Abstract
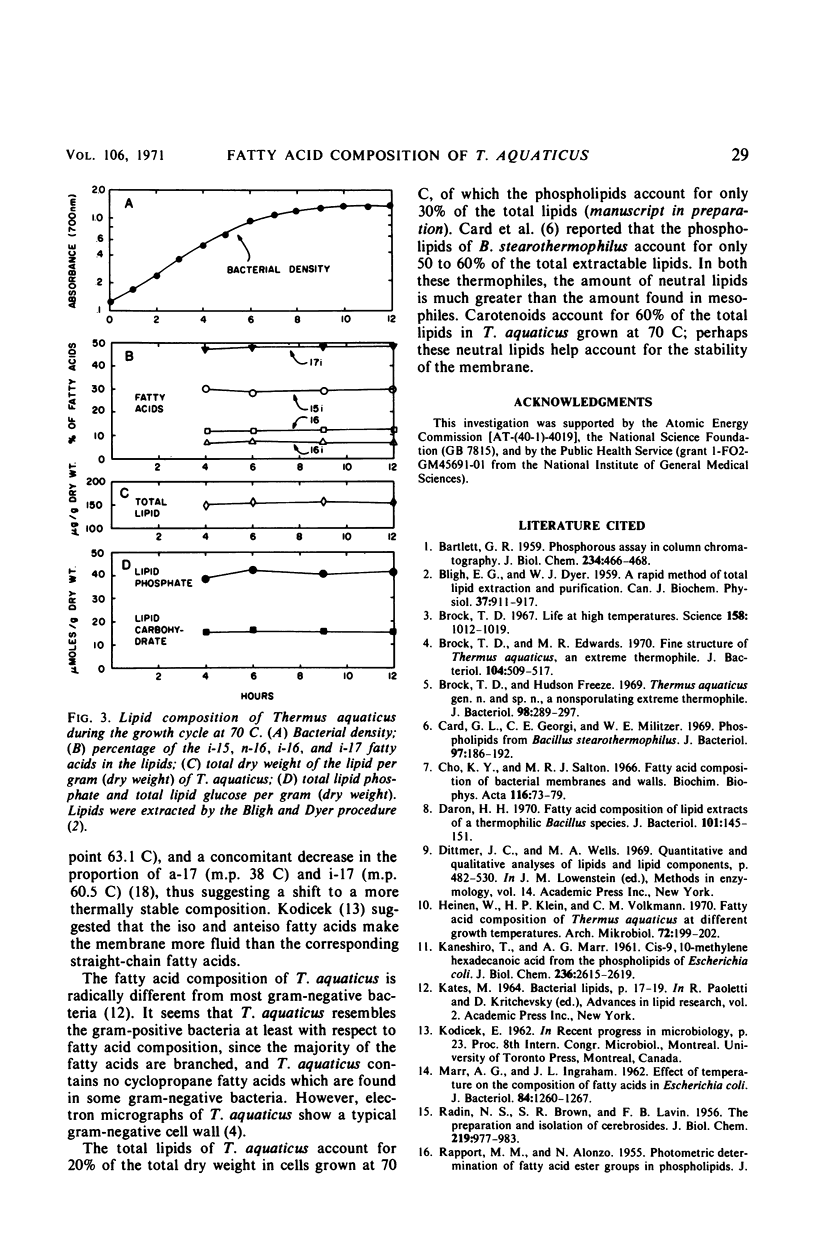
Thermus aquaticus contains four major fatty acids, iso-C15 (28%), iso-C16 (9%), normal-C16 (13%), and iso-C17 (48%), when grown at 70 C, as determined by gas chromatography and mass spectrometry. Small amounts of iso-C12, normal-C12:1, iso-C13, normal-C14, iso-C14, and normal-C15:1 were also detected. A change in growth temperature (50 to 75 C at 5-C intervals) affects a shift in the proportions of some of the fatty acids. The proportions of the monoenoic and branched-C17 fatty acids decreased and the proportions of the higher-melting iso-C16 and normal-C16 fatty acids increased. Cells grown at 75 C contained 70% more total fatty acids than cells grown at 50 C. The largest increases, in absolute amounts, were in the content of iso-C16 and normal-C16 fatty acids, with only a 1.6-fold increase in the major iso-C15 and iso-C17 fatty acids. There was a 2.5-fold decrease in normal-C15:1 and at least a 24-fold decrease in anteiso-C17, which is present at 50 and 55 C but not at higher temperatures. There was no difference in proportion or amount of fatty acids between exponential and stationary-phase cells grown at 70 C. When cells were grown on glutamate instead of yeast-extract and tryptone at 70 C, the total fatty acid content remained constant, but there was an increase in the proportions of iso-C16 and normal-C16 fatty acids concomitant with a decrease in the proportions of the iso-C15 and iso-C17 fatty acids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Edwards M. R. Fine structure of Thermus aquaticus, an extreme thermophile. J Bacteriol. 1970 Oct;104(1):509–517. doi: 10.1128/jb.104.1.509-517.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Card G. L., Georgi C. E., Militzer W. E. Phospholipids from Bacillus stearothermophilus. J Bacteriol. 1969 Jan;97(1):186–192. doi: 10.1128/jb.97.1.186-192.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Y., Salton M. R. Fatty acid composition of bacterial membrane and wall lipids. Biochim Biophys Acta. 1966 Feb 1;116(1):73–79. doi: 10.1016/0005-2760(66)90093-2. [DOI] [PubMed] [Google Scholar]
- Daron H. H. Fatty acid composition of lipid extracts of a thermophilic Bacillus species. J Bacteriol. 1970 Jan;101(1):145–151. doi: 10.1128/jb.101.1.145-151.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen W., Klein H. P., Volkmann C. M. Fatty acid composition of Thermus aquaticus at different growth temperatures. Arch Mikrobiol. 1970;72(2):199–202. doi: 10.1007/BF00409525. [DOI] [PubMed] [Google Scholar]
- KANESHIRO T., MARR A. G. cis-9,10-Methylene hexadecanoic acid from the phospholipids of Escherichia coli. J Biol Chem. 1961 Oct;236:2615–2619. [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADIN N. S., BROWN J. R., LAVIN F. B. The preparative isolation of cerebrosides. J Biol Chem. 1956 Apr;219(2):977–983. [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- Shen P. Y., Coles E., Foote J. L., Stenesh J. Fatty acid distribution in mesophilic and thermophilic strains of the genus Bacillus. J Bacteriol. 1970 Aug;103(2):479–481. doi: 10.1128/jb.103.2.479-481.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Sweeley C. C. Characterization of the iso-branched sphinganines from the ceramide phospholipids of Bacteroides melaninogenicus. Biochim Biophys Acta. 1969 Dec 17;187(4):527–532. doi: 10.1016/0005-2760(69)90050-2. [DOI] [PubMed] [Google Scholar]


