Abstract
We demonstrate how to construct calibrated, stable, and inexpensive tissue-like phantoms for near-infrared (NIR) fluorescence imaging applications. The bulk phantom material is composed of gelatin, intralipid, hemoglobin, and indocyanine green (ICG). Absorbance, scatter, background fluorescence, and texture can be tuned as desired. NIR fluorescent inclusions are comprised of ICG-labeled polystyrene divinylbenzene beads and Pam78-labeled hydroxyapatite crystals. The former mimic tumor masses of controllable size and contrast agent concentration, and the latter mimic microcalcifications in breast cancer. NIR-fluorescent inclusions can be positioned precisely in phantoms, with one or more regions having different optical properties, and their position can be verified independently using micro-computed tomography. We demonstrate how these phantoms can be used to calibrate and compare imaging systems, and to train surgeons to operate under NIR fluorescence image guidance.
Keywords: Near-Infrared Fluorescence, Optical Imaging, Phantoms, Indocyanine Green, Hydroxyapatite, Surgical Training
Introduction
Living tissue has relatively low photon absorption in the near-infrared (NIR) range of the electromagnetic spectrum (700 − 1000 nm). Endogenous fluorophores are strong contributors to autofluorescence in the UV and visible regions of the spectrum, but are significantly weaker in the NIR. Hence from an absorbance and autofluorescence standpoint, NIR light has distinct advantages. Scatter is significant at all wavelengths, and is a complex function of tissue composition (discussed in [1]). However, for many tissues, the absolute value of scatter tends to be lower in the NIR.
In the last few years, there has been a dramatic rise in the publication of imaging systems that exploit NIR light, and in some cases, exogenous NIR fluorophores. The three basic types of imaging systems are steady-state (also known as continuous wave or CW), time-domain, and frequency-domain. Within each, source/detector geometry can be based on point or area illumination, and point or area detection, leading to over 12 different imaging system configurations (reviewed in [2]).
Paralleling the explosion of imaging systems is a similar one for exogenous NIR fluorophores. Several comprehensive reviews of NIR fluorophores used for in vivo imaging have recently been published [3-5]. Briefly, NIR fluorescent contrast agents are now available for quantitation of vascular mapping, tissue perfusion, tissue calcification, protease activity, cell injury, tissue response to injury, and for specific applications such as sentinel lymph node mapping and tumor imaging.
As imaging systems and contrast agents converge, there is an increasing need for standardized NIR fluorescent phantoms that can be compared among laboratories, and that assist with the training of surgeons and technologists. Cubeddu et al. [6] described agar-based phantoms utilizing intralipid for scattering and India ink for absorption. Wagnieres et al. [7], described agarose-based phantoms containing silica powder and intralipid for scattering, and India ink and erythrocyte concentrate for absorption. The former optical phantom utilizes non-physiological absorbers, the latter is difficult to construct, and both utilize a galactose polymer as base material. Quan et al. [8] and Kelly et al. [9] utilized gelatin, derived from the hydrolysis of vertebrate connective tissue, as a phantom base material for photoacoustic and radiation dosimetry studies, respectively. Two recent studies [10,11] have utilized gelatin-based phantoms for NIR fluorescence studies; however, neither describes the construction of precise NIR fluorescent inclusions or a general purpose phantom platform. Other approaches to NIR imaging phantoms include hard resin or soft RTV silicon materials [12] into which holes are drilled; however, this system is inflexible with respect to inclusion size, placement, and geometry, and multiple interfaces are formed at each hole site. In this study, we demonstrate how to construct simple, yet stable, gelatin-based phantoms that can be tailored to the optical properties of various tissues using readily available materials. We also describe two different types of NIR fluorescent inclusions that simulate tumor spheroids and/or breast cancer microcalcifications.
Materials and Methods
Reagents
NF-grade gelatin (catalog #EM-GX0048−1) was from VWR (West Chester, PA). Sterile Intralipid™ (20% w/v) was purchased from Baxter (Deerfield, IL). Bovine hemoglobin (catalog #H-2625) and indocyanine green (ICG; catalog #I-2633) were from Sigma (St. Louis, MO). AG1-X8 anion exchange resin (catalog #140−1422) was from Bio-Rad (Hercules, CA). Pam78 was prepared as described (Zaheer et al., 2001). 100 μm hydroxyapatite (HA) crystals (catalog #391947) were from Calbiochem (La Jolla, CA).
Phantom Mixture Construction
The buffer for all phantoms was Tris-buffered saline (TBS) composed of 50 mM Tris, pH 7.4 and 150 mM sodium chloride. Sodium azide was added to a final concentration of 0.1% (15 mM; TBS/azide) to bind methemoglobin, block re-oxygenation, and to act as a preservative. Gelatin was suspended at the desired concentration (% w/v) in TBS (GTS), and warmed to 37−50°C with constant stirring until completely dissolved. GTS was then cooled to 25°C and the desired amounts of hemoglobin and intralipid added, with constant stirring. Solidification of the GTS mixture should be performed as rapidly as possible in order to avoid separation of the intralipid. We have found that pouring the room temperature solution into the desired pre-chilled mold at 4°C will minimize this effect. Depending on the size and shape of the mold, solidification should take 5−20 min.
NIR Fluorescent Inclusions
NIR fluorescent inclusions were made using indocyanine green (ICG)-labeled polystyrene divinylbenzene beads (AG1-X8) or Pam78-labeled hydroxyapatite crystals. These latter inclusions were used to simulate microcalcifications found in breast cancer [13]. The concentration of fluorophore associated with each inclusion was measured precisely, and expressed as “ICG Equivalence” (defined below). Beads were sieved twice to a diameter of 1000 ± 100 μm using a 20-mesh (850 μm square holes) brass sieve (VWR catalog #57334−112). For bead loading, 10 mM ICG in DMSO was diluted in TBS to twice the desired loading concentration and mixed 1:1 with a 50% bead slurry, vortexed for 3 sec, incubated 20 min, and washed 4X with TBS. Pam78 was loaded onto HA in a similar fashion. For dual-labeling with iodine contrast and ICG, 60% iothalamate meglumine (stock concentration 2.22 M iodine; Conray™, Mallinckrodt, St. Louis, MO) was diluted in TBS to a final iodine concentration of 88.8 mM prior to the addition of ICG and bead loading. Prior to embedding into phantoms, NIR fluorescent inclusions were calibrated against ICG standards in DMSO having the same path length to determine their “ICG Equivalence” (see below).
Fluorescent inclusions, having ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) from 0 to 5 μM, could be varied in size from 75 to 1000 μm. 3-D positioning of fluorescent inclusions was verified independently using an eXplore Locus micro-computed tomography system (GE Healthcare Biosciences, Waukesha, WI) to within ± 90 μm.
Phantom Construction
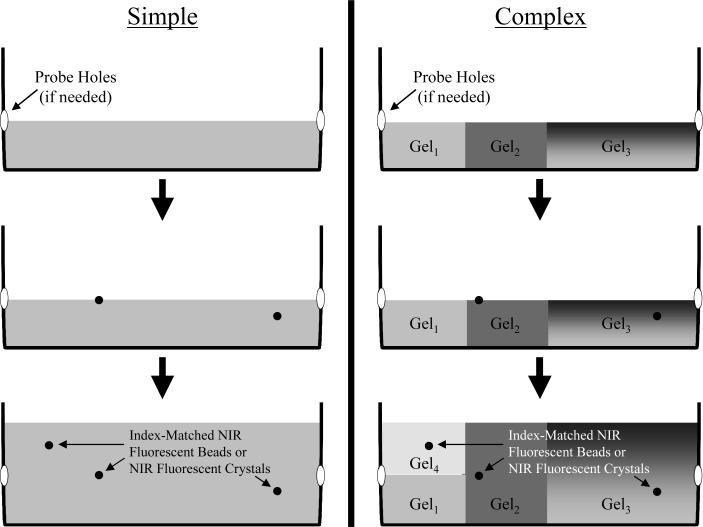
For phantoms with uniform bulk optical properties, a mold is filled and gelled to the desired level of the inclusion, then the inclusions are mixed with a 30°C phantom solution (5−100 μL depending on inclusion) and pipetted onto the surface of the solidified mold. The warmth of the solution softens the top of the mold and allows the inclusion solution to adhere to the surface. Once the inclusion solution has set, more of the same warm phantom mix can be poured on top, thus removing any interfaces. Different levels of the same phantom can have different inclusions inserted into it without having to create separate molds for each level (Figure 1). Moreover, various regions of the same phantom can have different properties, without artificial refractive interfaces.
Figure 1. Tissue-Like Phantoms for NIR Fluorescence Imaging.
By choosing the scatter, absorbance, and background fluorescence of the phantom medium, simple (left) or complex (right) phantoms can be created. In complex phantoms, optical properties can be varied in three dimensions, including the use of gradients (e.g., Gel3), without refractive interfaces. For optical tomography, probe holes are pre-drilled and sealed before filling, or the container is coated with vegetable oil for easy removal of the entire phantom. Fluorescent inclusions can be placed at any position in 3-D within the phantom.
Phantoms with different optical properties in different compartments can be produced by placing smaller molds inside the larger main mold. These smaller sub-molds can then be filled with different phantom mixtures, with the inclusions inserted into these molds in a similar manner as described above. Alternatively, different compartments can be constructed separately, then added to the main mold using warm gelatin as described above for inclusions. Remaining gaps are filled in using the desired warm phantom mixture. To minimize glare in color images due to uneven surfaces, complex phantoms are often covered with an additional 1−3 mm layer of GTS, which has negligble effect on scatter and absorption. Once a phantom has been constructed, it can be stored for long periods in a sealed container, preferably a humidified one, at 4°C.
Optical Measurements
Absorbance spectrometry was performed using a 1-cm path length quartz cuvette (Starna, Atascadero, CA), HR2000 fiberoptic spectrometer and a DH2000-BAL light source (Ocean Optics, Dunedin, FL). Spectral reflectance measurements were made using the same spectrometer and light source equipped with a R400−7-VIS/NIR probe (Ocean Optics), and normalization was performed using a homogenously reflecting surface.
Absolute absorption and reduced scattering were measured on a Lambda 19 (Perkin Elmer, Boston, MA) UV/Vis/NIR spectrophotometer equipped with a 150 mm integrating sphere. For long-term stability experiments, phantoms were measured in this apparatus over a 4-week period, being stored at 4°C in a humidified chamber on intervening days. To assess diffusion during long-term stability testing, samples were prepared between two 2” × 3” glass slides, and were composed of a central 0.5” × 1.385” rectangle (area of measurement) of one material with the remaining periphery composed of a second material. The edges were sealed with epoxy. The scattering and absorbing properties of samples were computed using the inverse adding-doubling method from 400−1000 nm ([14]; the algorithm is available for download at http://omlc.ogi.edu/software/iad/).
We compared the results provided by the integrating sphere and inverse adding-doubling method to Mie theory using samples of known properties. 903 nm polystyrene (PS) beads (Catalog #3900A) were purchased from Duke Scientific (Palo Alto, CA). The Mie theory calculator was accessed at http://omlc.ogi.edu/calc/mie_calc.html. Inputs to the calculator included an index of refraction of 1.33; real index of refraction of the sphere of 1.6, 1.582, 1.575, and 1.57 for 400 nm, 600 nm, 800 nm, and 1000 nm, respectively; imaginary index of refraction of −0.00045, −0.0008, −0.001, and −0.002 for 400 nm, 600 nm, 800 nm, and 1000 nm, respectively; 200 angles; and, a density of 0.0073 spheres/μm3 [15].
Fluorescence spectrometry was performed with a 225 μL, 3-sided quartz cuvette (Starna), HR2000 fiberoptic spectrometer and CUV-ALL-UV 4-way cuvette holder (Ocean Optics), and 770 nm NIR laser diode light source (Electro Optical Components, Santa Rosa, CA) set to 8 mW with output through a 300 μm core diameter, NA 0.22 fiber (Fiberguide Industries, Stirling, NJ).
Intraoperative NIR fluorescence imaging was performed as described in detail previously [16], using filtered (Chroma #HHQ750/50x) 725−775 nm NIR excitation light, and custom light collection optics equipped with a long-pass (≥ 795 nm) emission filter (Chroma #HQ795LP).
Quantification of Autofluorescence in Living Tissues
Animal protocols were in accordance with Institutional Animal Care and Use Committee Guidelines. Thirty-five kg adult male Yorkshire pigs (n = 3) were purchased from E.M. Parsons and Sons (Hadley, MA). All animals acclimated to the animal facility for at least 48 hours prior to experimentation. Pig anesthesia was induced with 4.4 mg/kg intramuscular Telazol (Fort Dodge Labs, Fort Dodge, IA) and anesthesia maintained through a 7 mm endotracheal tube with 1.5% isoflurane/98.5% O2 at 5 L/min.
Tissues and organs were surgically exposed, then imaged using NIR excitation light of 725−775 nm, with an excitation fluence rate of 5 mW/cm2 (as measured with a calibrated Orion-TH digital light power meter equipped with an Orion model 2A-SH thermopile detector head). In each field of view, and on non-absorbing and non-reflecting black matte paper (Canson Mi-Teintes, Stygian Black #425), were placed 1 mm path length ICG standards in DMSO of 0, 31, 63, 125, 250, 500, 1000, and 2000 nM, and calibration curves were created for camera exposure times of 10, 34, 67, 134, 500 and 1000 msec. From these six curves, the average the autofluorescence of the tissue or organ under study was calculated, and expressed as ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm).
After each study, anesthetized animals are euthanized by rapid intravenous injection of 10 ml of Fatal-Plus (Vortech Pharmaceuticals, Dearborn, MI). This method of euthanasia is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Results
Preparation of Phantoms
Phantoms were prepared in covered plastic food storage containers of the desired size and geometry. The basic building block is a gelatin-containing medium having the desired absorbance, scatter, and background fluorescence. Absorbance of the medium is controlled by the concentration of oxy- and deoxyhemoglobin (the ratio of which can be adjusted using azide), and/or other biomolecules. Scatter is controlled by the concentration of intralipid, with virtually no contribution from the gelatin, even at high concentrations.
Characterization of Phantom Optical Properties
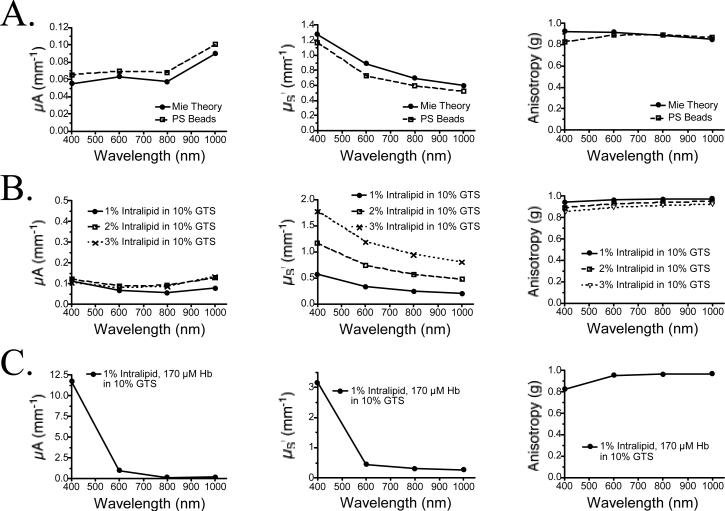
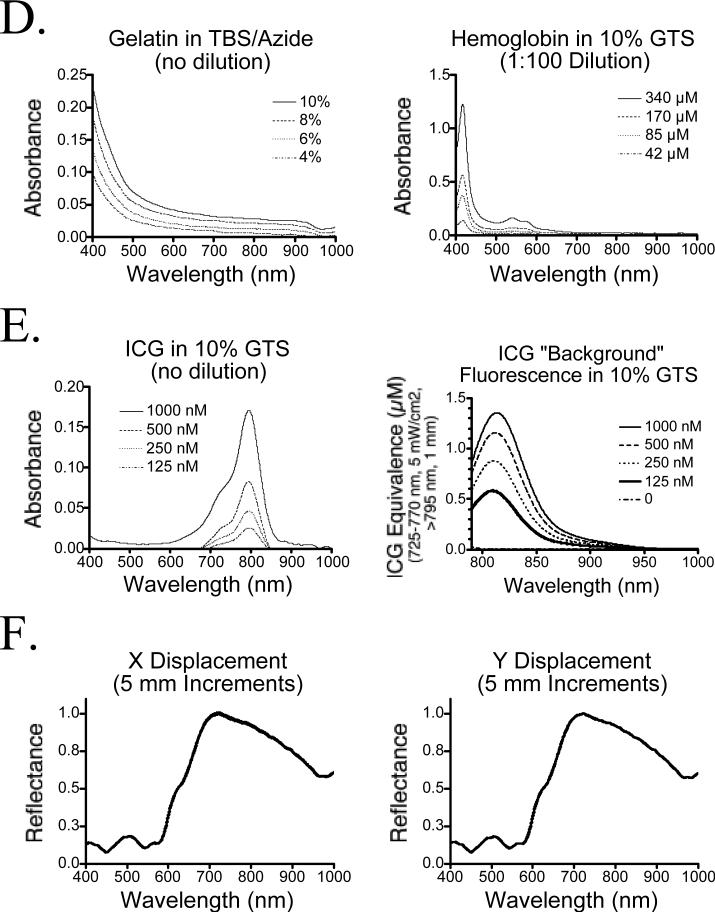
Optical properties of the phantoms were measured using an integrating sphere as described in Materials and Methods. Validation of the inverse adding-doubling algorithm employed was performed by comparing results of Mie scattering theory with calibrated polystyrene beads of defined optical properties. As shown in Figure 2A, theoretical and empirical values for μA, μS’, and anisotropy were closely correlated over the wavelength range 400−1000 nm. Optical properties of the various phantom components over this wavelength range are shown in Figure 2B (various concentrations of intralipid in 10% GTS) and Figure 2C (complete phantom). It should be noted that our values for μS’ of 1% intralipid in GTS are approximately two-fold lower than previously published values for 1% intralipid in water, which likely reflects lot-to-lot variation in particle size. We also found unexpectedly high scatter at 400 nm in the presence of concentrated Hb (Figure 2C), possibly due to Rayleigh scatter from the Hb molecules themselves. Absorbance of the phantom is controlled by the hemoglobin concentration, with a negligible contribution by gelatin at blue wavelengths (Figure 2D).
Figure 2. Optical Characterization of Phantom Components.
A) Validation of the employed inverse adding-doubling method by comparing measurements using polystyrene (PS) beads of defined optical properties (PS Beads) to Mie scattering theory (Mie Theory). Shown are μA (left), μS’ (middle) and anisotropy (right).
B) Optical properties of various intralipid concentrations as measured using an integrating sphere and the inverse adding-doubling method. Shown are μA (left), μS’ (middle) and anisotropy (right).
C) Optical properties of a typical phantom mixture compose of 1% intralipid and 170 μM Hb in 10% GTS. This composition most closely matches scattering human tissue with an 8% blood volume. Shown are μA (left), μS’ (middle) and anisotropy (right).
D) Absorbance of various gelatin concentrations in TBS/azide (left), and Hb in 10% GTS (right; diluted 1:100 in TBS/azide prior to measurement).
E) Absorbance (left) and “background” fluorescence (ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm), described in text; right) of ICG in 10% GTS.
F) Homogeneity of a 1% intralipid/170 μM Hb phantom in 10% GTS measured in 5 mm increments in the X (left) and Y (directions), over a total distance of 10 cm. Shown are the mean ± SEM of the 20 reflectance spectra at each wavelength from 400−1000 nm.
Varying the concentration of ICG in the medium controls background fluorescence, and also adds a NIR component to absorption (Figure 2E). Background fluorescence could be varied from 0 to 1.4 μM ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) by varying ICG concentration from 0 to 1 μM. The enhancement of ICG quantum yield after adsorption to proteins, such as gelatin, is well known [17].
The absorbance, scatter, and background fluorescence of the phantom can be varied in three dimensions; hence, complex phantoms having any desired optical properties and geometry can be constructed inexpensively and easily using this system (Figure 1; see also Figure 6 below). Using the same base material for all compartments minimizes refractive interfaces. Interestingly, phantoms can be re-melted, NIR fluorescent inclusions retrieved, and all materials recycled into new phantoms as needed.
Figure 6. Complex Phantom for the Calibration and Assessment of a NIR Fluorescence Imaging System, and Independent Confirmation by Micro-Computed Tomography.
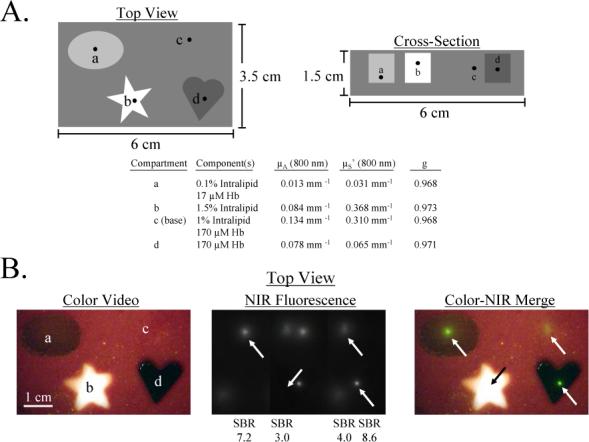
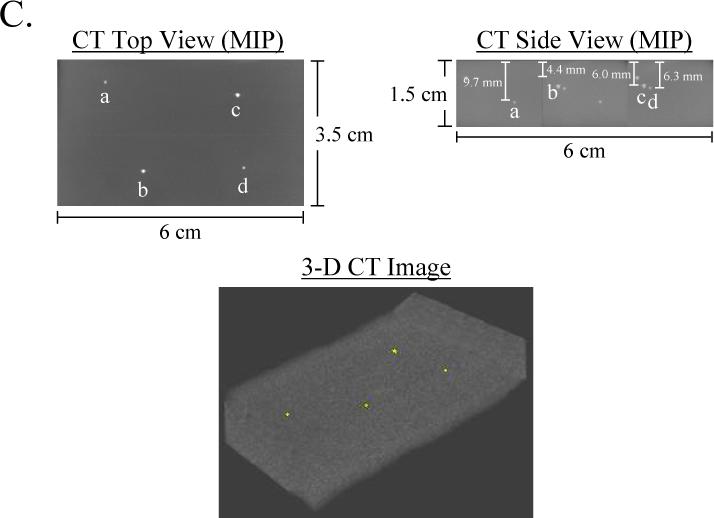
A) Schematic design of a complex phantom containing four compartments with optical properties shown in the table. Each compartment contained a 1 mm diameter bead of 880 nM ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) and 88.8 mM Conray placed at the desired depth.
B) Simultaneous color video/ NIR fluorescence imaging (top view) of the phantom using a NIR excitation fluence rate of 5 mW/cm2 and 100 msec exposure time. Shown are color video (left), NIR fluorescence (middle), and a pseudocolored (lime green) merge of the two (right). All pixel values were within the linear range of the NIR camera. Below each NIR fluorescent bead (arrows) is shown its SBR.
C) Confirmation of precise position of NIR fluorescent inclusions using micro-computed tomography. Shown are the maximum intensity projections (MIP) for top view and side view, along with a 3-D rendering of the phantom with beads in yellow.
Characterization of Phantom Homogeneity
Phantom homogeneity was tested by taking 20 full spectrum (400−1000 nm) reflectance measurements each in the X and Y directions, at 5 mm intervals. As shown in Figure 2F, variation over this 10 cm distance, in either direction, was less than 2%.
Characterization of Phantom Stability
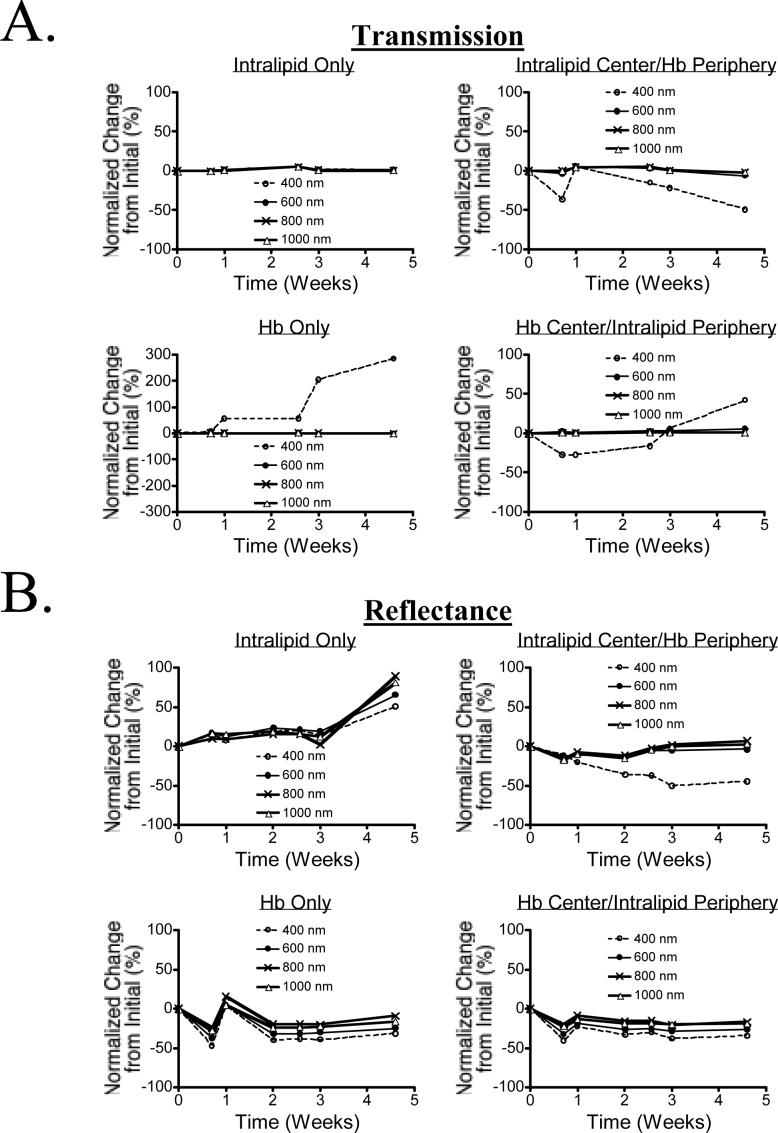
The stability of the phantom over time was measured at 400 nm, 600 nm, 800 nm, and 1000 nm over a 4-week period. Shown in Figure 3A is the variation in transmission, and in Figure 3B the variation in reflectance. At wavelegnths from 600−1000 nm, there was virtually no change in optical properties of the phantom components. However, at 400 nm and starting at approximately 2 weeks, there was evidence of Hb transition from the oxy-, deoxy- and possibly met-Hb states, as well as evidence of diffusion of Hb within the phantom. Although these results should have no impact on NIR fluorescence experiments, use of multi-compartment phantoms for measuring Hb concentration over periods greater than 2 weeks must be performed with these results in mind.
Figure 3. Long-Term Stability of Tissue-Like Phantoms and their NIR Fluorescent Inclusions.
A) Transmission over a 4-week interval for 1% Intralipid in 10% GTS (top left), 1% intralipid in a central location with Hb around the periphery, both in 10% GTS (top right), 170 μM Hb in 10% GTS (bottom left) and 170 μM Hb in a central location with 1% intralipid around the periphery, both in 10% GTS (bottom right).
B) Reflectance over a 4-week interval for the samples described in (A).
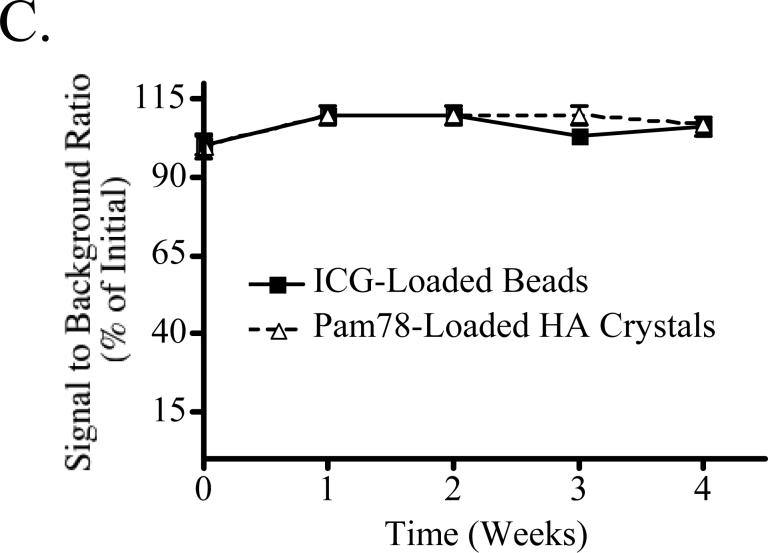
C) Stability of NIR fluorescent inclusions. NIR fluorescent beads and HA inclusions, each with a 1 μM ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm), were placed randomly throughout a phantom (1% intralipid and 170 μM Hb in 10% GTS) at a depth of 5 mm, and measurements were taken weekly. Between measurements, the phantom was sealed tightly in a storage container at 4°C. On the ordinate is signal to background ratio (SBR) expressed as mean ± SEM.
The Concept of “ICG Equivalence”
To solve the problem of extrapolating results from one embedded fluorescent inclusion to another, and from one phantom to another, we report all fluorescence emissions in terms of “ICG Equivalence,” that is, the measured fluorescence emission over a defined wavelength range, excited with NIR light of a defined wavelength and fluence rate, which is equivalent to an ICG standard in DMSO of a defined path length and concentration. For simplicity, the following notation is employed: ICG EquivalenceExcitation Wavelength(s), Fluence Rate, Emission Wavelength(s), Path Length. DMSO is one of the few solvents in which ICG is stable [18], and for which an absolute quantum yield (13%) has been reported [19]. Hence, by expressing measured fluorescence as ICG Equivalence, the performance of virtually any other NIR fluorophore, with similar excitation/emission wavelengths, can be extrapolated. For example, IRDye78 (LI-COR, Lincoln, NE), a NIR fluorophore commonly used by our laboratory, has a quantum yield of ≈14% in aqueous buffer [20], hence fluorescent inclusion results expressed as ICG Equivalence would be roughly equivalent to the expected performance of IRDye78.
Autofluorescence of Living Tissue
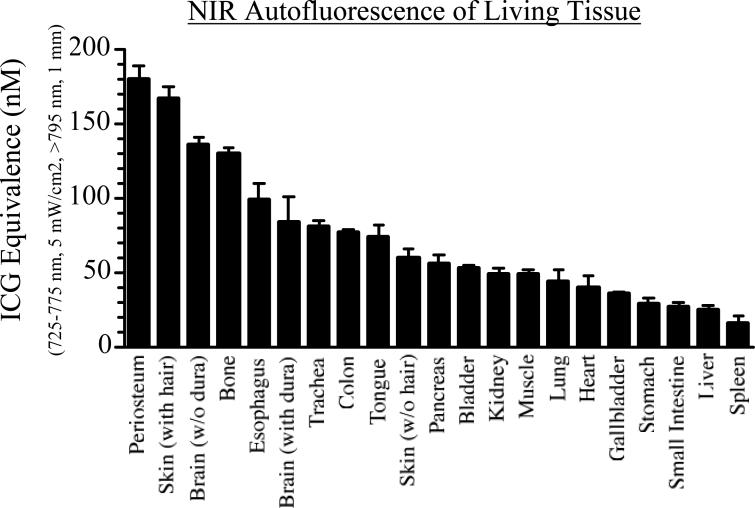
Living tissue has a low, but measurable, autofluorescence, which needs to be known before construction of a phantom with the proper amount of “background” fluorescence. Figure 4 shows the autofluorescence of virtually every tissue and organ in the body, expressed as ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm). The path length of the ICG standards in DMSO used to make these measurements must be specified since the reflectance imaging system employed in these studies is sampling an unknown depth of tissue. Since fluorescence emission often scales non-linearly with inclusion size, it is best to match the inclusion size to the typical tumor size of the model system employed. The values in Figure 4 can be used to add the appropriate level of phantom background fluorescence for the tissue type under study. This is especially importance since the relative autofluorescence of different tissues varies by over nine-fold.
Figure 4. Relative and Absolute NIR Autofluorescence of Living Tissues.
ICG Equivalence of major tissue types of the pig. Results shown are the average of three separate animals (mean ± SEM), and are expressed as ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) (nM).
NIR Fluorescent Inclusions
Similar to the sub-millimeter radioscintigraphic calibration sources previously reported by our group [21], the beads used in this study are available in wet bead diameters from 45 μm to 1180 μm, which can be sieved to any desired size, and are also available with cross-linking ranging from 2% to 8%. The latter value is important, since it determines the exclusion limit of the bead interior. The 8% bead used in this study does not exclude 775 Da ICG, resulting in relatively homogeneous fluorescence over the entire volume of the bead. Bead loading with ICG is distinctly non-linear (Figure 5), with stacking and quenching limiting the ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) per bead to approximately 4.2 μM. Due to high viscosity, Conray iodine contrast is diluted to 88.8 mM prior to bead loading. As expected, iodine contrast shifts the ICG loading curve shown in Figure 5 to the right (data not shown), emphasizing again the importance of confirming the ICG Equivalence of each fluorescent inclusion prior to placement in the phantom.
Figure 5. Characterization of NIR Fluorescent Inclusions.
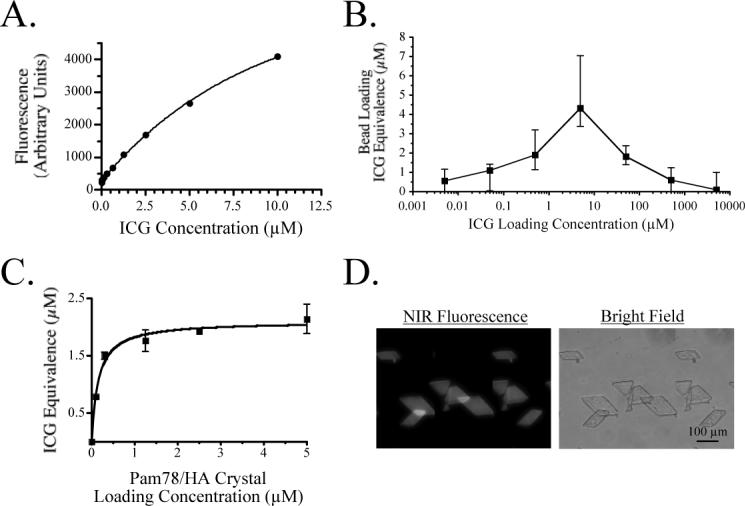
A) 0.79 μL ICG standards in DMSO.
B) ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) (μM; mean ± S.D.) of individual 1 mm (0.79 μL) ICG-loaded anion exchange beads expressed as a function of loading concentration.
C) ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) (μM; mean ± S.D.) of individual 100 μm HA crystals loaded with Pam78 and calibrated against 100 μm path length ICG standards in DMSO.
D) 100 μm Pam78-labeled HA crystals loaded with a 1 μM ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) as viewed on a NIR fluorescence microscope.
A second NIR fluorescent inclusion used in this study is hydroxyapatite (HA) crystals loaded with Pam78, a NIR fluorescent derivative of the bisphosphonate pamidronate [22]. Pam78 has been used previously for in vivo imaging of sites of osteoblastic activity and the simulated calcification of breast cancer [23]. Pam78-loaded HA is an ideal fluorescent inclusion for tissue-like phantoms since it has biologic and medical relevance, and can be loaded with physiologically relevant concentrations of fluorophore (Figure 5).
The stability of the NIR fluorescent inclusions was tested over 4 weeks with no significant changes in fluorescence emission found (Figure 3C). Given the stability of the base material and NIR fluorescent inclusions, phantoms could theoretically be shipped to any other laboratory for calibration or comparative assessment of different imaging systems.
Imaging System Calibration and Performance Assessment
One of the major uses for these phantoms is in assessing the performance of tomographic and reflectance imaging systems. This is illustrated in Figure 6 using a NIR fluorescence imaging system designed for large animal and human studies [16]. The complex phantom shown is particularly relevant to in vivo conditions where nearby tissues have distinctly different optical properties. The signal-to-background ratio (SBR), as well as visual discrimination of the inclusions, could be measured as a function of fluence rate, system optics, and camera integration time, and the precise 3-D position of inclusions measured using micro-computed tomography (Figure 6C). Of note, radiodense HA crystals require no additional contrast to be co-localized by micro-computed tomography (data not shown).
Training in Imaging System Operation and Image-Guided Surgery using NIR Fluorescent Phantoms
We have previously described an intraoperative NIR fluorescence reflectance imaging system that permits the surgeon to view surgical anatomy (via color video) and tumor or lymph node location (via NIR fluorescence) simultaneously, in real-time, and without moving parts ([16,20]; see also Figure 6B). As with any new imaging system, there is a learning curve associated with use of system software, and the surgeon must also be trained at NIR fluorescence-guided dissection, resection, and post-resection inspection of the surgical field. The phantoms described in this study may prove useful for such training and should permit quantitative assessment of surgical expertise.
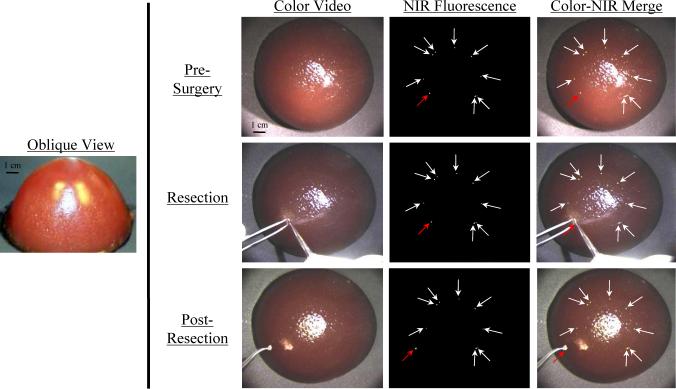
Shown in Figure 7 is a breast-shaped phantom with optical properties similar to the human breast. Embedded within the breast are nine 1 mm NIR fluorescent beads, corresponding to a tumor of approximately 8 × 105 cells (discussed below). Each bead has an ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) of 1 μM, corresponding to approximately 6 × 105 fluorophore molecules per cell. Given the close index matching between inclusions and phantom, it is virtually impossible to find inclusions using the naked eye or color video enhancement. Using the NIR fluorescence channel, however, the inclusions are easily found (Pre-Surgery), and the surgeon can then resect the desired inclusion (Resection) and inspect the field to ensure completeness (Post-Resection).
Figure 7. Training in Image-Guided Surgery using NIR Fluorescent Phantoms.
Nine 1 mm beads (each approximating a collection of 8 × 105 cells; arrows) with a 1 μM ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) were placed concentrically, and 0.5 cm below the surface, of a breast-shaped phantom (see oblique view). The phantom composition was 10% gelatin, 1% intralipid, and 17 μM hemoglobin, corresponding to μA = 0.056 mm−1, μS’ = 0.310 mm−1 and g = 0.968 at 800 nm. Shown is NIR fluorescence image-guided resection of one (red arrow) of the otherwise invisible beads using a scalpel and forceps. NIR fluorescence images have identical exposure times (100 msec) and normalizations.
Discussion
In this study, we demonstrate how to construct simple, inexpensive phantoms for NIR fluorescence imaging applications. The significant advantages of these phantoms include complete control over geometry and optical properties, the ability to create both simple and complex conditions, the ability to independently confirm the 3-D position of fluorescent inclusions using computed tomography, the ability to incorporate any pigmented biomolecule (e.g., hemoglobin), high stability over time in the NIR, no artificial interface between volumes having different optical properties or between fluorescent inclusions and medium, and the representation of fluorescence emission as “ICG Equivalence.” Moreover, these phantoms should be compatible with tomographic imaging systems having either circular [24-26] or planar [27] geometry (detailed in Figure 1).
Interestingly, cross-linking of the phantoms for various periods of time (typically 1−2 hours) with various concentrations of glutaraldehyde (0.1−1%) permits fine-tuning of their mechanical properties, with more highly cross-linked phantoms remarkably similar to living tissue when cut with a scalpel. Glutaraldehyde presumably cross-links both the buffer (Tris) and protein components of the phantom, and of course, requires re-measurement of all optical properties. Glutaraldehyde does not appear to affect significantly NIR fluorescence emission from either bead/ICG or HA/Pam78 inclusions (data not shown).
The fluorescent inclusions described in this study were chosen for their relevance to important medical applications. For tumor cell receptor imaging, an abundant receptor will be present at 100,000 to 600,000 copies on the cell surface. Assuming a cell volume of 1 pL, and no endocytosis, the maximum concentration of contrast agent achievable is 160 nm to 1 μM. As demonstrated in Figure 5, the anion exchange beads used in this study can be loaded with ICG Equivalence throughout this physiologic range. The beads are also available in volumes ranging from 45 pL to 820 nL, corresponding to roughly 45 to 820,000 cells. Pam78-loaded HA also has significant biologic relevance in light of recent reports that the malignant calcification of breast cancer is composed of HA, and not other calcium salts [13], and since the ICG Equivalence(725−775 nm, 5 mW/cm2, >795 nm, 1 mm) of Pam78-loaded HA is within the range obtained during in vivo imaging [22,23]. When combined with our results from tissue autofluorescence measurements, and published values for tissue optical properties [28], one should be able to use these phantoms to estimate, prior to in vivo experimentation, the performance of virtually any molecularly targeted NIR fluorophore having similar excitation/emission wavelengths as ICG in DMSO, embedded in virtually any simulated tissue of interest.
We have previously described a simultaneous color video/NIR fluorescence reflectance imaging system for intraoperative use and demonstrated its utility in image-guided sentinel lymph node mapping [29-33], optical angiography [16,20], and tumor resection (discussed in [16]). Others have described optical tomography systems for detecting embedded NIR fluorescent targets in the human breast (reviewed in [34]). The translation of NIR fluorescence technology to the clinic will require the training of medical technologists (e.g., for tomographic breast imaging) and surgeons (e.g., for intraoperative reflectance imaging systems). Such training should be greatly facilitated by the tissue-like phantoms described in this study. As optical imaging approaches clinical use, inexpensive training systems that do not require animal or human subjects during the steepest phase of the learning curve will be particularly helpful.
Acknowledgements
We thank Barbara L. Clough for editing and Grisel Rivera for administrative assistance. This work was supported by Department of Energy (Office of Biological and Environmental Research) grant #DE-FG02-01ER63188, a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (non-animal experiments), an Application Development Award from the Center for Integration of Medicine and Innovative Technology (CIMIT) and grants #R33-CA-88245 and #R21-CA-11018 from the National Cancer Institute of the National Institutes of Health to JVF.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Lim YT, Kim S, Nakayama A, Stott NE, Bawendi MG, Frangioni JV. Selection of quantum dot wavelengths for biomedical assays and imaging. Molecular Imaging. 2003;2:50–64. doi: 10.1162/15353500200302163. [DOI] [PubMed] [Google Scholar]
- 2.Hielscher AH, Bluestone AY, Abdoulaev GS, Klose AD, Lasker J, Stewart M, Netz U, Beuthan J. Near-infrared diffuse optical tomography. Dis Markers. 2002;18:313–37. doi: 10.1155/2002/164252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sevick-Muraca EM, Houston JP, Gurfinkel M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr Opin Chem Biol. 2002;6:642–50. doi: 10.1016/s1367-5931(02)00356-3. [DOI] [PubMed] [Google Scholar]
- 4.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 5.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Cubeddu R, Pifferi A, Taroni P, Torricelli A, Valentini G. A solid tissue phantom for photon migration studies. Phys Med Biol. 1997;42:1971–9. doi: 10.1088/0031-9155/42/10/011. [DOI] [PubMed] [Google Scholar]
- 7.Wagnieres G, Cheng S, Zellweger M, Utke N, Braichotte D, Ballini JP, van den Bergh H. An optical phantom with tissue-like properties in the visible for use in PDT and fluorescence spectroscopy. Phys Med Biol. 1997;42:1415–26. doi: 10.1088/0031-9155/42/7/014. [DOI] [PubMed] [Google Scholar]
- 8.Quan KM, Christison GB, MacKenzie HA, Hodgson P. Glucose determination by a pulsed photoacoustic technique: an experimental study using a gelatin-based tissue phantom. Phys Med Biol. 1993;38:1911–22. doi: 10.1088/0031-9155/38/12/014. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RG, Jordan KJ, Battista JJ. Optical CT reconstruction of 3D dose distributions using the ferrous-benzoic-xylenol (FBX) gel dosimeter. Med Phys. 1998;25:1741–50. doi: 10.1118/1.598356. [DOI] [PubMed] [Google Scholar]
- 10.Giller CA, Liu H, Gurnani P, Victor S, Yazdani U, German DC. Validation of a near-infrared probe for detection of thin intracranial white matter structures. J Neurosurg. 2003;98:1299–306. doi: 10.3171/jns.2003.98.6.1299. [DOI] [PubMed] [Google Scholar]
- 11.Boehm T, Hochmuth A, Malich A, Reichenbach JR, Fleck M, Kaiser WA. Contrast-enhanced near-infrared laser mammography with a prototype breast scanner: feasibility study with tissue phantoms and preliminary results of imaging experimental tumors. Invest Radiol. 2001;36:573–81. doi: 10.1097/00004424-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Pogue BW, McBride TO, Paulsen KD. Quantitative analysis of near-infrared tomography: sensitivity to the tissue-simulating precalibration phantom. J Biomed Opt. 2003;8:308–15. doi: 10.1117/1.1559692. [DOI] [PubMed] [Google Scholar]
- 13.Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res. 2002;62:5375–80. [PubMed] [Google Scholar]
- 14.Prahl SA, van Gemert MJC, Welch AJ. Determining the optical properties of turbid media by using the adding-doubling method. Appl Opt. 1993;32:559–568. doi: 10.1364/AO.32.000559. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Lu JQ, Brock RS, Jacobs KM, Yang P, Hu XH. Determination of complex refractive index of polystyrene microspheres from 370 to 1610 nm. Phys Med Biol. 2003;48:4165–72. doi: 10.1088/0031-9155/48/24/013. [DOI] [PubMed] [Google Scholar]
- 16.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–562. doi: 10.1177/153303460300200607. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic Alternatives to Quantum Dots for Intraoperative Near-Infrared Fluorescent Sentinel Lymph Node Mapping. Mol Imaging. 2005 doi: 10.1162/15353500200505127. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan R, Uetrecht P, Bugaj JE, Achilefu SA, Dorshow RB. Stabilization of the optical tracer agent indocyanine green using noncovalent interactions. Photochem Photobiol. 2000;71:347–50. doi: 10.1562/0031-8655(2000)071<0347:sotota>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Benson C, Kues HA. Absorption and fluorescence properties of cyanine dyes. J. Chem. Eng. Data. 1977;22:379–383. [Google Scholar]
- 20.Nakayama A, del Monte F, Hajjar RJ, Frangioni JV. Functional near-infrared fluorescence imaging for cardiac surgery and targeted gene therapy. Molecular Imaging. 2002;1:365–377. doi: 10.1162/15353500200221333. [DOI] [PubMed] [Google Scholar]
- 21.English JR, Accorsi R, Idoine JD, Parker JA, Renze JT, Lanza RC, Frangioni JV. Sub-millimeter technetium-99m calibration sources. Mol Imag Biol. 2002;4:380–384. doi: 10.1016/s1536-1632(02)00084-7. [DOI] [PubMed] [Google Scholar]
- 22.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148–1154. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 23.Lenkinski RE, Ahmed M, Zaheer A, Frangioni JV, Goldberg SN. Near-infrared fluorescence imaging of microcalcification in an animal model of breast cancer. Acad Radiol. 2003;10:1159–64. doi: 10.1016/s1076-6332(03)00253-8. [DOI] [PubMed] [Google Scholar]
- 24.Intes X, Ripoll J, Chen Y, Nioka S, Yodh AG, Chance B. In vivo continuous-wave optical breast imaging enhanced with Indocyanine Green. Med Phys. 2003;30:1039–47. doi: 10.1118/1.1573791. [DOI] [PubMed] [Google Scholar]
- 25.Dehghani H, Pogue BW, Poplack SP, Paulsen KD. Multiwavelength three-dimensional near-infrared tomography of the breast: initial simulation, phantom, and clinical results. Appl Opt. 2003;42:135–45. doi: 10.1364/ao.42.000135. [DOI] [PubMed] [Google Scholar]
- 26.Iftimia N, Gu X, Xu Y, Jiang H. A compact, parallel-detection diffuse optical mammography system. Rev. Sci. Instrum. 2003;74:2836–2842. [Google Scholar]
- 27.Reynolds JS, Troy TL, Sevick-Muraca EM. Multipixel techniques for frequency-domain photon migration imaging. Biotechnol Prog. 1997;13:669–80. doi: 10.1021/bp970085g. [DOI] [PubMed] [Google Scholar]
- 28.Cheong WF, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. IEEE J. Quantum Electronics. 1990;26:2166–2195. [Google Scholar]
- 29.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parungo CP, Ohnishi S, De Grand AM, Laurence RG, Soltesz EG, Colson YL, Kang PM, Mihaljevic T, Cohn LH, Frangioni JV. In vivo optical imaging of pleural space drainage to lymph nodes of prognostic significance. Ann Surg Oncol. 2004;11:1085–92. doi: 10.1245/ASO.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Parungo CP, Colson YL, Kim SW, Kim S, Cohn LH, Bawendi MG, Frangioni JV. Sentinel lymph node mapping of the pleural space. Chest. 2005;127:1799–804. doi: 10.1378/chest.127.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parungo CP, Ohnishi S, Kim SW, Kim S, Laurence RG, Soltesz EG, Chen FY, Colson YL, Cohn LH, Bawendi MG, Frangioni JV. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. J Thorac Cardiovasc Surg. 2005;129:844–50. doi: 10.1016/j.jtcvs.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltesz EG, Kim S, Laurence RG, DeGrand AM, Parungo CP, Dor DM, Cohn LH, Bawendi MG, Frangioni JV, Mihaljevic T. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 2005;79:269–77. doi: 10.1016/j.athoracsur.2004.06.055. discussion 269−77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntziachristos V, Chance B. Probing physiology and molecular function using optical imaging: applications to breast cancer. Breast Cancer Res. 2001;3:41–6. doi: 10.1186/bcr269. [DOI] [PMC free article] [PubMed] [Google Scholar]