Abstract
Purpose
To determine whether the sustained intravitreous delivery of CNTF modulates cortical response thresholds to electrical retinal stimulation in the RCS rat model of retinal degeneration.
Methods
Animals were assigned to four groups: untreated, nonsurgical control and infusion groups of 10 ng/d CNTF, 1 ng/d CNTF, and PBS vehicle control. Thresholds for electrically evoked cortical potentials (EECPs) were recorded in response to transcorneal electrical stimulation of the retina at p30 and again at p60, after a three-week infusion.
Results
As the retina degenerated over time, EECP thresholds in response to electrical retinal stimulation increased. Eyes treated with 10 ng/d CNTF demonstrated significantly greater retinal sensitivity to electrical stimulation when compared with all other groups. In addition, eyes treated with 1 ng/d CNTF demonstrated significantly greater retinal sensitivity than both PBS-treated and untreated control groups.
Conclusions
Retinal sensitivity to electrical stimulation was preserved in animals treated with chronic intravitreous infusion of CNTF. These data suggest that CNTF-mediated retinal neuroprotection may be a novel therapy that can lower stimulus thresholds in patients about to undergo retinal prosthesis implantation. Furthermore, it may maintain the long-term efficacy of these devices in patients.
Retinitis pigmentosa (RP) is a clinical term that refers to a heterogeneous group of inherited or sporadic retinal dystrophies that affect 1 in 4000 worldwide.1-3 This degeneration is characterized by a progressive loss of photoreceptors in the outer retina and later, a remodeling of higher-order neuronal circuits within the relatively spared inner retina.4-6 Ultimately, patients with RP may become blind due to failure of the retina to process visual input as a consequence of these changes. Although there are currently no effective treatments for these diseases, patients who have lost their vision because of retinal degeneration may benefit from a retinal prostheses designed to restore sensory inputs to the remaining retinal circuits.
Pioneering human trials have demonstrated that electrical stimulation of the retina can produce rudimentary visual percepts, called electrophosphenes.7-9 These studies have also revealed, however, that electrophosphene thresholds are significantly higher in patients with more advanced stages of retinal degeneration. In addition, electrical stimulation studies of retinal wholemounts in rd1 mice have confirmed that more advanced stages of retinal degeneration are associated with higher electrical stimulation thresholds.10 Thus, as a patient’s retinal degeneration progresses, the effectiveness of a sensory replacement retinal prosthesis may progressively diminish. Increased electrophosphene thresholds constrain the resolution of sensory replacement retinal prosthetics by necessitating larger electrodes with greater charge-carrying capacity. Heat generation may also increase as the currents required for electrophosphene percepts rise.
Consequently, we hypothesized that methods for retinal neuroprotection may preserve or enhance retinal sensitivity to electrical stimulation. Electrical stimulation devices have, in the past, been shown to be neuroprotective against a multitude of neuronal injuries and disease states, including those that afflict the eye. After transection of the optic nerve, the ganglion cells of the retina rapidly succumb to apoptosis within weeks.11,12 However, transcorneal electrical stimulation was found to rescue axotomized retinal ganglion cells for 1 to 2 weeks after injury.13,14 In addition to rescue of ganglion cells, electrical stimulation may be neuroprotective against inherited retinal degenerations.15 Pardue et al.15 found that subretinal implantation of a microphotodiode array in the RCS rat preserves electroretinograms (ERGs) for up to 6 weeks after surgery. The treatment effect was transient, however, and no significant functional preservation was seen at postoperative week 8.
The mechanisms of neuroprotection after electrical stimulation most likely include the modulation of endogenous growth factors. A multitude of growth factors have long been shown to enhance survival of retinal neurons.16 After injury or stress, activated microglia and Müller cells have demonstrated increased expression of CNTF, glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and basic fibroblast growth factor (bFGF or FGF2), which act to enhance photoreceptor survival.17,18 Expanding on the knowledge that growth factor upregulation has a protective effect, a number of groups have administered exogenous growth factors intraocularly to rodent models of RP and found that they reduced the degree of retinal degeneration.19-22 The neurotrophin CNTF has been found to be efficacious in slowing the progression of retinal degeneration, with prolonged administration to the RCS rat, S334ter-3 rat, and rcd1 canine animal models of retinal degeneration.21,22 Recently, a phase I clinical trial demonstrated the safety of chronic intravitreous delivery of CNTF over a 6-month period in patients with RP.23 These clinical trials have now entered phase II in patients with RP and dry aged-related macular degeneration. The lack of adverse outcomes from chronic CNTF delivery is an encouraging finding, suggesting that CNTF may be an efficacious treatment to slow the progression of RP.
The purpose of this study was to determine whether retinal neuroprotection in the RCS rat via chronic intravitreous infusion of CNTF is associated with a reduction in electrically evoked cortical potential (EECP) stimulation thresholds.
Methods
Experimental Design
The effect of CNTF on the responsiveness of the retina to electrical stimulation was assessed in homozygous rdy pink-eyed Royal College of Surgeon (RCS) rats. All animals were bred from stock originally provided by Werner Noell (retired). Retinal responsiveness to electrical stimulation was assessed at 4 weeks of age, or postnatal day (p)30, and again at 8 weeks of age (p60) using EECPs. Animals were randomly assigned to four experimental groups (1); untreated, nonsurgical control animals, (2) 10 ng/d intravitreous CNTF infusion, (3) 1 ng/d intravitreous CNTF infusion, and (4) phosphate-buffered saline (PBS) infusion control. Animals that underwent surgery were 5 weeks old, and received chronic intravitreous infusions for 3 weeks. They were euthanatized after the p60 EECP measurements. All animal procedures were performed in accordance with institutional guidelines regarding animal experimentation and conformed to the standards of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cannula Construction
Transscleral intravitreous cannulae (Fig. 1) were constructed with 30-gauge hypodermic 304 stainless steel tubing, connected to fine-gauge (300 μm inner diameter × 640 μm outer diameter) silicone tubing (Silastic; Dow Corning Corp., Midland, MI) by using a medical grade silicone adhesive (Nusil Technologies, Carpinteria, CA). Small sheets (3 × 7 mm) of 50-μm-thick polyimide (Kapton; duPont Corp., Wilmington, DE) were glued to the cannula with gel cyanoacrylate. The cannulae were sterilized with steam and UV light. Cannula flow and integrity were verified before surgical insertion.
Figure 1.
Intravitreous cannula. Hash marks: millimeters. Black arrow: polyimide flap.
Surgical Procedure
At 5 weeks of age, the animals underwent surgery for intravitreous cannula placement. They were anesthetized with 1% to 2% isoflurane, shaved, prepped with a 10% povidone iodine solution, and draped. A 2.0-cm rostrocaudal incision was made between the scapulae, and blunt dissection was used to create a subcutaneous pocket tract to the orbit and into the subconjunctival space of the right eye. Lid retraction allowed adequate exposure for the creation of a limbal peritomy of the superior temporal conjunctiva. A 20-gauge needle was inserted under the fornix-based conjunctiva flap (bevel side up) and advanced subcutaneously toward the interscapular incision. Sterile monofilament was then threaded through the lumen of the needle, before the gentle withdrawal of the needle. The monofilament was tied to the unconnected end of the cannula tubing and used to pull the cannula tubing under the conjunctiva into the subcutaneous space, where it exited into the interscapular subcutaneous pocket. The cannula was then primed with infusate and an osmotic minipump (Alzet Model 2002; Durect Corp., Cupertino, CA) was connected to the cannula tubing and secured with silicone adhesive. The cannula needle tip was then inserted through the sclera like a thumbtack at the ora serrata into the vitreous, and the polyimide flap was trimmed away from the cornea with scissors, to avoid touching the cornea, and secured with ethyl cyanoacrylate. The conjunctival edge was drawn over the polyimide, aligned with the limbus, and affixed with a small amount of cyanoacrylate adhesive applied to the anterior edge of the polyimide with the tip of a 30-gauge needle. The osmotic minipump was secured subcutaneously with a monofilament suture, and the skin incision was closed with staples. The animals received 0.5 mg/kg SC butorphanol to prevent postoperative pain.
Infusate Preparation and Minipump Priming
Osmotic minipumps were primed the evening before surgery with a sterile infusate and incubated at 37°C in 0.9% NaCl to ensure a flow rate of 0.5 μL/hr. This flow rate was chosen to fall within the aqueous outflow capacity of the rat eye. Consequently, the infusion pump did not increase intraocular pressure. Minipumps were loaded with the following sterile infusates: (1) vehicle control (0.1% bovine serum albumin [BSA] in 0.01 M PBS), (2) 10 ng/d CNTF, and (3) 1 ng/d CNTF. Recombinant rat CNTF (R&D Systems, Minneapolis, MN) was reconstituted from stock the evening before surgery.
Electrically Evoked Cortical Potentials
One week before cannula implantation and 3 weeks after surgery, EECPs were acquired from animals anesthetized with ketamine (87 mg/kg, intraperitoneally [IP]) and xylazine (10 mg/kg; IP). The pupils were dilated with phenylephrine hydrochloride (2.5%) and tropic-amide (1%). Topical proparacaine (0.5%) was administered to both corneas for anesthesia. The corneas were kept moist throughout the duration of the experiment by frequent application of balanced salt solution (BSS; Alcon Corp., Houston, TX) and topical ophthalmic ointment (GenTeal; Norvartis Ophthalmics, East Hanover, NJ). EECPs were performed with platinum corneal stimulating electrodes and a platinum needle recording electrode placed subcutaneously above the primary visual cortex. Both eyes were stimulated separately in each animal. Reference electrodes were placed through the ears. Electrical stimulation was performed with an isolated, voltage-controlled, constant-current source (model 2200; A-M Systems, Carlsborg, WA). Control voltages were produced, and analog-to-digital conversion was performed with a multifunction-DAQ board (Model 6071e; National Instruments, Austin, TX). Recordings were amplified (gain, 5000; band-pass, 1–300 Hz) with an AC preamplifier (model CP511; Grass-Telefactor, West Warwick, RI). Electrical stimulation, data acquisition, signal processing, and threshold determination programs were written in commercial software (Labview 2000, ver. 6.0; National Instruments). Data acquisition was performed at 2000 samples per second. Thresholds for stimulation were determined by averaging 30 EECP traces and analyzing the amplitude of the response peak (N1/P1) after the stimulus artifact of the resulting EECP waveform (Fig. 2). The response peak of the EECP was identified as present if and only if the amplitude of response rose above baseline. Threshold currents for EECPs were determined for biphasic charge-balanced stimulus durations of 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, 2.5, and 3.0 ms per phase. These were acquired in random order and were then use to generate a strength–duration curve. For each stimulus duration, the range of current intensities necessary to establish EECP thresholds were as follows: 0.4 (30–1500 μA), 0.6 (5–1300 μA), 0.8 (5–900 μA), 1.0 (2–700 μA), 1.5 (2–550 μA), 2.0 (0.5–350 μA), 2.5 (0.5–150 μA), and 3.0 (0.5–125 μA) ms. The cortical origin of these responses was confirmed 15 minutes after euthanasia resulted in abolition of the cortical response waveform with preservation of the stimulus artifact (data not shown).
Figure 2.
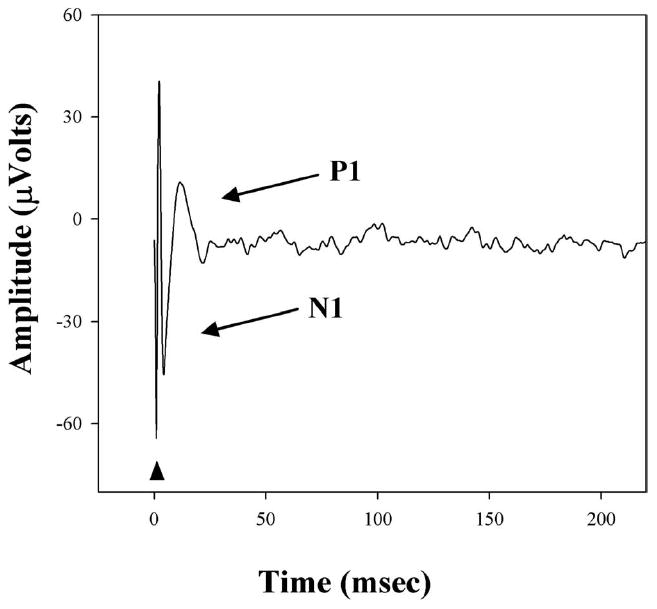
Representative EECP in response to transcorneal stimulation in a p30 RCS rat. Triangle: stimulus artifact. N1 and P1: the first downward and upward deflections of the cortical response used to determine the threshold.
Calculation of Rheobase, Chronaxie, Data Analysis, and Statistics
Rheobase and chronaxie are values that can be extrapolated from strength–duration curves (Fig. 3). The rheobase represents the minimum stimulus intensity needed to bring cells to threshold response levels. The asymptote of the strength–duration curve denotes this value. Chronaxie is a useful measure to determine the excitability of neural tissue. The chronaxie is the minimum stimulus duration necessary for threshold response when the current level is set to twice that of the rheobase. This duration can be measured from the strength–duration curve, an example of which is provided in Figure 3. The stimulus duration necessary to reach excitation threshold at an electrical current strength of twice the rheobase provides the chronaxie. The rheobases were calculated by transforming the strength–duration curve into a plot of charge versus stimulus duration. A best-fit line was then created from the linear plot, the slope of which is the rheobase. The chronaxie was calculated by solving for the y-intercept of this line, as described previously.24,25
Figure 3.
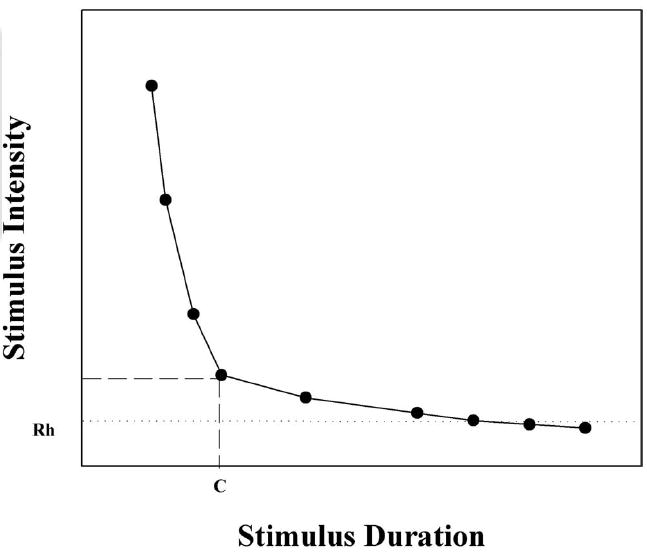
Example strength–duration curve denoting the corresponding rheobase (Rh) and chronaxie (C) values. Rheobase is defined as the minimum stimulus intensity needed to reach threshold response. Chronaxie is the stimulus duration necessary to reach threshold response at a stimulus intensity set to twice the rheobase current.
Group data are expressed as the mean ± SEM (n = 5 for each group). Significant differences (α = 0.05) were determined by Student’s t-test or one-way analysis of variance (ANOVA), and when differences reached P < 0.05, the least significant difference (LSD) post hoc analysis was run. All statistics were performed with commercial software (SPSS software; SPSS Inc., Chicago, IL).
Results
Association of Progressive Retinal Degeneration and Electrical Stimulation Thresholds
strength–duration and charge–threshold curves were obtained on naïve animals (p30), to measure baseline EECP thresholds to transcorneal electrical stimulation of the retina (Figs. 4-7). One-way ANOVAs were performed on all strength–duration and charge threshold curve data. F(13,61-66) ranged between 2.923 and 4.402, and probabilities were within the range of P < 0.001 to 0.002. After performing the LSD post hoc analysis, we found no significant differences in the baseline strength–duration or charge–threshold curves between the right and left eyes of individual animals, or between groups for any of the stimulation durations tested (P = 0.157–0.996). strength–duration and charge–threshold curves observed in the untreated control group demonstrated increased EECP stimulation thresholds over time. Figure 4A illustrates that strength–duration curves acquired at baseline (p30) were significantly lower than those acquired 4 weeks later (p60). This finding was consistent for all stimulus durations tested. Paired Student’s t-test probabilities ranged between 0.002 and 0.008. Figure 4B demonstrates similar findings for the charge–threshold curves. Values acquired at p30 demonstrated significantly lower stimulation thresholds than those acquired at p60 for all stimulus durations tested. Paired Student’s t-test probabilities ranged between 0.002 and 0.008.
Figure 4.
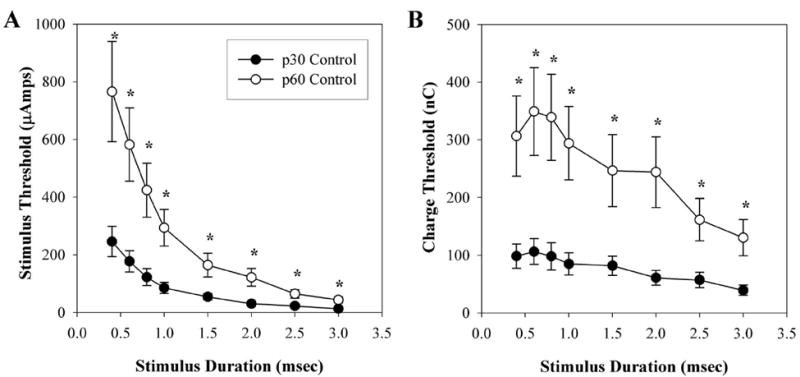
Mean EECP thresholds in untreated control, acquired at p30 and p60. (A) strength–duration curve relative to stimulus duration. (B) Charge threshold curve relative to stimulus duration. *P < 0.05 **P < 0.001 p30 vs. p60. Error bars, ±SEM.
Figure 7.
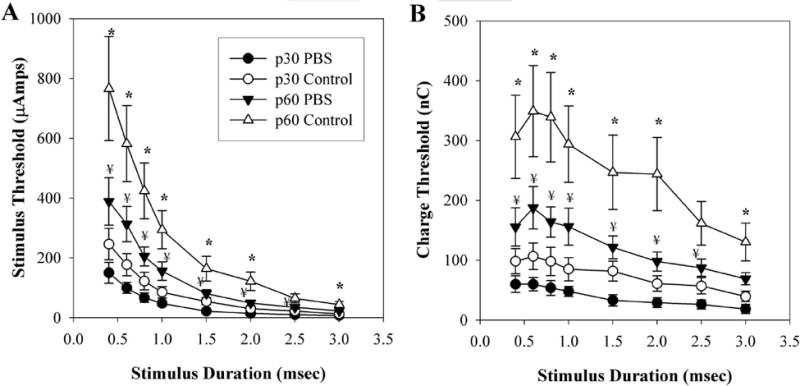
Effects of PBS infusion on mean EECP thresholds. Data points represent the mean of both control and PBS-treated animals acquired at both p30 and at p60. (A) strength–duration curve relative to stimulus duration. (B) Charge threshold curve relative to stimulus duration. *P < 0.05, ** P < 0.001 (PBS p60 vs. control p60); ¥P < 0.05 PBS p60 vs. PBS p30. Error bars, ±SEM.
CNTF and EECP Thresholds
The strength–duration curves for the 10 ng/d CNTF treatment group acquired after 3 weeks of continuous infusion (p60) demonstrated significantly lower thresholds to electrical stimulation relative to age-matched (p60) untreated control animals at every time point tested (Fig. 5A). LSD post hoc analysis probabilities ranged from < 0.001 to 0.004. No significant differences in stimulation thresholds existed between the strength–duration curves for the 10-ng/d CNTF–treated (p60) animals and the curves for the naïve (p30) animals. The charge–threshold curves in Figure 5B also demonstrated that the 10 ng/d CNTF treatment group had significantly lower charge thresholds at p60 when compared with p60 untreated controls. Statistical significance was observed at all stimulus durations (post hoc probabilities ranged between < 0.001 and 0.004). The charge threshold curves for the 10-ng/d CNTF p60 animals did not demonstrate the same increases observed in other groups over the 4-week study period and were not significantly different from the p30 charge–threshold curves in the RCS rats.
Figure 5.
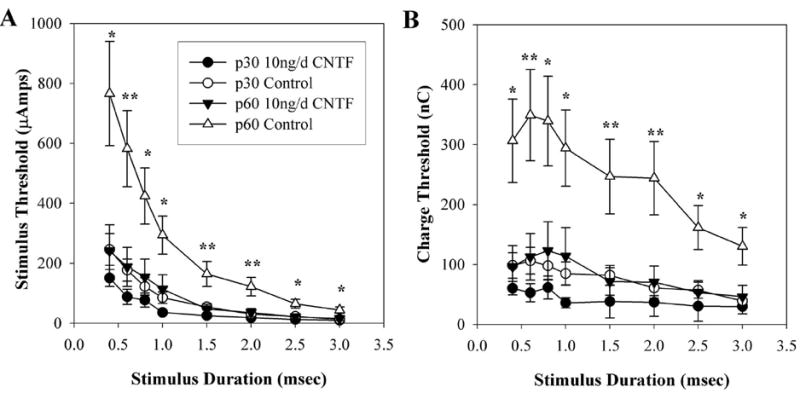
Effects of 10-ng/d CNTF treatment on mean EECP thresholds. Data points represent the mean of both control and 10-ng/d CNTF–treated animals acquired at both p30 and at p60. (A) strength–duration curve relative to stimulus duration. (B) Charge threshold curve relative to stimulus duration. *P < 0.05, **P < 0.001 (10 ng/d CNTF p60 vs. control p60). Error bars denote ± SEM.
Figure 6A demonstrates that strength–duration curves for the 1-ng/d CNTF–treated p60 animals had significantly lower thresholds to electrical stimulation than those of the p60 untreated controls at all time points studied. LSD post hoc analysis probabilities ranged from < 0.001 to 0.003. The strength–duration curves for 1-ng/d CNTF–treated animals at p60 were not significantly different from baseline p30 strength–duration curves. Charge–threshold curves (Fig. 6B) also demonstrated lower thresholds in the 1-ng/d CNTF–treated animals at p60 relative to p60 untreated control animals. This was evident at all stimulus durations with probabilities from LSD post hoc analysis ranging between < 0.001 and 0.003. There was no difference between p30 and p60 charge–threshold curves within the 1 ng/d CNTF treatment group.
Figure 6.
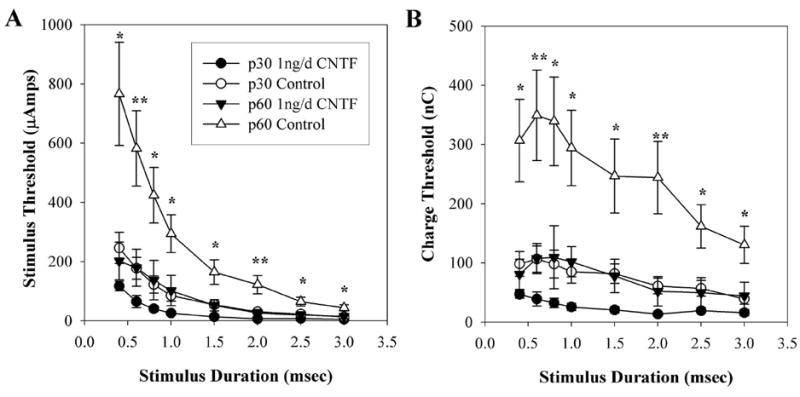
Effects of 1-ng/d CNTF treatment on mean EECP. Data points represent the mean of both control and 1 ng/d-CNTF–treated animals acquired at both p30 and at p60. (A) strength–duration curve relative to stimulus duration. (B) Charge threshold curve relative to stimulus duration. *P <.0.05, **P < 0.001 (1 ng/d CNTF p60 vs. control p60). Error bars denote ± SEM.
strength–duration and charge–threshold curves for the PBS-treated animals at p60 also demonstrated significantly lower stimulation thresholds than those in the untreated p60 control rats (Figs. 7A, 7B), as determined by LSD post hoc analysis. This result was observed at all stimulation durations except 2.5 ms (P = 0.06), with significant probabilities ranging between 0.002 and 0.023. For both the strength–duration and charge–threshold curves, the p60 PBS-treated animals demonstrated significantly higher thresholds when compared with the PBS p30 baseline curve, at all stimulus durations except 3.0 ms (P = 0.057). LSD post hoc analysis probabilities ranged between 0.008 and 0.026. It should be noted, however, that the stimulation thresholds for PBS-infused eyes were higher that those of the 1- and 10-ng/d CNTF infusion groups, although this difference did not reach statistical significance (LSD post hoc analysis probabilities ranged between 0.298 and 0.738).
Table 1 lists the mean chronaxie and rheobase values determined for all treatment groups at p30 and p60. CNTF infusion demonstrated a dose-dependent reduction of rheobase values, compared with PBS-infused and untreated animals. However, these differences were not found to be statistically significant.
Table 1.
Mean Rheobase and Chronaxie Values Extrapolated from Strength-Duration Curves with Correlations of Linear Approximation
| 10 ng/day CNTF
|
1 ng/day CNTF
|
PBS
|
Untreated Controls
|
|||||
|---|---|---|---|---|---|---|---|---|
| Treated | Untreated | Treated | Untreated | Treated | Untreated | OQ | OS | |
| Baseline | ||||||||
| Rheobase | 5.00 ± 1.04 | 5.06 ± 1.63 | 3.40 ± .81 | 3.05 ± .83 | 5.29 ± 1.23 | 6.51 ± 1.85 | 11.02 ± 4.18 | 9.69 ± 2.67 |
| Chronaxie | 4.29 ± 1.24 | 3.80 ± .98 | 3.32 ± 2.18 | 1.82 ± .83 | 3.36 ± .83 | 1.73 ± .45 | 3.58 ± .83 | 3.23 ± .76 |
| Model fit: R2 | 0.93 ± .02 | 0.95 ± .02 | 0.96 ± .02 | 0.97 ± .02 | 0.94 ± .01 | 0.95 ± .01 | 0.96 ± .01 | 0.96 ± .02 |
| Postoperative Week 3 | ||||||||
| Rheobase | 10.73 ± 3.53 | 19.84 ± 7.38 | 14.42 ± 6.61 | 16.05 ± 8.38 | 16.43 ± 2.59 | 18.86 ± 4.61 | 27.62 ± 7.21 | 39.78 ± 14.33 |
| Chronaxie | 2.68 ± .75 | 1.95 ± .87 | 1.45 ± .26 | 1.96 ± .75 | 3.52 ± .74 | 2.84 ± .70 | 3.53 ± 1.19 | 2.32 ± .35 |
| Model fit: R2 | 0.96 ± .01 | 0.91 ± .01 | 0.97 ± .01 | 0.96 ± .01 | 0.96 ± .01 | 0.94 ± .01 | 0.95 ± .02 | 0.97 ± .01 |
Data are expressed as the mean ± SEM.
Effect of CNTF on Retinal Sensitivity to Electrical Stimulation and EECP Retinal Stimulation Thresholds
Retinal degeneration in patients and in animal models can be an asymmetrical process. Significant differences in the rate of degeneration between animals of the same age and even between eyes of the same animal are commonly observed. To allow for comparison of EECP response thresholds between animals, we normalized EECP threshold data to account for any asymmetrical degeneration. Thus, data were interpreted in terms of retinal sensitivity, defined as the ratio of the retina’s EECP response threshold at baseline measurements (p30) to EECP measurements acquired after a 3-week infusion (p60) in the same eye. If the retinal sensitivity pre-to-post ratio was equal to 1, there was no change in the charge threshold of retinal stimulation. However, a ratio less than 1 indicated that p60 thresholds were greater than p30 thresholds.
Over time, RCS rats in the untreated control group demonstrated increased retinal stimulation thresholds relative to baseline measurements with a mean retinal sensitivity of 0.419 ± 0.041 (Fig. 8), which corresponds to a nearly 60% increase in retinal stimulation thresholds from p30 to p60. Differences in retinal sensitivity between groups were tested using one-way ANOVAs that were significant (F(6,27) = 6.691; P < 0.001). Subsequently, LSD post hoc analyses were performed to establish significance. Animals treated with 10 ng/d CNTF had retinal sensitivities of 0.937 ± 0.106. These were significantly higher than those of the untreated control group (P < 0.001). The 1-ng/d CNTF group’s mean retinal sensitivity was 0.621 ± 0.062 which was significantly higher than the untreated control group (P = 0.021). Mean retinal sensitivity for the untreated control group (0.419 ± 0.041) was not significantly different from the mean retinal sensitivity in PBS-treated eyes (0.350 ± 0.030; P = 0.420) or fellow untreated eyes of all treatment groups (P = 0.297–0.928).
Figure 8.
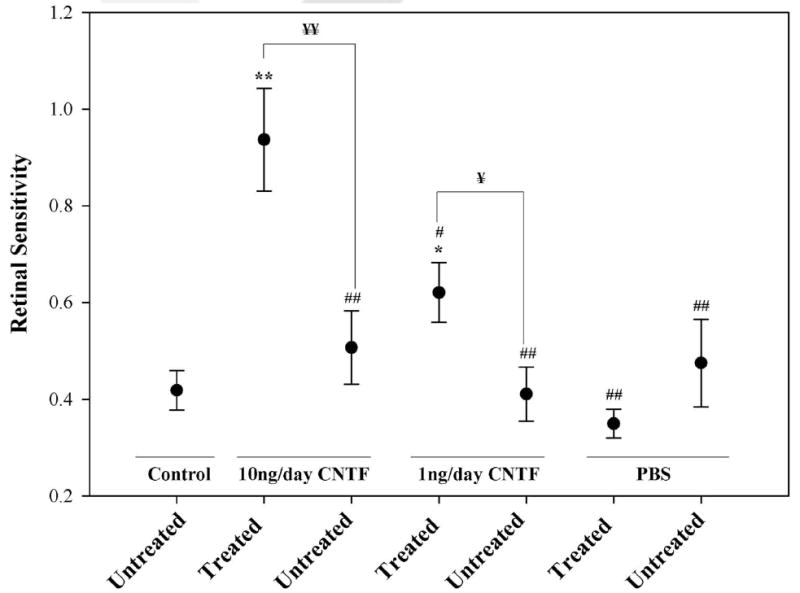
Retinal sensitivity to electrical stimulation as determined from p30 and p60 EECPs thresholds. Data are the mean ± SEM. Statistical significance denoted by *P < 0.05, **P < 0.001 when compared to untreated control animals; ¥P < 0.05, ¥¥P < 0.001 treated eyes versus untreated eyes within the same treatment group; #P < 0.05, ##P < 0.001 when compared to 10 ng/d-CNTF–treated animals.
The chronic intravitreous administration of 10 ng/d CNTF resulted in a significantly higher mean retinal sensitivity than in fellow untreated eyes (0.937 ± 0.106 vs. 0.507 ± 0.076, t-test P < 0.001). In addition, 10-ng/d CNTF–treated eyes exhibited significantly higher mean retinal sensitivities to electrical stimulation than the fellow untreated eyes of all other treatment groups (P < 0.001 for each) or those treated with 1 ng/d CNTF (0.621 ± 0.062, P = 0.002) or PBS infusion (0.350 ± 0.030, P < 0.001). The 1-ng/d CNTF-treated group demonstrated significantly higher mean retinal sensitivity than the fellow untreated eyes (0.621 ± 0.062 vs. 0.411 ± 0.056, t-test P = 0.041). PBS-infused eyes did not demonstrate a significant difference in mean retinal sensitivity compared with their fellow untreated eyes (0.350 ± 0.030 vs. 0.475 ± 0.090, t-test P = 0.206).
Discussion
The goal of artificial vision is to use electrical stimulation devices to excite retinal cells, thereby generating visual percepts in blind patients. To be effective, the retina must be healthy enough to respond adequately to electrical currents that are within a physiologically and electrochemically tolerable range. Maintaining the health of the electrode-tissue interface is a major challenge for the successful long-term implementation of retinal prosthetic devices. If electrophosphene thresholds significantly increase as a consequence of a progressive degeneration, retinal stimulators will fail due to a breakdown of the electrode-tissue interface. The goals of the present study were to investigate the changes in stimulation thresholds of degenerating retinas over time and to determine whether CNTF-mediated neuroprotection could preserve stimulation thresholds in the RCS rat retinal degeneration model.
In our study, as the retina progressively deteriorated, the amount of electrical current needed to reach threshold increased. This increase is evident in our strength–duration curves for the untreated control animals, which show marked differences in thresholds between p30 and p60. In addition, as the retina deteriorated, the amount of charge needed to elicit a response increased. These findings have also been shown in a study of a murine model for retinal degeneration which demonstrated that the amount of charge needed to elicit a response was directly related to the health of the retina.10 Pharmacologic methods for retinal neuroprotection in RP provide novel means with which to preserve the health of the electrode–tissue interface and maintain stable electrical stimulation thresholds over time.
Neurotrophins such as CNTF have been shown to be efficacious therapeutic molecules that slow the progression of retinal degeneration. Previous studies have shown that administration of exogenous CNTF to the retina promotes survival of both the photoreceptors22,26,27 in retinal degeneration models and the cells of the inner retina28,29 in optic nerve injury models, most likely through upregulation of the Bcl-xL family of antiapoptotic proteins.30,31 Although the CNTF receptor has been localized to the outer segments of the photoreceptors,32 the mechanism of CNTF neuroprotection to the photoreceptors may be indirectly mediated through Müller cells.33-35 Whether CNTF is neuroprotective to the photoreceptors through a direct or indirect mechanism, it remains an effective neuroprotectant among multiple neuronal populations, across many species of animal neurodegenerative models.21,22,36 Our study demonstrates that prolonged, intravitreous administration of CNTF lowered the current thresholds needed for electrical stimulation of the retina in RCS rats as measured by EECPs. Retinal sensitivity to electrical stimulation was significantly enhanced in 10-ng/d CNTF-treated eyes when compared with untreated eyes and all other treatment groups (Fig. 8). The CNTF-induced preservation of retinal sensitivity allowed for more desirable stimulation parameters, with lower electrical currents needed to bring cells to the threshold. Similar results were reported with chronic administration of CNTF and BDNF to chemically deafened animals with cochlear implants.36 Neurotrophic intervention with a cochlear implant resulted in decreased stimulation thresholds and an increased density of spiral ganglion cells, allowing the cochlear implants to be much more effective. It is important to note that while we delivered electrical current to evaluate retinal sensitivity, we did not use the stimulation parameters employed by others to effect neuroprotection.13,14 Although it is possible that the electrical stimulation we used to elicit EECPs conferred a degree of neuroprotection on the degenerating retina, it is unlikely, because the short duration of stimulation used and the 4-week interval between EECP threshold measurements.
PBS-infused eyes also demonstrated significantly lower EECP thresholds at p60 relative to p60 untreated control animals (Figs. 7A, 7B). Although the data suggest that PBS infusion may have neuroprotective properties, it has been shown that surgical procedures can slow retinal degeneration in RCS rats.37-39 This effect was not superior to CNTF infusion, however. Although the EECP thresholds for the p60 PBS-treated eyes were lower than those for p60 untreated controls, the p60 PBS-treated eyes demonstrated significantly higher thresholds than the p30 PBS treatment group (Figs. 7A, 7B). Only the CNTF infusion groups demonstrated significant stabilization of retinal stimulation thresholds and retinal sensitivity from p30 to p60. In addition, retinal sensitivity of PBS-treated eyes did not significantly differ from those of fellow untreated eyes, or untreated control eyes (Fig. 8), whereas all CNTF-infused eyes demonstrated significantly greater retinal sensitivity at p60 compared with their fellow untreated eyes and eyes of the untreated control group.
Although in our study we saw enhancement of retinal function after the application of CNTF, others have reported reductions in retinal function.27,40,41 It is important to note, however, that those studies evaluated retinal function in terms of the ERG, which measured light responses. It has recently been shown that CNTF reduces levels of rhodopsin in degenerating retinas.42 Rhodopsin is essential in the photoreceptor signal transduction cascade that is needed for normal light responses. It is conceivable that the reduction in retinal function reported by others is a result of CNTF-induced downregulation of rhodopsin. Although the ERG is an effective method of evaluating retinal photoreceptor function, animals with severely degenerated retinas may fail to demonstrate an appreciable ERG response to light, yet their retinas may remain functional in response to electrical stimulation.8 In the present study, we assessed retinal function by measuring cortical responses to electrical stimulation of the retina. Using EECPs, we were able to demonstrate that CTNF administration resulted in preservation of retinal responses to electrical stimulation in p60 RCS rats at a time when light-flash ERGs are nearly extinguished.43
Currently, retinal prosthetic devices are used to generate visual percepts by electrically stimulating the diseased retinas of patients with progressive degeneration. As the health of the retina deteriorates, the electrical currents required to bring neurons to threshold increases. Consequently, electrical stimulation thresholds may rise over time to physiologically intolerable levels, thereby resulting in the failure of prosthetic devices. Our findings demonstrate that CNTF-mediated neuroprotection slows the progression of retinal degeneration thereby maintaining the long-term health of the retina and the electrode-tissue interface, maintaining electrical stimulation thresholds at low levels. Thus, CNTF-mediated neuroprotection is likely to enhance the long-term efficacy of retinal prosthetic devices.
Acknowledgments
Supported by the Ligon Research Center of Vision, a Research to Prevent Blindness Career Development Award (RI), an unrestricted Grant from Research to Prevent Blindness, National Institutes of Health Loan Repayment Program (RI), and the National Science Foundation Integrative Graduate Education and Research Traineeship Program (TLK).
Footnotes
Disclosure: T.L. Kent, None; I.V. Glybina, None; G.W. Abrams, None; R. Iezzi, None
References
- 1.Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 2.Massof RW, Dagnelie G, Benzschawel T, Palmer RW, Finkelstein D. First order dynamics of visual field loss in retinitis pigmentosa. Clin Vis Sci. 1990;5:1–26. [Google Scholar]
- 3.Pagon RA. Retinitis pigmentosa. Surv Ophthalmol. 1988;33:137–177. doi: 10.1016/0039-6257(88)90085-9. [DOI] [PubMed] [Google Scholar]
- 4.Humayun M, Sato Y, Propst R, de Juan E., Jr Can potentials from the visual cortex be elicited electrically despite severe retinal degeneration and a markedly reduced electroretinogram? German J Ophthalmol. 1995;4:57–64. [PubMed] [Google Scholar]
- 5.Stone JL, Barlow WE, Humayun MS, de Juan E, Jr, Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110:1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- 6.Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–147. doi: 10.1385/MN:28:2:139. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci. 2003;44:5355–5361. doi: 10.1167/iovs.02-0819. [DOI] [PubMed] [Google Scholar]
- 8.Humayun MS, de Juan E, Jr, Dagnelie G, Greenberg RJ, Propst RH, Phillips DH. Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol. 1996;114:40–46. doi: 10.1001/archopht.1996.01100130038006. [DOI] [PubMed] [Google Scholar]
- 9.Humayun MS, de Juan E, Jr, Weiland JD, et al. Pattern electrical stimulation of the human retina. Vision Res. 1999;39:2569–2576. doi: 10.1016/s0042-6989(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Humayun MS, Weiland JD, et al. Comparison of electrical stimulation thresholds in normal and retinal degenerated mouse retina. Jpn J Ophthalmol. 2004;48:345–349. doi: 10.1007/s10384-004-0084-9. [DOI] [PubMed] [Google Scholar]
- 11.Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- 13.Miyake K, Yoshida M, Inoue Y, Hata Y. Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Invest Ophthalmol Vis Sci. 2007;48:2356–2360. doi: 10.1167/iovs.06-1329. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto T, Miyoshi T, Matsuda S, Tano Y, Fujikado T, Fukuda Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest Ophthalmol Vis Sci. 2005;46:2147–2155. doi: 10.1167/iovs.04-1339. [DOI] [PubMed] [Google Scholar]
- 15.Pardue MT, Phillips MJ, Yin H, et al. Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci. 2005;46:674–682. doi: 10.1167/iovs.04-0515. [DOI] [PubMed] [Google Scholar]
- 16.Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem. 2003;88:57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- 17.Harada T, Harada C, Kohsaka S, et al. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joly S, Pernet V, Chemtob S, Di Polo A, Lachapelle P. Neuroprotection in the juvenile rat model of light-induced retinopathy: evidence suggesting a role for FGF-2 and CNTF. Invest Ophthalmol Vis Sci. 2007;48:2311–2320. doi: 10.1167/iovs.06-1205. [DOI] [PubMed] [Google Scholar]
- 19.Frasson M, Picaud S, Leveillard T, et al. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40:2724–2734. [PubMed] [Google Scholar]
- 20.Miyazaki M, Ikeda Y, Yonemitsu Y, et al. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003;10:1503–1511. doi: 10.1038/sj.gt.3302028. [DOI] [PubMed] [Google Scholar]
- 21.Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- 23.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geddes LA. Accuracy limitations of chronaxie values. IEEE Trans Biomed Eng. 2004;51:176–181. doi: 10.1109/TBME.2003.820340. [DOI] [PubMed] [Google Scholar]
- 25.Holsheimer J, Dijkstra EA, Demeulemeester H, Nuttin B. Chronaxie calculated from current-duration and voltage-duration data. J Neurosci Methods. 2000;97:45–50. doi: 10.1016/s0165-0270(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 26.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- 28.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 29.Zhang CW, Lu Q, You SW, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005;46:1497–1503. doi: 10.1167/iovs.04-0664. [DOI] [PubMed] [Google Scholar]
- 30.Bonni A, Frank DA, Schindler C, Greenberg ME. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993;262:1575–1579. doi: 10.1126/science.7504325. [DOI] [PubMed] [Google Scholar]
- 31.Kirito K, Watanabe T, Sawada K, Endo H, Ozawa K, Komatsu N. Thrombopoietin regulates Bcl-xL gene expression through Stat5 and phosphatidylinositol 3-kinase activation pathways. J Biol Chem. 2002;277:8329–8337. doi: 10.1074/jbc.M109824200. [DOI] [PubMed] [Google Scholar]
- 32.Valter K, Bisti S, Stone J. Location of CNTFRalpha on outer segments: evidence of the site of action of CNTF in rat retina. Brain Res. 2003;985:169–175. doi: 10.1016/s0006-8993(03)03130-5. [DOI] [PubMed] [Google Scholar]
- 33.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhee KD, Ruiz A, Duncan JL, et al. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2007;48:1389–1400. doi: 10.1167/iovs.06-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Müller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 36.Shinohara T, Bredberg G, Ulfendahl M, et al. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 38.Li LX, Sheedlo HJ, Gaur V, Turner JE. Effects of macrophage and retinal pigment epithelial cell transplants on photoreceptor cell rescue in RCS rats. Curr Eye Res. 1991;10:947–958. doi: 10.3109/02713689109020331. [DOI] [PubMed] [Google Scholar]
- 39.Silverman MS, Hughes SE. Photoreceptor rescue in the RCS rat without pigment epithelium transplantation. Curr Eye Res. 1990;9:183–191. doi: 10.3109/02713689008995205. [DOI] [PubMed] [Google Scholar]
- 40.Schlichtenbrede FC, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 41.Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- 42.Zeiss CJ, Allore HG, Towle V, Tao W. CNTF induces dose-dependent alterations in retinal morphology in normal and rcd-1 canine retina. Exp Eye Res. 2005;2:2. doi: 10.1016/j.exer.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Skoog KO. Fast and slow artificial diurnal rhythms (light-darkness-light) and the rate of progression of retinal degeneration in dystrophic RCS rats. An electroretinographic study. Br J Ophthalmol. 1985;69:468–470. doi: 10.1136/bjo.69.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]


