leading to the tendency to respond PUPIL can interfere with the correct response HOLLOW.
Figure 1.
Schematic illustrating double- and single-function pairs. A. Words were randomly sampled from the noun pool and assigned to either the single- or the double-function list. B. Pairs were formed from each word list. The order of pair presentation was shuffed. Pairs were presented one item at a time in the center of the screen. Pairs were distinguished from each other by an inter-pair delay longer than the intra-pair delay.
Because single-function lists do not contain pairs with overlapping items there is no source of associative interference, thereby leading to better performance for single-function pairs. Item-context binding should have a facilitatory effect on learning of single-function pairs. Likewise, item-context binding should facilitate the strength of associations between the cue and the correct response in double-function pairs, but at the cost of also increasing the level of response competition, leading to an overall decrease in performance.
Global tests of age-mediated performance differences can be problematic because younger adults typically out-perform older adults. As such, we can make predictions that are not explicable in terms of a generalized deficit. If aging is associated with reduced item-context binding (Naveh-Benjamin, 2000; Howard et al., 2006) and if TCM’s account of the role of item-context binding in PAL (Howard et al., 2005) is correct, then we would expect older adults to be less effective at forming remote associations, and hence, to exhibit fewer backward and transitive intrusions than younger adults for double-function cues. Paradoxically, the associative deficit, that older adults should suffer from less response competition on the double-function pairs, should have a facilitatory effect on older adults’ double-function correct recall. Therefore, we can make two specific predictions regarding age-mediated performance differences on double-function pairs. First, an item-context binding deficit should be manifest as an interaction of pair-type and age, such that older adults perform better than expected on double-function pairs compared to their performance on single-function pairs. Second, if older adults make fewer backward and transitive associations then they should demonstrate fewer backward (B-A) and transitive (A-C) intrusions in response to double-function pairs than younger adults, after overall levels of correct recall and intrusion rates are controlled for.
Experiment
We presented younger and older adults with lists of paired associates composed of both single-function pairs with no contextual overlap (e.g., J-K, L-M), and double-function pairs consisting of chains of pairs (e.g., A-B, B-C). The double-function pairs were arranged in a circular linked-list so that the stimulus of the first pair and the response of the last pair were joined into another pair, e.g., DARLING-ABSENCE in Figure 1B.
Method
Participants
A total of 77 younger adults and 75 older adults participated. Of these participants, two younger adults were excluded from further analyses due to technical problems. To ensure participants understood the task, all participants included in the subsequent analysis met two criteria: criteria of probability of correct recall greater than zero for either pair type by the third trial, and probability of having made no response less than .5 for either pair type by the third trial . The first criteria resulted in the exclusion of two older adults and the second criteria resulted in the exclusion of one younger adult. Thus, 74 younger adults and 73 older adults were included in analyses. Younger adults were recruited from the Syracuse community area and consisted of a combination of Syracuse University undergraduate and graduate students. Older adults were recruited through a registry of participants from a longitudinal study run by Syracuse University. The younger adult group was recruited from flyers posted around campus during the summer and was constrained to an age range of 18 to 40 years. All of the younger participants were either undergraduate or graduate students, such that the minimum years of education for each younger participant was 13. The older adults in this study were older than in many other cognitive studies of memory: mean age for the older adult group was 80.8 (SD = 5.5) years. Mean years of education for the older adult group was 15.3 (SD = 2.4) years. All of the older participants were concurrently participating in cognitive experiments. None of these experiments, however, involved PAL. Younger adults had not previously participated in any cognitive experiments conducted in our laboratories. Both older and younger adults also performed a battery of standard cognitive tests (operation span task, numbering matching task, and a mental count/keep track task).
Materials
Study lists were composed of 8 double-function pairs consisting of 8 distinct words and 7 single-function pairs consisting of 14 distinct words. The double-function pairs were formed for each participant by randomly sampling 8 words without replacement from the noun subset of the Toronto word pool (Friendly, Franklin, Hoffman, & Rubin, 1982). The first two words were assigned to the first pair, the second and third word the second pair, etc., through the seventh pair. The eighth pair was formed by pairing the eighth word with the first word, thereby creating a circular list. Single-function pairs were formed for each participant by sampling another 14 words without replacement from the Toronto word pool. The first two words were assigned to the first pair, the third and fourth word to the second pair, etc., until the 13th and 14th words were assigned to the seventh pair. The order of the single function pairs was recorded for the purpose of obtaining a control measure for the double-function pairs. Each single-function pair’s backward associate was an item assigned from the list of ordered pairs. For instance the response term from the eighth single-function pair was assigned as the “backward associate” for the ninth single function pair despite the fact that those words were never actually presented as part of the same pair. “Remote responses” for the single-function pairs were assigned in an analogous manner. The observation of backward and remote responses to single function items could not be a consequence of specific associative learning.
Procedure
Participants were given a paired-associate learning task, followed by a 20 s distractor task, and a cued-recall test over three separate testing sessions. Each pair was presented once per trial for five consecutive trials. Stimuli were presented visually and participants were instructed to read each word aloud at presentation. The order of pair presentation was randomized.
Each word was displayed in capital letters for 1000 ms, followed by a 100 ms blank interval. Following the presentation of a pair a blank screen was presented for an additional 2000 ms before the next pair presentation. Because our interest was in examining the effect of temporal context on associative learning, pairs were presented sequentially, rather than simultaneously (Kahana et al., 2002; Howard et al., 2006).
Immediately following the study list, participants were given a 20 s arithmetic distractor task. The distractor task consisted of individually presented arithmetic problems of the form A + B + C =?, where A, B and C were positive, single-digit, integers. Participants were required to read each equation aloud and state the answer aloud. Younger participants typed the answer using the computer keyboard while older participants had an experimenter present who typed the stated answer for them. Subjects were allotted as much time as necessary on each arithmetic problem for the duration of the 20 s task.
After the distractor task the cue word from each pair was presented individually on the screen for 1000 ms. The stimulus was followed by the presentation of a row of asterisks accompanied by an auditory tone that signaled participants to recall the correct response word. Subjects were instructed to read each cue word aloud and recall the correct response to the stimulus. Subjects were encouraged to respond with the correct pair item, however, they were also encouraged to respond even if they were not completely certain of the correct response. Subjects had 7s for recall in response to each cue word. The order of test cue presentation was randomized.
The second and third experimental sessions were conducted at least one day after the previous session. New word pairs were chosen for each session with replacement. The three sessions were identical in procedure with the exception that younger participants completed a consent form and a demographic measure at the beginning of session one and did not do so for sessions two and three. Older participants had already completed consent and demographic forms for prior testing and so were not required to re-complete these forms.
There were three, presumably minor, procedural differences between the age groups. First, the experimenter accompanied the older participants the entire testing session across all three sessions. Younger participants were accompanied by the experimenter only during the instructions and first trial of session one. Second, the groups had different experimenters and some of the older participants were familiar with the experimenters from previous testing, while the younger participants had no previous experience with the experimenters. Finally, the groups were tested in different locations. The older participants were tested at an assisted living community center in Syracuse and the younger participants were tested in a lab at Syracuse University.
Results and Discussion
We examine the results of correct recall from the paired associate cued-recall data, followed by intrusion analyses.
Correct-recall Analysis
Figure 2 plots the probability of correct recalls for both age groups on single- and double-function pairs across trials. Examination of this figure indicates that single-function pairs were learned better than double-function pairs for both age groups. In addition, younger participants outperformed older participants across all trials on both pair types.
Figure 2. Correct recall analysis.
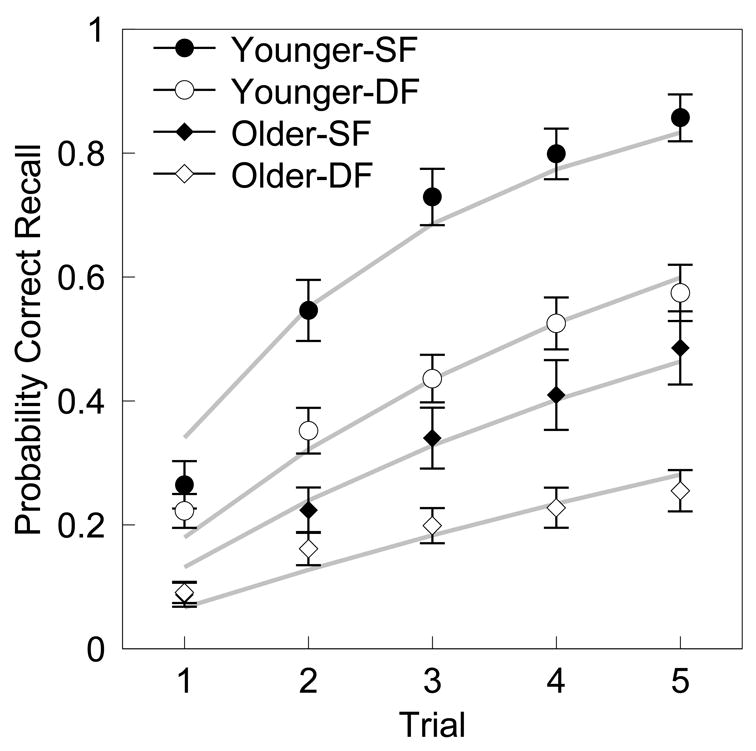
Probability of correct recall for double-function and single function pairs for each age group at each trial. There are significant main effects of age and pair-type, as well as a significant interaction of pair-type and age. The grey lines represent the negative exponential fits for probability of correct recall for each pair-type and age across trials.
An ANOVA with probability of correct-recall as the dependent measure, and age (younger or older) and pair-type (single- vs. double-function) as factors, and trial (1–5) as a regressor, showed main effects of age, F(1, 1462) = 987.8, MSe = 29.3, p < .001, η2 = .26; pair-type, F(1, 1462) = 357.9, MSe = 10.6, p < .001, η2 = .09; and trial, F(1, 1462) = 845.8, MSe = 25.1, p < .001, η2 = .22. There were also significant interactions of pair-type with trial, F(1, 1462) = 81.6, MSe = 2.4, p < .001, η2 = .02; of trial with age, F(1, 1462) = 54.3, MSe = 1.6, p < .001, η2 = .01; and of pair-type with age, F(1, 1462) = 27.7, MSe = .8, p < .001, η2 = .007. A three way interaction of pair-type by age by trial was not significant, F(1, 1462) = 0.001, MSe = .05, η2 = 8.8e-06.
The main effect of age demonstrates that younger adults had higher levels of correct recall across both pair types. The main effect of pair-type reflects the fact that the probability of correct recall was higher for single-function pairs than for double-function pairs. In other words, the contextually-related double-function pairs were more diffcult to recall than the non-overlapping single-function pairs. The main effect of trial indicates that the probability of correct recall for both pair types increased with each trial. The pair-type by trial interaction demonstrates that the probability of correct recall for single-function pairs increased more rapidly across trials than did probability of correct recall for double-function pairs. The trial by age interaction quantifies that there were greater increases in the probability of correct recall for younger adults across trials.
Importantly, though the older adults had lower overall correct recall probabilities, the pair-type by age interaction suggests that the older adults performed better on double-function pairs than might be predicted from both their single-function pair performance and the younger adults’ performance on the double-function pairs. This could be indicative of increased associative interference for younger adults as a result of backward and transitive associations. However, it is also possible that the interaction is an artifact resulting from the different levels of overall performance of the different groups.
To control for possible scaling effects we transformed the data using two standard statistical transformations for examining proportions, the arcsine and logit. An ANOVA with arcsine-transformed probability of correct-recall as the dependent measure, and age (younger or older) and pair-type (single- vs. double-function) as factors, and trial (1–5) as a regressor, showed main effects of age, F(1, 1462) = 984.2, MSe = 119217, p < .001; pair-type, F(1, 1462) = 332.9, MSe = 40329, p < .001; and trial, F(1, 1462) = 835.8, MSe = 101233, p < .001. There were also significant interactions of pair-type with trial, F(1, 1462) = 79.8, MSe = 9672, p < .001; of trial with age, F(1, 1462) = 39.6, MSe = 4800, p < .001; and of pair-type with age, F(1, 1462) = 28.7, MSe = 3479, p < .001. A three way interaction of pair-type by age by trial was not significant, F(1, 1462) = 0.3, MSe = 31, p = .6. An ANOVA with logit-transformed probability of correct-recall as the dependent measure, and age (younger or older) and pair-type (single- vs. double-function) as factors, and trial (1–5) as a regressor, showed main effects of age, F(1, 1462) = 902.3, MSe = 867.6, p < .001; pair-type, F(1, 1462) = 276.2, MSe = 265.6, p < .001; and trial, F(1, 1462) = 771.3, MSe = 741.7, p < .001. There were also significant interactions of pair-type with trial, F(1, 1462) = 74.3, MSe = 71.5, p < .001; of trial with age, F(1, 1462) = 21.8, MSe = 20.9, p < .001; and of pair-type with age, F(1, 1462) = 30.0, MSe = 28.9, p < .001. A three way interaction of pair-type by age by trial was not significant, F(1, 1462) = 2.1, MSe = 2.0, p = .1. Importantly, the critical interaction of age and pair-type remained significant after applying both the arcsine transformation, F(1, 1462) = 28.7, MSe = 3479, p < .001; and the logit transformation, F(1, 1462) = 30.0, MSe = 28.9, p < .001.
In addition to those two standard statistical transformations, we also directly modeled participants’ learning curves. In an experiment like this one, the probability of a correct response on any trial is a function of learning, but does not directly measure it. We fit a negative exponential function to the participants’ learning curves across trials, P(C) = 1 –;exp–lt), where P(C) is the probability of a correct response, l is the learning rate and t is the trial number. The model assumes that with suffcient training, P(C) eventually saturates at 1.0 and that the accuracy at trial zero (prior to any learning) is 0.0. These simplifying assumptions enable us to summarize the entire learning curve with a single parameter interpretable as the learning rate. The negative exponential was fit using the nonlinear mixed modeling procedure SAS (PROC NLMIXED). Separate models were fit for single-function and double-function lists and for younger and older participants. Learning rate was treated as a random effect and allowed to vary across individuals. This method provides an unbiased estimator of learning rate even as performance tends to zero or one. As such, it should be insensitive to age differences in the level of recall attained.
The younger adults’ mean learning rate was .44± .03 for the single-function pairs, and .21± .01 for the double-function pairs. The older adults’ mean learning rate was .15± .01 for the single-function pairs and .07 ± .01 for the double-function pairs. The difference in the learning rates between single-function and double-function pairs was significantly greater than zero for both younger, .23 ± .025, t(144) = 11.2, and older, .08 ± .008, t(144) = 11.2 adults. Critically, the difference between learning rates for single- and double-function pairs was greater for younger adults than for older adults, t(144) = 8.7; p < .001.
These analyses provide convergent support for our initial observation of a significant interaction of age and pair-type on the probability of correct recall. We suggest older adults formed fewer backward and transitive associations for the double-function pairs, resulting in less associative interference on the double-function list. If there were fewer competing responses then, perhaps paradoxically, an associative deficit in item-context binding actually facilitated older adults’ double-function correct recall rates.
Intrusion Analysis
An age-mediated associative deficit, which resulted in fewer backward and transitive associations formed among double-function pairs, may have facilitated older adults’ double-function correct recall rates. However, the age by pair-type interaction does not directly measure backward and transitive associations. We examined the pattern of intrusions for younger and older adults. Responses to a cue word can be divided into 6 exhaustive non-overlapping categories (see Figure 3). Figure 4 illustrates an example of these categories using the illustrative pairs used in Figure 1. A participant can either not respond or respond to a cue. Given that there was a response, the response can either be a correct recall or an intrusion. An intrusion can either be a word that was not presented at study or a word that was presented at study. Intrusions that were not on the list are classified extra-list intrusions. Intrusions that were presented at study can be divided into two categories: non-associative intrusions and associative intrusions.
Figure 3.
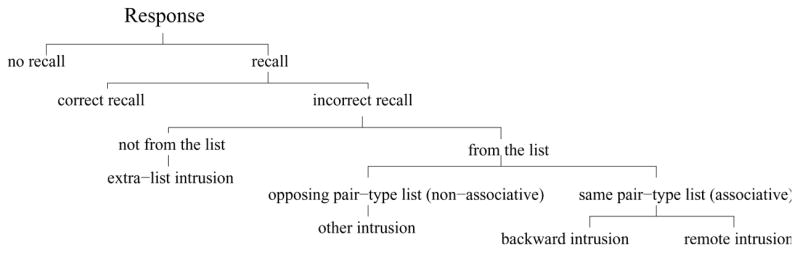
Types of response categories.
Figure 4.
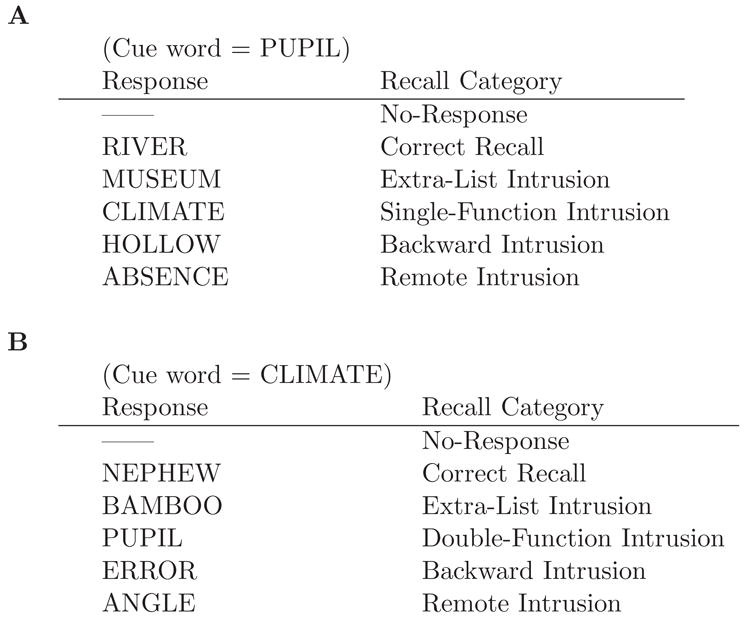
Example of the recall categories for a given cue word. The pairs refer to those listed in Figure 1. A. Double-function. B. Single-function.
Non-associative intrusions
Intrusions that were presented on the study list but that came from the other pair-type list than the cue word will be referred to as an other intrusion. For example, an intrusion of a single-function word in response to a double-function cue would be an other intrusion, or vice versa for a single-function cue (see Figure 4 for a concrete example). Other intrusions are non-associative because they cannot be the consequence of an association made between pairs.
Associative intrusions
An intrusion of the same pair type as the cue word can be classified as either a backward or as a remote intrusion. For double-function pairs, a backward intrusion would be a response to a cue that was the stimulus in the pair in which the cue word was the response. For example, given D as a cue after study of the list C-D, D-E, a response of C would be a backward intrusion. Although the cue from a single-function pair was never presented as the response of another pair, a response from a specific pair was assigned as the word that would be counted as a “backward” intrusion to that single-function pair. Each response word was assigned as the backward intrusion for precisely one cue word. For example, if the participant studied a list that included the pairs J-K, L-M, and N-O1 and was given L as a cue, then a response of K would be classified as a backward intrusion; if the cue were N, then a response of M would be classified as a backward intrusion. Remote intrusions were responses which originated from the same type of pairs as the probe item, but were neither the correct response nor a backward intrusion. For instance, if C were the cue after study of a list including the pairs A-B, B-C, C-D, and D-E, then a response of A or E would be classified as a remote intrusion. If transitive associations are formed across pairs (Bunsey & Eichenbaum, 1996; Slamecka, 1976; Howard et al., Revised), then this would be a cause of an excess of remote intrusions. For single-function cues, any single-function item that was not chosen as the backward intrusion for that cue would be classified as a remote intrusion. For instance, given the single-function cue L from the list J-K, L-M, N-O, responses of J, N, and O would be classified as remote intrusions. Because there are no overlapping items for the single-function items, the single-function remote intrusions serve as a control for the remote intrusions to double-function cues.
Table 1 displays response probabilities for each category for double-function (Table 1A) and single-function (Table 1B) probes. Each row of the table sums to 1 (up to rounding error) and the last cell of each column gives the average across all five trials. With respect to the intrusions for both pair-types, initial inspection of the table indicates that younger adults made far fewer extra-list intrusions (μDF = .06; μSF = .06) than older adults did (μDF = .18; μSF = .20). It is possible that methodological differences between the groups could have influenced group differences in extra-list intrusions. That is, older adults had an experimenter sitting with them through the experiment across all sessions, who reminded them of the instructions to guess if there were not sure of a response. The younger adults received the same instructions for recall but did not have an experimenter sitting with them after the instructions on the first session. However, the trend for older adults to produce more errors in the form of extra-list intrusions is consistent with other episodic recall data (Kahana, Dolan, Sauder, & Wingfield, 2005; Kahana et al., 2002).
Table 1.
Raw probabilities for the different types of responses to probes across trials. The column labeled “N” gives the probability of no responses. “C” gives the probability of correct recalls. “X” gives the probability of an extra-list intrusion–a word that was not presented during study of either list. “O” gives the probability of reporting an intrusion from the list opposite the cue-word list. “B” gives the probability of a backward intrusion.“R” gives the probability of a remote intrusion (see text for details). The numbers in parentheses are 95% confidence intervals. A. Double-function. B. Single-function
| A | B | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | N | C | X | O | B | R | Trial | N | C | X | O | B | R | ||||
| Younger | 1 | .14 (.04) | .22 (.03) | .11 (.03) | .17 (.03) | .18 (.02) | .17 (.03) | Younger | 1 | .18 (.05) | .26 (.04) | .12 (.03) | .25 (.03) | .01 (.005) | .16 (.03) | ||
| 2 | .08 (.03) | .35 (.04) | .06 (.01) | .14 (.02) | .21 (.02) | .15 (.02) | 2 | .09 (.03) | .55 (.05) | .07 (.02) | .15 (.03) | .007 (.004) | .13 (.03) | ||||
| 3 | .06 (.02) | .44 (.04) | .05 (.02) | .10 (.02) | .22 (.02) | .14 (.02) | 3 | .05 (.02) | .73 (.05) | .05 (.01) | .08 (.02) | .01 (.006) | .07 (.02) | ||||
| 4 | .04 (.02) | .53 (.04) | .04 (.01) | .08 (.02) | .20 (.03) | .11 (.02) | 4 | .03 (.01) | .80 (.04) | .04 (.01) | .06 (.02) | .004 (.003) | .06 (.02) | ||||
| 5 | .03 (.01) | .57 (.05) | .04 (.02) | .06 (.02) | .19 (.03) | .11 (.02) | 5 | .02 (.01) | .86 (.04) | .04 (.01) | .05 (.02) | .005 (.004) | .03 (.01) | ||||
| μ | .07 | .42 | .06 | .11 | .20 | .13 | μ | .08 | .64 | .06 | .12 | .007 | .09 | ||||
|
| |||||||||||||||||
| Older | 1 | .18 (.05) | .09 (.02) | .29 (.05) | .15 (.03) | .09 (.02) | .17 (.03) | Older | 1 | .18 (.05) | .09 (.02) | .32 (.05) | .26 (.04) | .007 (.004) | .13 (.02) | ||
| 2 | .10 (.04) | .16 (.03) | .19 (.05) | .22 (.03) | .14 (.02) | .18 (.03) | 2 | .11 (.03) | .22 (.04) | .22 (.04) | .24 (.04) | .01 (.006) | .18 (.03) | ||||
| 3 | .10 (.04) | .20 (.03) | .15 (.04) | .21 (.03) | .16 (.02) | .17 (.03) | 3 | .10 (.04) | .34 (.05) | .16 (.04) | .22 (.03) | .02 (.007) | .16 (.03) | ||||
| 4 | .08 (.03) | .23 (.03) | .16 (.04) | .18 (.03) | .19 (.03) | .15 (.02) | 4 | .09 (.03) | .41 (.06) | .15 (.04) | .17 (.03) | .02 (.008) | .15 (.03) | ||||
| 5 | .07 (.03) | .26 (.03) | .13 (.03) | .19 (.03) | .20 (.03) | .16 (.02) | 5 | .06 (.02) | .49 (.06) | .13 (.03) | .16 (.03) | .01 (.006) | .15 (.03) | ||||
| μ | .11 | .19 | .18 | .19 | .16 | .17s | μ | .11 | .31 | .20 | .21 | .01 | .15 | ||||
Another notable difference between the groups, with respect to intrusion trends, is the proportion of associative (backward and remote) to non-associative errors made on double-function pairs (Table 1A). Across trials, younger adults made a larger proportion of backward (μ = .20) and remote (μ = .13) intrusions than other (μ = .11) intrusions. By the fifth trial, younger adults made about three times more backward than other intrusions, and about twice as many remote intrusions as other intrusions to double-function probes. The younger adults’ propensity to make backward intrusions may be because backward intrusions occur in the same temporal context as the cue item. For example, if the cue item is D from the list C-D, D-E, the backward associate, C, was presented in the same temporal context as D. This trend to make backward intrusions, however, does not follow suit for older adults. By trial five older adults made a larger proportion of other intrusions (μ = .19) relative to both backward (μ = 16) and remote (μ = .17) intrusions. The older adults’ propensity to make more other intrusions may be a consequence of guessing words from the experiment. Given a double-function cue there were 14 possible words that comprised the single-function list. Given the same double-function cue, there were only four remote items left from which to guess from the double-function list. For example, consider the list A-B, B-C, C-D, D-E, E-F, F-G, G-H, H-A. If the cue item was D, a remote intrusion by definition excludes the item itself (D), the correct response (E), and the backward intrusion (C). The low probability of remote intrusions for older adults on double-function pairs is consistent with a guessing hypothesis because there were more other response options. The younger adults higher probability of remote intrusions than other intrusions, however, does not fit with a guessing hypothesis. Indeed, in response to the single function probes younger and older adults showed roughly similar ratios of remote intrusions to other intrusions (Table 1B). Instead, we suggest younger adults made significantly more remote intrusions than other intrusions to double-function probes due an intact associative mechanism that allowed item-context binding among items not presented together in time.
Initial inspection of the raw intrusion data suggest that younger adults made a higher proportion of associative intrusions than older adults. However, each row of the table is constrained to sum to one, such that an increase in one category of the figure necessarily means decreases in all other categories. For example, younger adults made substantially more correct recalls than older adults. Therefore a direct comparison of younger adult intrusion probabilities is negatively biased in favor of older adult intrusions. To quantify the differences between associative and non-associative intrusions and to control for differences in the magnitude of intrusions across age groups we calculated an associative intrusion ratio. The associative intrusion ratio was calculated for each participant on each trial, as the difference in the probability of making an associative vs. a non-associative intrusion divided by the sum of the probabilities of making an associative or non-associative intrusion:
where B and R are the rate of associative intrusions and O is the rate of non-associative intrusions. This method ensures the intrusion analysis includes only study list items and ignores extra-list intrusions, while controlling for differences in overall intrusion magnitudes (and probability of recall) across groups. The associative intrusion ratio can take values from −1 to +1. An index of −1 would indicate that the intrusions were all non-associative (i.e., B = 0 and R = 0 while O > 0). An index of zero would indicate that an equivalent proportion of non-associative and associative intrusions was made. An index of +1 would indicate that the intrusions were all either backward or remote intrusions (i.e., B or R).
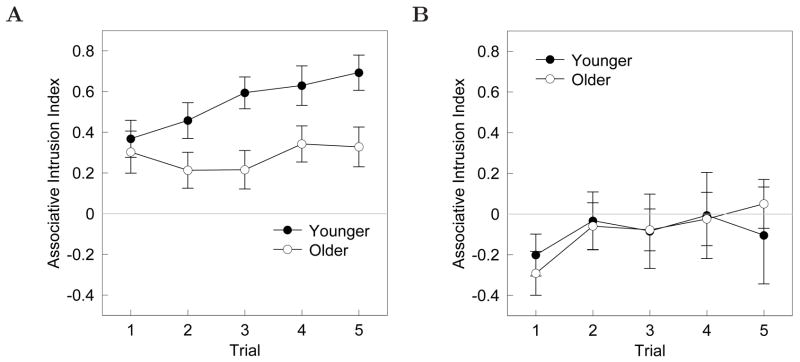
Figure 5 illustrates the associative intrusion ratios for double- (Figure 5A) and single-function (Figure 5B) pairs. The younger adults made a greater proportion of associative than non-associative intrusions on the double-function cues and the proportion of associative intrusions increased with trial. The older adults made a smaller proportion of backward and remote intrusions to double-function pairs than the younger adults, and the proportion of associative intrusions the older adults made did not significantly increase with trial. The single-function associative intrusion ratio (Figure 5B) demonstrates that younger and older adults did not have significantly different intrusion trends for the non-overlapping pairs. Specifically, younger and older adults tended to make slightly more non-associative errors in response to single-function pairs, though the error bars indicate both groups had an index of approximately zero on trials two through five. An ANOVA with the associative intrusion ratio as the dependent measure, age and pair-type as factors, and trial as a regressor, showed main effects of trial, F(1, 1384) = 33.0, MSe = 7.8, p < .001, η2 = .02; pair-type, F(1, 1384) = 359.9, MSe = 84.5, p < .001, η2 = .19; and age, F(1, 1384) = 36.8, MSe = 8.7, p < .001, η2 = .2. Additionally there was an interaction of pair-type and age, F(1, 1384), = 25.5, MSe = 6.0, p < .001, η2 = .01; and a three-way interaction of age by pair-type by trial, F(1, 1384) = 8.4, MSe = 2.0, p < .01, η2 = .005. The main effects of trial, pair-type, and age indicate that the associative intrusion ratio changed significantly across trial, differed for the single- and double-function pairs, and was reliably different across the age groups, respectively. The interaction of pair-type and age confirms that the younger and older adults produced disproportionate amounts of associative and non-associative intrusions, such that younger adults made more associative intrusions to double-function cues than older adults did. The pair-type by age by trial interaction demonstrates that older and younger adults not only differed with respect to the proportions of associative to non-associative intrusions to double-function cues, but that the older and younger adults’ intrusion trends, across trials, followed different trajectories.
Figure 5. Intrusion analysis.
Associative intrusion ratios plotted as a function of age across trial. Associative intrusions are responses from the same list as the cue word. Positive numbers indicate a higher probability of making an associative intrusion with a maximum score of +1. Non-associative intrusions are responses from the opposite list of the cue word. Negative numbers indicate a greater probability of making an non-associative intrusion with a maximum score of −1. The grey line indicates the probability of an equivalent proportion of associative and non-associative intrusions. Error bars are 95% confidence intervals. A. Double-function. B. Single-function.
Taken in isolation, the effect observed in Figure 5A could be argued to be due to an item repetition effect. Although single-function and double-function pairs were presented an equal number of times, the double-function words were presented in two different pairs, once as a stimulus and once as a response, and so received twice as many exposures as the single-function words. If younger adults benefited from extra presentations of the double-function words, then they would also be more likely to make double-function intrusion errors in response to single-function probes. The intrusions to single-function pairs provide a control measure against which to compare the intrusions to double-function pairs. The item repetition effect hypothesis would be supported if younger adults made a greater proportion of O intrusions in response to single-function probes than older adults did. The single-function associative intrusion ratio (Figure 5B) illustrates that both the younger and older adults made similar proportions of associative and non-associative intrusions on single-function pairs across all five trials. This argues against an item repetition effect.
In addition to providing a control measure for the item repetition effect, the single-function associative intrusion ratio also provides a control for type selection effects. Suppose that participants identified cues as a belonging to a particular pair-type (single- or double-function) and used that information to guess from the corresponding list. If younger adults were better at identifying the types of cues, or perhaps at tagging responses as members of a pair of a particular type, then this could account for the result that younger adults made more associative intrusions to double-function cues. If this were the case, however, then the younger adults should also have made more associative intrusions on the single-function pairs, despite the fact that there is no overlapping context between single-function pairs. Inspection of Figure 5B reveals that younger adults did not make more associative than non-associative intrusions to the single-function cues, nor did they differ from older adults in this region. This strongly suggests that the type selection effects do not explain the age differences in associative intrusion proportions manifest in Figure 5A.
To differentiate the B and R intrusions, as well as to further quantify differences among backward, transitive, and non-associative intrusions, we performed further analyses. We first transformed the B, R and O intrusion rates to describe the proportion of intrusions of study items that belonged to each category. For instance, . We then performed Wilcoxon rank-sum tests with Bonferroni’s correction between the age groups for B′ and O′, O′ and R′, and R′ and B′ intrusions across trials by pair-type. As suggested by the single-function associative intrusion ratio (Fig 5B), there were no significant group differences between the three single-function pairwise intrusion comparisons. There were, however, group differences among the double-function pairwise intrusion comparisons. For the first comparison, given either B′ or O′ intrusions, younger adults made a larger proportion of B′ intrusions than older adults across all five trials, (p < .005). For the second comparison, given either O′ or R′ intrusions, both groups made equivalent proportions of each intrusion type for trials one and two. However, younger adults made larger proportions of R′ intrusions than older adults on trials three though five (p < .005). For the third comparison, given either B′ or R′ intrusions, younger adults made larger proportions of B′ intrusions across all trials (p < .05). Taken together, these pairwise comparisons suggest that the B′ associate was the primary source of response interference for the younger adults on double-function cues.
The older adults may be impaired at item-context binding, which resulted in more guessing from the lists. This guessing is demonstrated in the older adults’ propensity to have larger overall proportions of O and R intrusions. Importantly, when the O′ and R′ intrusions were directly compared, the older adults made approximately equivalent proportions of the two responses across all trials. However, the younger adults made a larger proportion of R′ intrusions on the last three trials, suggesting the younger adults were learning the associative structure.
A methodological difference between the groups was that only the older adult group had an experimenter sitting with them the entire experiment. It is possible that the age by pair-type interaction in the associative intrusion ratio is due to reminders from the experimenter to guess if unsure. That is, rather than differences in the associative intrusion ratio being driven by a decrease in B′ and R′ for older adults, perhaps it was driven by an artifactual increase in O′ for older adults.2 If this guessing artifact accounted for our results, we would expect to see the age difference reverse, or disappear, if we replaced O intrusions with the rate at which participants failed to make any response at all, N. That is, encouragement to guess for the older adults should inflate O, but necessarily decrease N. Accordingly, we calculated an alternate associative intrusion ratio:
precisely analogous to that calculated above except that we replace the non-associative intrusion rate with the rate at which participants failed to make any response to the probes. An ANOVA with the alternate associative intrusion ratio as the dependent measure, age and pair-type as factors, and trial as a regressor, showed a marginally significant main effect of age, F(1, 1368) = 3.7, MSe = 1.3, p = .05, η2 = .002; and main effects of pair-type, F(1, 1368) = 101.1, MSe = 34.9, p < .001, η2 = .07; and trial, F(1, 1368) = 63.8, MSe = 22.1, p < .001, η2 = .04. Critically, there was a significant interaction of age and pair-type, F(1, 1368) = 9.1, MSe = 3.1, p < .01, η2 = .006. The interaction results from the fact that the mean alternate associative intrusion ratio for younger subjects for double-function pairs, .70± .04, is much larger than that for older subjects, .55± .06, t(145) = 2.27, p < .03, whereas for the single-function pairs, there is no significant difference between the mean for younger, .26 ± .08, and older adults, .34 ± .07, t(145) = .78.
These results, which control for the overall rate of incorrect responses, are closely analogous to those observed for the associative intrusion ratio. Because the methodological differences across age groups would be expected to reverse the direction of the age effect in the alternate associative intrusion ratio, the absence of this finding leads us to conclude that older adults failed to make as many associative intrusions as younger adults.
General Discussion
We employed a paired-associate task to quantify age differences in the associative structure induced by standard paired-associate learning (PAL) pairs and pairs with overlapping contexts. Standard PAL single-function pairs with non-overlapping contexts (e.g., J-K, L-M) and double-function pairs with overlapping contexts (e.g., A-B, B-C) were randomly presented to groups of younger and older adults. According to the temporal context model (TCM, Howard & Kahana, 2002; Howard et al., 2005), if older adults are impaired at binding items to the temporal contexts in which they were presented (Naveh-Benjamin, 2000; Howard et al., 2006), then we should see a decrease in backward (e.g. B-A) and transitive (e.g. A-C) associations for older adults. Younger adults showed higher probabilities of correct recall for both single- and double-function pairs (Figure 2). Additionally, both negative exponential fits to the correct recall data (Figure 2), as well as two standard statistical transformations, provide convergent evidence of an age by pair-type interaction on correct recall. The age by pair-type interaction, which suggests the older adults had better double-function pair performance than would be expected from their single-function pair performance, is consistent with the hypothesis that the older adults formed weaker backward and transitive associations among the double-function items than did the younger adults.
We were also able to find direct support for the hypothesis that older adults were impaired at forming backward and transitive associations by examining intrusion rates (see Table 1). To allow a fair comparison of older adults’ rates of backward and remote intrusions we calculated the proportion of intrusions of list items that were backward, remote, or other intrusions (see Figures 3 and 4). We found that a higher proportion of younger adults’ intrusions of list items to double-function cues were backward and remote intrusions (Figure 5A). No such relationship was observed for intrusions to single-function cues (Figure 5B), allowing us to rule out several uninteresting accounts of the excess of backward and remote intrusions for younger adults. Instead, as suggested by TCM coupled with the associative deficit hypothesis, the younger adults’ propensity to make a larger proportion of backward and remote intrusions to double-function pairs may be a natural consequence of intact item-context binding. The hypothesis that older adults are particularly impaired at item-context binding is also supported by evidence arguing for a greater effect of aging on recollection than on familiarity in recognition memory (Healy et al., 2005; Howard, Bessette-Symons, Zhang, & Hoyer, 2006; Light et al., 2004; Prull et al., 2006).
Although older adults made disproportionately fewer associative intrusions than younger adults, this deficit was not due to an inability to generate responses. Consistent with previous findings, the older adults made more incorrect recalls (intrusions) than the younger adults (Kliegl & Lindenberger, 1993; Zacks, Radvansky, & Hasher, 1996; Balota et al., 1999; Naveh-Benjamin, 2000; Kahana et al., 2002, 2005). Associative retrieval accounts of episodic memory characterize response generation as a process in which potential recalls are first sampled, followed by an editing process that should limit responses to those items that were part of the target list/pair (Raaijmakers & Shiffrin, 1981). The older adults’ tendency to globally produce more intrusions than younger adults may be due to a reduced ability to recognize that a generated intrusion was not part of the target list/pair (Zaromb et al., 2005; Kahana et al., 2005). Hasher and Zacks (1988) suggest this may reflect an inability to inhibit extraneous associations formed in previous contexts, and is consistent with prior research demonstrating an age-related deficit in the ability to inhibit non-list items generated at recall (Kahana et al., 2005). The importance of inhibition-based accounts of cognitive aging may lead one to consider the hypothesis that reason older adults made fewer associative intrusions due to an increased ability to inhibit these incorrect responses rather than a decreased ability to form such associations in the first place. While this “enhanced-inhibition” hypothesis is possible, it does not account for the increase in extra-list and other intrusions (which presumably should be subject to inhibition as well), the reduced difference between correct recall on single- and double-function pairs for older adults, nor the decrease in overall levels of recall. It is much more parsimonious to suppose that older adults are impaired at forming and/or utilizing backward and remote associations. This is a natural outcome of the hypothesis that older adults are impaired at forming item-to-context bonds as described in TCM.
The finding that older adults generated a reduced proportion of associative intrusions to double-function cues could suggest the mechanism(s) that mediates the formation of flexible declarative associations undergoes damage with age. Gluck and Myers (1997) suggested the hippocampus is necessary for making arbitrary associations between abstract stimuli. Recent neurophysiological and neuroanatomical evidence from humans and non-human primates demonstrates that one role of the hippocampal formation is to facilitate such associative learning (Eichenbaum, 2000; Heckers, Zalesak, Weiss, Ditman, & Titone, 2004; Henke, Weber, Kneifel, Wieser, & Buck, 1999; Henke, Buck, Weber, & Wieser, 1997; Wallenstein, Eichenbaum, & Hasselmo, 1998; Squire & Zola-Morgan, 1991). Bunsey and Eichenbaum (1996) specifically examined associations among arbitrary stimuli with olfactory learning in rats. Much as in human PAL tasks, the rats formed specific associations between stimuli and later identified the associated choice that followed each stimulus. Rats with hippocampal lesions successfully learned associations between odors A and B, and between B and C. However, in contrast to non-lesioned rats, lesioned rats did not demonstrate either a transitive generalization to the association A →C or a backward generalization to the association B →A. The authors suggest these results demonstrate that the hippocampus is involved in forming flexible representations of associations between stimuli. TCM expands on this statement by describing the computations—item-context binding and successful retrieval—necessary for such associations (Howard et al., 2005). Generalizing these lesioning results to aging, if the hippocampus is suggested to mediate associative learning and associative learning is decremented with age, then the integrity of the hippocampus could be compromised with age. In fact, hippocampal dysfunction has been shown to contribute to the associative memory deficits observed during normal aging in old humans, monkeys, and rats (Erickson & Barnes, 2003). The hypothesis that the hippocampus is important in item-context binding and that this functioning is compromised with normal aging provides a natural account of the findings in the present study.
The PAL task provides a unique situation in which both correct and incorrect responses to studied cues provide informative indicators of associative functioning. However, due to the generative nature of PAL tests, intrusions are subject to confounds specific to verbal stimuli. For example, lists of semantically or phonologically related verbal stimuli are potentially vulnerable to the possibility of differential strategy production. Prior studies have demonstrated that differential strategy production, concordant with age, may unduly influence item encoding (Dunlosky & Hertzog, 1998; Kausler & Lair, 1966; Kausler, 1994). Because the present study examined age-related differences in temporally based associative processes, our findings are presumably unlikely to be attributable to differential strategy production, the role of semantic relatedness, or the role of phonological similarities between list items and subsequent intrusions (Deese, 1959; Roediger & McDermott, 1995, e.g., false memory paradigms). Any such residual differences should have affected O′ intrusions as much as B’ or R’ intrusions.
Another confound specific to verbal generation measures is disproportionate recall output between age groups. Consistent with previous findings, the older adults demonstrated comparatively lower overall correct recall rates (Naveh-Benjamin, 2000; Kahana et al., 2005) and made more extra-list intrusions than younger adults (Kahana et al., 2005). To circumvent group differences in recall output other studies of associative learning have employed recognition paradigms (Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003, 2004; Bastin & Van der Linden, 2006; Castel & Craik, 2003; Light et al., 2004; Healy et al., 2005; Prull et al., 2006). Recognition paradigms remove sources of additional variance specific to recall paradigms. Moreover, false alarm rates provide a means to directly probe “intrusions” to specific combinations of stimuli. We suggest the associative recognition paradigm capitalizes upon the benefits of the recognition methodology while still allowing for manipulation of context in the specific lags of re-arranged test pairs. Tests of associative recognition could be beneficial toward the end of identifying the underlying components of age-mediated associative deficits and a coherent picture of aging.
Conclusions
We studied age differences in the associative structure induced by learning mixed lists of double-function and single-function pairs and found evidence, from both correct recall probabilities and intrusion analysis, of age-mediated associative differences. Motivated by the theoretical framework of temporally-defined episodic associations that depend upon contextual retrieval (TCM Howard & Kahana, 2002) and the hypothesis that aging is associated with decreased context-binding (Howard et al., 2006; Naveh-Benjamin, 2000), we predicted that intact associative memory would result in associations among items with overlapping contexts and create additional response interference for the double-function pairs. Learning rates estimated from participants’ learning curves, as well as two standard statistical transformations, all argue that this finding is not an artifact in overall levels of recall across age groups. To directly measure associative interference we performed an intrusion analysis, constrained to include only items that were presented at study. Younger adults made a greater proportion of associative intrusions to double-function cues than did older adults. We suggest an age-mediated associative deficit, due to an inability to generate item-context binding, was manifest as fewer associative intrusions to highly associative cues. Thus, we provide evidence from intrusion analysis that contextually overlapping associations decrease as a function of age and suggest that in addition to traditional PAL correct recall analysis, intrusion analysis can contribute a novel analytic technique for studying the mechanisms suggested to account for age-related associative deficits.
Acknowledgments
This research was supported in part by grants from the National Institute of Mental Health (MH-069938 to MWH) and the National Institute on Aging (AG-12448 to MJS). The authors thank Paul Verhaeghen, Randall Jorgensen, Moshe Naveh-Benjamin, and an anonymous reviewer for their helpful comments on an earlier version of this article. Thanks also to Donna Bridge, George Gerard, Donna Martello-Boyce, Lise Mayo, Clarion Mendes, Susan Sliwinski, Vijay Venkatadass, Tess Youker, Michael Zaremba, and Yaofei Zhang for assistance with data collection. Correspondence concerning this article should be addressed to Jennifer Provyn, Syracuse University, 570 Huntington Hall, Syracuse, NY, 13244–2340, jpprovyn AT syr DOT edu.
Footnotes
This list does not refer to the order in which the pairs were presented, but would correspond to the list formed after sampling the word pool to construct the pairs. See also the methods section.
This was potential confound was pointed out by an anonymous reviewer.
References
- Balota DA, Cortese M, Duchek J, Adams D, Roediger H, McDermott K, Yerys B. Veridical and false memories in healthy older adults and in dementia of the alzheimer’s type. Neuropsychology. 1999;16:361–384. [Google Scholar]
- Bastin C, Van der Linden M. The effects of aging on the recognition of different types of associations. Experimental Aging Research. 2006;32(1):61–77. doi: 10.1080/03610730500326291. [DOI] [PubMed] [Google Scholar]
- Brinley JF. Cognitive sets, speed and accuracy of performance in the elderly. In: Welford AT, Birren JE, editors. Behavior, aging and the nervous system. Springfield, IL: Charles C Thomas; 1965. pp. 114–149. [Google Scholar]
- Bunsey M, Eichenbaum HB. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379(6562):255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Castel AD, Craik FI. The effects of aging and divided attention on memory for item and associative information. Psychology and Aging. 2003;18(4):873–85. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Cerella J. Information processing rates in the elderly. Psychological Bulletin. 1985;98(1):67–83. [PubMed] [Google Scholar]
- Cerella J. Age effects may be global, not local: comment on Fisk and Rogers (1991) Journal of Experimental Psychology: General. 1991;120(2):215–23. doi: 10.1037/0096-3445.120.2.215. [DOI] [PubMed] [Google Scholar]
- Cerella J. Generalized slowing in brinley plots. Journal of Gerontology. 1994;49(2):P65–71. doi: 10.1093/geronj/49.2.p65. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Collie A, Myers C, Schnirman G, Wood S, Maruff P. Selectively impaired associative learning in older people with cognitive decline. Journal of Cognitive Neuroscience. 2002;14(3):484–92. doi: 10.1162/089892902317361994. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, Hertzog C. Aging and deficits in associative memory: what is the role of strategy production? Psychology and Aging. 1998;13(4):597–607. doi: 10.1037//0882-7974.13.4.597. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews, Neuroscience. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Experimental Gerontology. 2003;38(1–2):61–9. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Friendly M, Franklin PE, Hoffman D, Rubin DC. The Toronto Word Pool: Norms for imagery, concreteness, orthographic variables, and grammatical usage for 1,080 words. Behavior Research Methods and Instrumentation. 1982;14:375–399. [Google Scholar]
- Gluck MA, Myers CE. Psychobiological models of hippocampal function in learning and memory. Annual Review of Psychology. 1997;48:481–514. doi: 10.1146/annurev.psych.48.1.481. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review of a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: evidence from receiver operating characteristics. Journal of Experimental Psychology : Learning, Memory, and Cognition. 2005;31:768–88. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14(2):153–62. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7(3):249–56. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proceedings of the National Academy of Science, USA. 1999;96(10):5884–9. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: Evidence from modeling and roc curves. Psychology & Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: Toward a common explanation of medial temporal lobe function across domains. Psychological Review. 2005;112(1):75–166. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Jing B, Rao VA, Provyn JP, Datey AV. Bridging the gap: Transitive associations are formed from learning items in similar temporal contexts. doi: 10.1037/a0015002. Revised. [DOI] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A distributed representation of temporal context. Journal of Mathematical Psychology. 2002;46(3):269–299. [Google Scholar]
- Howard MW, Kahana MJ, Wingfield A. Aging and contextual binding: Modeling recency and lag-recency effects with the temporal context model. Psychonomic Bulletin and Review. 2006;13(3):439–445. doi: 10.3758/bf03193867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Dixon RA. The role of pre-experimental knowledge in text processing in adulthood. Experimental Aging Research. 1983;9(1):17–22. doi: 10.1080/03610738308258414. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Dolan ED, Sauder CL, Wingfield A. Intrusions in episodic recall: age differences in editing of overt responses. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2005;60(2):P92–7. doi: 10.1093/geronb/60.2.p92. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Howard MW, Zaromb F, Wingfield A. Age dissociates recency and lag-recency effects in free recall. Journal of Experimental Psychology : Learning, Memory, and Cognition. 2002;28:530–540. doi: 10.1037//0278-7393.28.3.530. [DOI] [PubMed] [Google Scholar]
- Kausler DH. Learning and memory in normal aging. San Diego: Academic Press; 1994. [Google Scholar]
- Kausler DH, Lair CV. Associative strength and paired-associate learning in elderly subjects. Journal of Gerontology. 1966;21(2):278–80. doi: 10.1093/geronj/21.2.278. [DOI] [PubMed] [Google Scholar]
- Kliegl R, Lindenberger U. Modeling intrusions and correct recall in episodic memory: adult age differences in encoding of list context. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19(3):617–37. doi: 10.1037//0278-7393.19.3.617. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–76. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL, Patterson MM, Chung C, Healy MR. Effects of repetition and response deadline on associative recognition in young and older adults. Memory & Cognition. 2004;32(7):1182–93. doi: 10.3758/bf03196891. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D’Esposito M. Aging and reflective processes of working memory: binding and test load deficits. Psychology and Aging. 2000;15(3):527–41. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult-age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology : Learning, Memory, and Cognition . 2000;26(5):1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: further support using face-name associations. Psychology and Aging. 2004;19(3):541–6. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: further support for an associative-deficit hypothesis. Journal of Experimental Psychology : Learning, Memory, and Cognition. 2003;29(5):826–37. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Primoff E. Backward and forward associations as an organizing act in serial and in paired-associate learning. Journal of Psychology. 1938;5:375–395. [Google Scholar]
- Prull MW, Dawes LL, Martin AM, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychology and Aging. 2006;21(1):107–18. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JGW, Shiffrin RM. Search of associative memory. Psychological Review. 1981;88:93–134. [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: remembering words not presented in lists. Journal of Experimental Psychology : Learning, Memory, and Cognition. 1995;21:803–814. [Google Scholar]
- Salthouse TA. A theory of cognitive aging. Amsterdam: North-Holland; 1985. [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological review. 1996;103(3):403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Slamecka NJ. An analysis of double-function lists. Memory & Cognition. 1976;4:581–585. doi: 10.3758/BF03213221. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Tun PA. Age differences in processing expository and narrative text. Journal of Gerontology: Psychological Sciences. 1989;44(1):P9–15. doi: 10.1093/geronj/44.1.p9. [DOI] [PubMed] [Google Scholar]
- Umemoto T, Hilgard ER. Paired-associate learning as a function of similarity: common stimulus and response items within the list. Journal of Experimental Psychology. 1961;62:97–104. doi: 10.1037/h0046961. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends in Neurosciences. 1998;21(8):317–23. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Young RK. A comparison of two methods of learning serial associations. American Journal of Psychology. 1959;72:554–9. [PubMed] [Google Scholar]
- Young RK. Paired-associate learning when the same items occur as stimuli and responses. Journal of Experimental Psychology. 1961;61:315–8. doi: 10.1037/h0047911. [DOI] [PubMed] [Google Scholar]
- Young RK, Jennings PC. Backward learning when the same items serve as stimuli and responses. Journal of Experimental Psychology. 1964;68:64–70. doi: 10.1037/h0043104. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Radvansky G, Hasher L. Studies of directed forgetting in older adults. Journal of Experimental Psychology : Learning, Memory, and Cognition. 1996;22(1):143–56. doi: 10.1037//0278-7393.22.1.143. [DOI] [PubMed] [Google Scholar]
- Zaromb FM, Howard MW, Dolan ED, Sirotin YB, Tully M, Wingfield A, Kahana MJ. Temporally-based false memories in free recall. Journal of Experimental Psychology : Learning, Memoary, and Cognition. 2005;32(4):792–804. doi: 10.1037/0278-7393.32.4.792. [DOI] [PubMed] [Google Scholar]