Abstract
Background
Self-injurious behavior (SIB), a disorder that afflicts many individuals within both clinical and non-clinical populations, has been linked to states of heightened stress and arousal. However, there are no published longitudinal data on the relationship between increases in stress and changes in the incidence of SIB. The present study investigated the short- and long-term behavioral and neuroendocrine responses of SIB and control monkeys to the stress of relocation.
Methods
Twenty adult male rhesus macaques were exposed to the stress of relocation to a new housing arrangement in a newly constructed facility. Daytime behavior, sleep, and multiple measures of hypothalamic-pituitary-adrenocortical (HPA) axis function were investigated before and after the move.
Results
Relocation induced a complex pattern of short- and long-term effects in the animals. The SIB animals showed a long-lasting increase in self-biting behavior as well as evidence of sleep disturbance. Both groups exhibited elevated cortisol levels in saliva, serum, and hair, and also an unexpected delayed increase in circulating concentrations of corticosteroid binding globulin (CBG).
Conclusions
Our results indicate that relocation is a significant stressor for rhesus macaques, and that this stressor triggers an increase in self-biting behavior as well as sleep disturbance in monkeys previously identified as suffering from SIB. These findings suggest that life stresses may similarly exacerbate SIB in humans with this disorder. The HPA axis results underscore the potential role of CBG in regulating long-term neuroendocrine responses to major stressors.
Keywords: self-injury, relocation, stress, cortisol, CBG, HPA axis, monkey
Introduction
Self-injurious behavior (SIB), which may be defined as any self-directed act that results in tissue damage (1), is a significant health problem within both the general population and several clinical disorders. SIB is expressed in various forms including but not limited to cutting, burning, biting, hair pulling, skin picking, and head banging. Approximately 40% of humans with learning disabilities living in hospitals are reported to engage in SIB (2). SIB is also a common symptom of autism (3), post-traumatic stress disorder (PTSD; 4, 5), borderline personality disorder (6), Tourette syndrome (7), and the genetic disorders Lesch-Nyhan syndrome (8) and Prader-Willi syndrome (9). However, SIB is not restricted to individuals suffering from psychiatric or genetic disorders, as approximately 4% of the general population engages in various forms of this behavioral pathology that typically involve cutting, scratching or burning (10). The heterogeneity of SIB with respect to both the form of expression of the pathology and its incidence has made it difficult to develop effective treatment strategies (11).
Although the factors involved in the etiology and maintenance of SIB are not yet fully understood, episodes of self-injury often serve to reduce tension, anxiety, or other dysphoric states. This notion is supported by results from both self-report (12–16) and psychophysiological (17) studies. In addition, Philipsen and coworkers (18) recently found that clonidine treatment of female patients with borderline personality disorder who had previously exhibited SIB resulted in parallel decreases in “aversive inner tension” and in the urge to injure themselves.
Given the ability of different environmental or social stressors to provoke tension and anxiety, it seems likely that stress would increase the incidence and/or severity of self-injury. Yet, this hypothesis has thus far received scant attention in the literature apart from a few relevant findings. Thus, management of stress was reported as one of the reasons for engaging in self-injury by 77% of a clinical sample that had experienced earlier trauma (mainly in the form of sexual abuse) and that was currently undergoing treatment for PTSD and/or other psychiatric disorders (10). Furthermore, Symons et al (19) found a significant positive correlation between salivary cortisol levels and SIB severity in a group of developmentally disabled adults, supporting a possible relationship between SIB and stress. Sachsse et al (20) also reported a case study of a women suffering from borderline personality disorder whose nocturnal urinary cortisol excretion showed large increases during the period leading up to an episode of self-mutilation. Finally, several studies have demonstrated a greater incidence of sleep disturbances (suggestive of increased stress) in subjects exhibiting SIB than in control subjects (21–23).
One powerful approach to investigating the relationship between SIB and stress would be to conduct a longitudinal study of behavioral and physiological responses to a major imposed stressor in subjects suffering from SIB. There are major practical as well as ethical difficulties in carrying out such a study in human subjects; however, these limitations can be overcome using an experimental animal model. Our laboratory has an ongoing research program investigating one such model, namely individually housed adult male rhesus macaques that spontaneously develop SIB. The behaviors seen in these animals range from hair pulling and head banging to severe self-biting, that occasionally results in self-wounding. Our model, which has been well-characterized both behaviorally and physiologically (24, 25), has a number of advantageous features including a similar behavioral topology to human SIB, a prevalence rate of 5–13% (26) that is similar to the prevalence of SIB in some clinical populations (27), and the fact that unlike most rodent models of SIB, the pathology arises spontaneously without the need for pharmacological manipulations. As discussed in Tiefenbacher et al. (25), we propose that our nonhuman primate model most closely resembles the impulsive SIB category of Simeon and Favazza (28).
In the present study, we took advantage of an administratively mandated relocation of our subject cohort to new cages in a newly constructed building and with unfamiliar animals now present within each colony room. Previous studies have demonstrated that relocation leads to behavioral and physiological alterations in elderly human subjects (29, 30) and a cortisol response in nonhuman primates (31). Therefore, we anticipated that the move would constitute a significant and possibly prolonged stressor in our animals. The aims of this study were to determine both the short- and long-term behavioral responses of SIB and control monkeys to the relocation, and to assess concomitant hypothalamic-pituitary-adrenocortical (HPA) axis reactivity through cortisol measurements in multiple sample matrices (plasma, saliva, and hair) along with measurement of circulating corticosteroid-binding globulin (CBG). We predicted that both groups would exhibit behavioral and endocrine signs of stress, but that the SIB animals would particularly respond with an increased incidence of self-biting behavior.
Methods and Materials
Subjects
The subjects were 20 adult male rhesus monkeys (M. mulatta) ranging in age from 8–22 years of age (SIB mean = 13.4; control mean = 15.4) and maintained at the New England Primate Research Center (NEPRC). Thirteen monkeys had wounded themselves at least once with sufficient severity to require veterinary treatment (SIB group), whereas the remaining seven animals constituted a control group that had never self-wounded. Monkeys were socially reared either with their mother (eight SIB, three controls) or in the NEPRC nursery (five SIB, four controls). See Supplementary Material for additional information on housing of the monkeys before and after relocation. All animal procedures were approved by the Harvard Medical Area Standing Committee on Animals and were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals.
Behavioral Testing
The effects of relocation on behavior of the SIB and control monkeys was assessed using a modified frequency sampling procedure (32) in which the presence/absence of 32 categories of behavior was recorded in 15-sec intervals for a 5-min sampling period. The behaviors deemed of greatest interest for the present study included self-bite, stereotypy (which includes a variety of repetitive behaviors such as self-grasp, eye poke, digit suck, hair pull, bounce, rock, body flip, and pace), eating, yawning, cage shaking, rump present (an affiliative or submissive behavior), exploration, and foraging (see Supplemental Material for additional information on the testing schedule as well as a list of all behavioral categories and their definitions). Reliability between observers was calculated over all categories by percent agreement scores and averaged over 90%.
In humans, stress is well known to induce disturbances in sleep (33, 34). Consequently, we monitored nighttime activity of the animals during the period from 2100 h to 0300 h by means of a camcorder with low-light capability (Sony Handycam DCR-DVD 200). Each animal was videotaped twice, once prior to relocation and the second time from 8 to 27 weeks (mean ≈ 20 weeks for the SIB group; ≈19 weeks for controls) following the move. Videotapes were later scored for awake vs. sleep state by two observers using a 1-min point sampling procedure (32). Sleep behavior was scored using a scale of 0–2 as follows: 0 - animal was in a posture typically associated with sleep (i.e., sitting with a hunched over posture or laying down, eyes are closed if visible to the observer); 1 - animal was awake but resting (i.e., eyes open, visually exploring, scratching, yawning, or adjusting body position); 2 - animal was awake and exhibiting whole-body movements such as locomotion. Reliability between observers was calculated by percent agreement scores and averaged over 92%. We also calculated Cohen’s kappa scores for a randomly selected subset (20%) of the videotapes, and these scores ranged from 0.847 to 0.966 (mean = 0.914).
Sampling Procedures
Blood
Blood samples were collected approximately 2 months prior to relocation (baseline), 7 days following the relocation (post-move), and again 1 year later to determine recovery from the stress of the move. Samples obtained well before the move were used for baseline purposes due to the fact that preparation for this event caused some disturbance within the colony and could therefore have artificially elevated the circulating cortisol levels in our animals. See Supplementary Material for additional information on blood, saliva, and hair sampling procedures.
Saliva
Samples were obtained from 16 monkeys (11 SIB, 5 control) that had previously been trained for saliva collection using the “pole” method (35). Four samples were obtained from each animal during the week immediately prior to relocation. Eight samples were subsequently collected over 2 weeks following relocation (beginning 2 days post-move).
Hair
Our laboratory has recently developed and validated a procedure for measuring hair cortisol as a unique tool for assessing long-term changes in HPA system activity. Hair cortisol data obtained during the present study were included in the paper describing that method (36) in order to demonstrate that a significant, prolonged stressor does in fact elevate cortisol content of the hair. However, we reproduce the hair results again here for the important purpose of comparing them with plasma and salivary cortisol levels obtained in the same animals. To obtain baseline levels of hair cortisol before the move, the animals were shaved twice: the first shaving was performed 3 months prior to relocation (those samples were discarded) to define the beginning of the sampling period, and the second shaving was performed immediately prior to relocation to collect the hair that had grown during that 3-month period. The hair obtained at that time constituted the pre-move samples. Additional hair samples were then collected at 4 months (post-move) and 1 year (recovery) following relocation.
Biochemical Analyses
Serum cortisol and CBG concentrations were determined by radioimmunoassay (RIA). Salivary and hair cortisol were analyzed by enzyme immunoassay (EIA) according to published procedures (35, 36). The free cortisol index (FCI, which represents the cortisol:CBG ratio) was determined by dividing serum cortisol concentrations (in nmol/l) by CBG concentrations (in mg/l) (37, 38). Additional information on the biochemical analyses is available in Supplementary Material.
Data Analysis
For all behavioral categories, data from each subject were pooled to obtain a mean modified frequency score at each time point (pre-move, post-move, and 1 year later). Selected categories were subsequently analyzed using mixed design analyses of variance (ANOVAs) with time point as a within-subjects variable, and group (SIB and control) as a between-subjects variable. Nighttime behaviors were analyzed using the same mixed design procedure, but only included pre- and post-move time points. Data analysis of nighttime behavior focused on scans spent sleeping vs. awake (i.e., scores of 0 vs. 1 and 2 combined). Following significant F values in the ANOVA, Tukey-Kramer multiple comparisons post-hoc tests were used to determine group differences at each time point, and univariate repeated measures ANOVAs were used to determine differences across time within a group. Self-biting behavior was not normally distributed due to extremely low rates exhibited by the control monkeys throughout the study as well as by some of the SIB animals during the pre-move period. Consequently, to determine the effects of relocation on this variable, we conducted separate Wilcoxon signed-ranks tests on each subject group comparing the pre-move values with either the post-move or the 1-year values. All physiological variables (serum, salivary, and hair cortisol, CBG, and FCI) were analyzed using mixed design ANOVAs, Tukey-Kramer post-hoc tests and univariate repeated measures ANOVAs as described above. One exception was that the main effects of FCI were followed with a two-sample t-test between groups. Salivary cortisol was not determined at the 1-year time point. One member of the SIB group was euthanized due to illness prior to the 1-year sampling and therefore did not contribute to the results at that time point. However, due to the lower statistical power that can occur using nonparametric tests such as the Wilcoxon, we did include this animal in the self-biting analysis at 1 year by using the same biting data obtained from that subject at the post-move interval (note that we did not compare post-move vs. 1-year biting data).
Results
Behavior
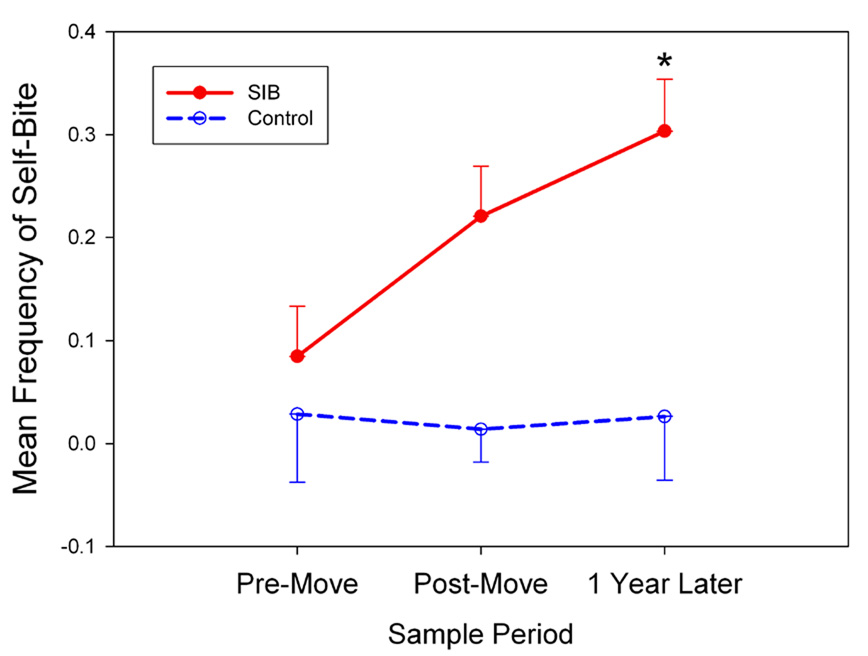
Relocation to a new environment induced substantial behavioral changes in our rhesus monkeys. Of particular importance was the effect of relocation on SIB. As illustrated in Figure 1, control monkeys showed low levels of self-biting throughout the course of the study. In contrast, monkeys in the SIB group exhibited an increase in self-biting from the pre-move period to the post-move time points. This increase reached statistical significance when the 1-year results were compared with the pre-move baseline rates of self-biting in the SIB group (W = 54, Z = 2.12, p < 0.05 by Wilcoxon signed-ranks test). A significant Group × Time difference was also determined in sleep patterns. The estimated amount of time spent sleeping declined from the pre-move to the post-move period in the SIB group (scans scored as sleep posture: 332.8 ± 3.3 vs. 317.1 ± 5.7 respectively, mean ± SEM); however, no such change occurred in the control group (319.9 ± 8.7 vs. 324.9 ± 8.4) (F[1,18] = 9.03, p < 0.05). Other behavioral changes associated with relocation are presented in Supplementary Material.
Figure 1.
Frequency scores for self-biting increased in SIB but not control monkeys from the pre-move to the 1-year sample period (data shown represent the mean ± SEM; *p < 0.05 compared to pre-move in the SIB group).
Cortisol
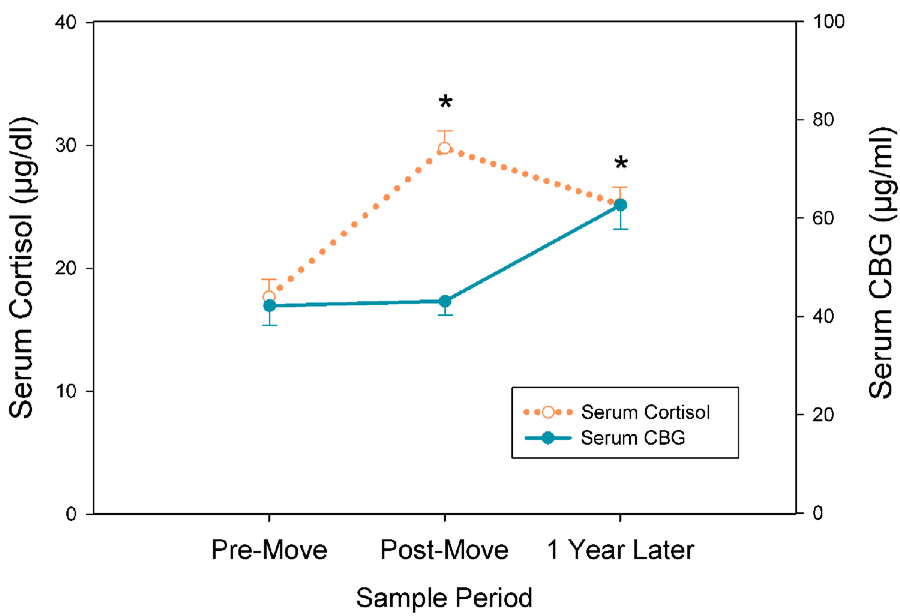
A significant increase of about 60% was seen in mean serum cortisol concentrations from the pre-move to the post-move samples (Figure 2, dotted line) that was reflected in a main effect of Time (F[2,41] = 16.98, p < 0.001). Surprisingly, however, there was no recovery to pre-move cortisol levels at the 1-year time point. Although there was a tendency for the SIB group to have lower serum cortisol than the controls at the pre- and post-move sampling periods, these differences were not statistically significant. Salivary cortisol results are presented in Supplementary Material.
Figure 2.
Relocation produced an overall increase in serum cortisol concentrations in monkeys, an effect that was maintained through the 1-year sample period (left Y-axis, dotted line, mean ± SEM; *p < 0.001 for pre-move compared to post-move, and *p < 0.01 for pre-move compared to 1 year later). Serum CBG concentrations at the post-move sample period showed no variation from the pre-move samples (right Y-axis, solid line, mean ± SEM; p = 0.91), but a significant increase occurred between the post-move and 1 year sample periods (*p < 0.005). No significant differences were found for serum cortisol or CBG between SIB and control monkeys at any of the three time points (not shown).
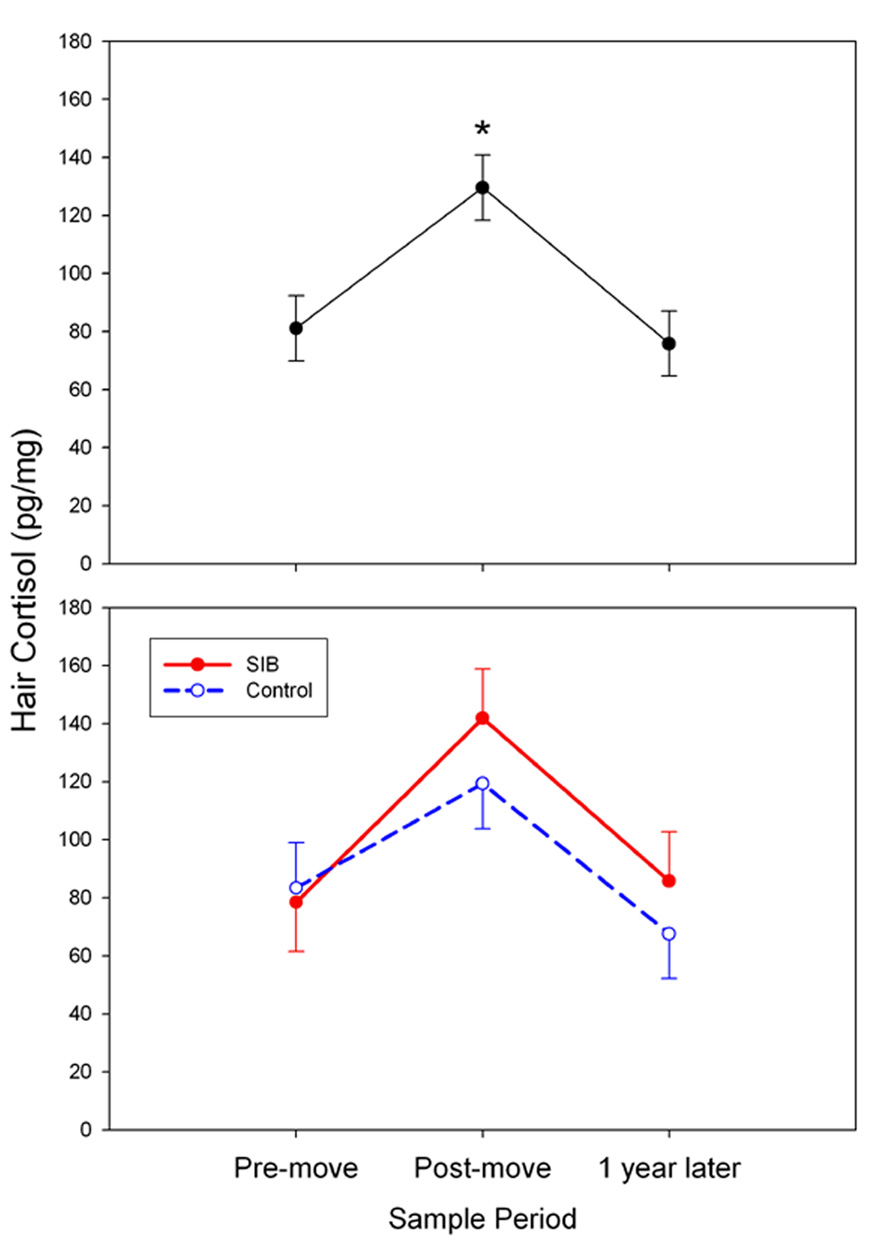
As mentioned earlier, hair cortisol concentrations from this study were previously published as part of the validation of our hair cortisol method (36); however, the results are presented again here for the purpose of comparison with the serum and salivary cortisol results. There was a significant overall increase in mean hair cortisol concentrations from the pre-move samples (representing the 3-month period prior to relocation) to the post-move samples (representing the 4-month period immediately following relocation); however, in contrast to the serum cortisol findings, hair cortisol had returned to pre-move concentrations by 1 year following the move (F [2,20] = 11.61, p < 0.001; Figure 3, upper panel). There were no significant differences between SIB and control animals at any time point (Figure 3, lower panel).
Figure 3.
Relocation produced an overall increase in hair cortisol concentrations at the post-move sample period (upper panel, mean ± SEM; *p < 0.05 compared to pre-move), and a subsequent return to baseline at the 1-year later sample period (n.s. compared to pre-move; *p < 0.001 compared to post-move). There was no significant group difference at any of the three sample periods (lower panel).
CBG and FCI
Measurement of CBG concentrations at each experimental time point revealed that changes in the amount of this binding protein did not parallel the changes observed for either serum or hair cortisol. Specifically, there was no alteration of CBG concentrations from pre-move to post-move samples, but there was a significant increase from the post-move to the 1-year time point (F[2, 32] = 11.43, p < 0.001; Figure 2, solid line). No differences were found between SIB and control animals in CBG concentrations at any time point.
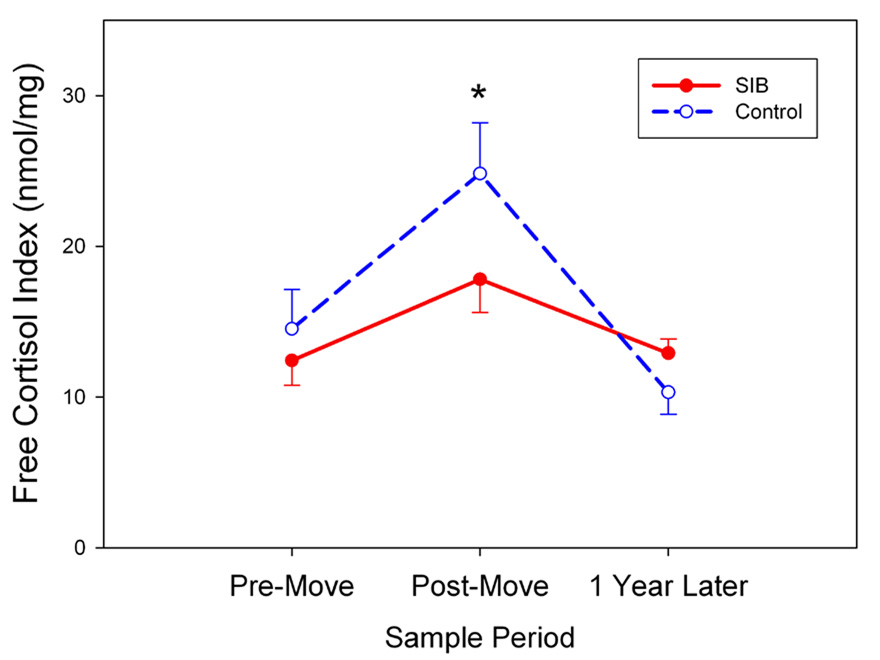
The amount of “free” cortisol available to enter tissues and activate glucocorticoid receptors depends not only on circulating cortisol concentrations but also on the levels of serum binding proteins, particularly CBG. Relative changes in cortisol availability can be assessed by means of the free cortisol index, a simple ratio of total cortisol to CBG measured in the same samples (37, 38). Therefore, it is interesting to note that despite the lack of any significant differences between the SIB and control monkeys on serum cortisol or CBG when considered alone, a difference between the groups did emerge when the FCI was analyzed across sampling times. Although there was an overall increase from pre-relocation (13.11 ± 1.37 nmol/mg) to post-relocation (20.28 ± 1.97 nmol/mg) FCI values when the data were collapsed across both groups, this response was noticeably blunted in the SIB monkeys as indicated by a significant Group × Time interaction followed by post-hoc testing (F[2, 24] = 12.74, p < 0.001; Figure 4). As can be seen in the figure, there were no group differences present at either the pre-move or 1-year time points.
Figure 4.
SIB monkeys showed significantly less free cortisol, as determined by FCI, than control monkeys at the post-move sample period (mean ± SEM; *p < 0.05).
Discussion
The present behavioral and physiological results demonstrate that the major stress of relocation induced substantial behavioral and physiological changes in male rhesus monkeys. Some of these perturbations were the same across both the SIB and control groups. Most importantly, however, the stressor elicited a particularly strong pathological response in the SIB animals, as evidenced by a significant increase in the frequency of self-biting as well as a disruption in sleep patterns. Also of interest are the complex long-term changes in various indices of HPA system function that occurred following relocation. These findings are discussed in greater detail below.
SIB in humans has often been associated with heightened stress or anxiety. Yet, there is relatively little evidence from the clinical literature to support this association. Thus, an important aim of the present study was to use a well-established primate model to determine the short- and long-term effects of a major stressor on the incidence of SIB. The results clearly show that SIB can be exacerbated by stress in this animal model. Indeed, the rate of self-biting in the SIB group not only increased following the move, it remained elevated for at least 1 year. In contrast, the stress of relocation did not cause the control monkeys to develop this behavioral pathology. These findings are consistent with one of the major components of our working model of rhesus monkey SIB, namely that stressful events are important in eliciting and maintaining this behavioral pathology (25, 26, 39). Confirmation of this hypothesis in human populations will require additional clinical studies specifically designed to investigate the relationship between stress and SIB.
Relocation also produced a selective sleep disruption in the SIB animals. Several studies have related SIB to sleep disturbances in both macaques (40) and humans (22–24), and one case study of a moderately retarded child with SIB found that sleep deprivation increased the incidence of self-injury (41). There is also a well-established relationship between stress and sleep disruption. Indeed, Reite and Short (42) found that infant monkeys stressed by maternal separation show decreases in the amount and quality of sleep. Nevertheless, the present results are the first to demonstrate either in humans or in non-human primates an association between SIB and increased vulnerability to stress-induced sleep disturbances.
The physiological stress response to relocation was measured in serum, saliva, and hair, each representing a unique assessment of HPA reactivity. Serum samples assessed the total cortisol response to the mild acute stress of restraint, sedation and venipuncture at each of the three time points. Saliva samples provided an index of free cortisol levels prior to relocation and during the first 2 weeks post-move. Finally, hair samples assessed chronic HPA activity integrated over long periods of time. The short-term (serum and saliva samples obtained 1–2 weeks post-move) and medium-term (hair samples obtained 4 months post-move) cortisol measurements yielded the expected stress-induced increases. Surprisingly, however, serum cortisol concentrations remained significantly elevated at the 1-year time point. This finding raises the possibility that in addition to the stress of relocation itself, the monkeys’ new physical and social environment was more stressful than their previous environment. Continued stress is also consistent with the persistently elevated self-biting behavior in the SIB group.
As shown in Figure 3, hair cortisol concentrations (which likely reflect the free fraction in the circulation; see 36) had completely returned to baseline levels by 1 year following the move, despite the elevated serum cortisol levels. We believe that this unexpected discrepancy can be accounted for by time-dependent changes in CBG. No change occurred in CBG levels from the pre-move to the post-move period; however, there was a significant increase from the post-move samples to the samples obtained at 1 year after relocation. Consequently, the increased FCI (an indicator of free cortisol; 37, 38) seen during the early post-move period was no longer present at the 1-year sampling time. This normalization (i.e., return to baseline) of the FCI in the face of chronic elevations in both cortisol and CBG may reflect a stress-induced allostatic shift in HPA system function (see 43).
There is an extensive literature demonstrating relatively rapid changes in CBG expression caused by glucocorticoid manipulations or stress (44–50). In contrast, little is known about the long-term effects of stress on CBG except for a study by Kanter and colleagues (51) who reported elevated CBG levels in Vietnam veterans later diagnosed with PTSD. Those results provided the first evidence that severe stress can precipitate a long-lasting increase in circulating CBG. Nevertheless, the mechanism underlying this effect has not been determined. In the case of the present study, we hypothesize that the persistent hypercortisolemia associated with the move led to enhanced hepatic CBG synthesis and/or a reduction in CBG metabolism and clearance. Further research is needed to test this hypothesis.
In humans, involuntary relocation may occur due to extreme circumstances such as war or natural disasters, but it may also occur under more common conditions such as a change in work site, admission of an elderly person to a long-term care facility, or a move from one such facility to another. Studies of elderly subjects have shown that even voluntary relocation can cause marked changes in behavior and physiology. For example, Hodgson and colleagues (30) found a significant increase in salivary cortisol concentrations 1 week following relocation of elderly nursing home residents to a new facility. Moreover, relocation of healthy elderly subjects from an independent living situation to a group living facility was associated with intrusive ideation, reduced vigor as measured by the Profile of Mood States, and decreased natural killer cell activity (29). Importantly, these effects had resolved within 3 months after the move. Thus, the long-term behavioral and endocrine changes observed in the present study differ to some extent from the transient effects typically reported in the human relocation literature (apart from the sometimes lasting consequences of traumatic relocation caused by war or other disasters).
There are several limitations to the present study. First, it is likely that spontaneously occurring SIB in rhesus monkeys only models certain types of SIB in humans. Elsewhere we have proposed that the syndrome of self-biting and self-wounding in socially reared monkeys is most closely related to Simeon and Favazza’s category of impulsive SIB (26, 52). Consequently, it is possible that stress plays a more important role in that form of SIB than in other forms of the disorder. Second, although involuntary relocation is a stressful event for many people, there are many other life stresses that hypothetically could precipitate an increase in SIB. Nevertheless, the propensity for rhesus monkeys to react strongly to changing environmental and social conditions suggests that the relocation manipulation used in the present study is reasonable for simulating at least some of the kinds of stressors that might impact humans with SIB. Indeed, the apparent prolonged increase in stress that occurred in our relocated monkeys could be seen as a model of major life stressors that are widely believed to trigger psychopathological responses in vulnerable individuals (53). Finally, the available sample sizes may have been a limiting factor in the study. If more animals had been available, we might have attained statistically significant group differences for a few outcome measures in which only trends were noted.
In summary, the present study used a nonhuman primate model of SIB to determine the effects of relocation on behavior and on HPA axis function. As predicted, the move to a new building with different caging and unfamiliar conspecifics in the colony room proved to be a significant stressor for both the SIB monkeys and controls. Most importantly, this stressor led to a long-term increase in self-biting behavior in the SIB group, as well as complex adaptations in the HPA axis involving changes in both circulating cortisol and CBG. Although various behavioral and pharmacological therapies have been developed for the treatment of SIB in humans (11, 54, 55), consistency of results and long-term efficacy remain to be established. Therefore, elucidating the factors that give rise to and maintain SIB is an important step in both preventing and treating this disorder. The present results demonstrate the value of our nonhuman primate model in showing a role for stress as a mediating factor in SIB.
Supplementary Material
Acknowledgments
This study was funded by NCRR grant #RR11122 to Melinda A. Novak and by grant #RR00168 to the New England Primate Research Center (NEPRC). We thank the research technicians Karen Stonemetz and Vanessa Maguire who assisted in the collection of behavioral data, and the NEPRC veterinary staff and technicians who assisted with the collection of biological samples. Finally we would like to thank the anonymous reviewers for their helpful insight and comments regarding this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest with respect to the funding or completion of the present work.
References
- 1.Tate BG, Baroff GS. Aversive control of self-injurious behavior in a psychotic boy. Behav Res Ther. 1966;4:281–287. doi: 10.1016/0005-7967(66)90024-6. [DOI] [PubMed] [Google Scholar]
- 2.Deb S. Self-injurious behavior as part of a genetic syndrome. Br J Psychiatry. 1998;172:388. doi: 10.1192/bjp.172.5.385. [DOI] [PubMed] [Google Scholar]
- 3.Bouvard MP, Leboyer M, Launay JM, Recasens C, Plumet MH, Waller-Perotte D, et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: a double-blind, placebo-controlled study. Psychiatry Res. 1995;58:191–201. doi: 10.1016/0165-1781(95)02601-r. [DOI] [PubMed] [Google Scholar]
- 4.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik C, Mattia JI, Zimmerman M. Clincal correlates of self-mutilation in a sample of general psychiatric patients. J Nerv Ment Dis. 1999;187:296–301. doi: 10.1097/00005053-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Sansone RA, Gaither GA, Songer DA. Self-harm behaviors across the life cycle: a pilot study of inpatients with borderline personality disorder. Compr Psychiatry. 2002;43:215–218. doi: 10.1053/comp.2002.32354. [DOI] [PubMed] [Google Scholar]
- 7.Robertson MM. Self-injurious behavior and Tourette syndrome. In: Chase TN, Friedhoff AJ, Cohen DJ, editors. Tourette Syndrome: Genetics, Neurobiology, and Treatment (Advances in Neurology vol 58) New York: Raven Press; 1992. pp. 105–114. [Google Scholar]
- 8.Herman BH. A possible role of proopiomelanocortin peptides in self-injurious behavior. Prog Neuropsychopharmacol Biol Psychiatry. 1990;(14 Suppl):S109–S139. doi: 10.1016/0278-5846(90)90091-t. [DOI] [PubMed] [Google Scholar]
- 9.Hellings JA, Warnock JK. Self-injurious behavior and serotonin in Prader-Willi syndrome. Psychopharmacol Bull. 1994;30:245–250. [PubMed] [Google Scholar]
- 10.Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609–620. doi: 10.1037/h0080369. [DOI] [PubMed] [Google Scholar]
- 11.Singh NN, Singh YN, Ellis CR. Psychopharmacology of self-injury. In: Luiselli JK, Matson JL, Singh NN, editors. Self-injurious behavior: Analysis, Assessment, and Treatment. New York: Springer-Verlag; 1992. pp. 307–351. [Google Scholar]
- 12.Herpertz S. Self-injurious behaviour. Psychopathological and nosological characteristics in subtypes of self-injurers. Acta Psychiatr Scand. 1995;9:57–68. doi: 10.1111/j.1600-0447.1995.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 13.Huband N, Tantam D. Repeated self-wounding: women’s recollection of pathways to cutting and of the value of different interventions. Psychol Psychother. 2004;77:413–428. doi: 10.1348/1476083042555370. [DOI] [PubMed] [Google Scholar]
- 14.Linehan MM. Dialectical behavior therapy for treatment of borderline personality disorder: implications for the treatment of substance abuse. NIDA Res Monogr. 1993;137:201–216. [PubMed] [Google Scholar]
- 15.Nixon MK, Cloutier PF, Aggarwal S. Affect regulation and addictive aspects of repetitive self-injury in hospitalized adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1333–1341. doi: 10.1097/00004583-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939–1948. doi: 10.1542/peds.2005-2543. [DOI] [PubMed] [Google Scholar]
- 17.Haines J, Williams CL, Brain KL, Wilson GV. The psychophysiology of self-mutilation. J Abnorm Psychol. 1995;104:471–489. doi: 10.1037//0021-843x.104.3.471. [DOI] [PubMed] [Google Scholar]
- 18.Philipsen A, Richter H, Schmal C, Peters J, Rusch N, Bohus M, et al. Clonidine in acute aversive inner tension and self-injurious behavior in female patients with borderline personality disorder. J Clin Psychiatry. 2004;65:1414–1419. doi: 10.4088/jcp.v65n1018. [DOI] [PubMed] [Google Scholar]
- 19.Symons FJ, Sutton KA, Walker C, Bodfish JW. Altered diurnal pattern of salivary substance P in adults with developmental disabilities and chronic self-injury. Am J Ment Retard. 2003;108:13–18. doi: 10.1352/0895-8017(2003)108<0013:ADPOSS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Sachsse U, von der Heyde S, Huether G. Stress regulation and self-mutilation. Am J Psychiatry. 2002;159:672. doi: 10.1176/appi.ajp.159.4.672. [DOI] [PubMed] [Google Scholar]
- 21.Matson JL, Hamilton M, Duncan D, Bamburg J, Smiroldo B, Anderson S, et al. Characteristics of stereotypic movement disorder and self-injurious behavior assessed with the Diagnostic Assessment for the Severely Handicapped (DASH-II) Res Dev Disabil. 1997;18:457–469. doi: 10.1016/s0891-4222(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 22.Brylewski J, Wiggs L. Sleep problems and daytime challenging behaviour in a community-based sample of adults with intellectual disability. J Intellect Disabil Res. 1999;43:504–512. doi: 10.1046/j.1365-2788.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 23.Symons FJ, Davis ML, Thompson T. Self-injurious behavior and sleep disturbance in adults with developmental disabilities. Res.Dev.Disabil. 2000;21:115–123. doi: 10.1016/s0891-4222(00)00028-7. [DOI] [PubMed] [Google Scholar]
- 24.Novak MA. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- 25.Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. The physiology and neurochemistry of self-injurious behavior: A non-human primate model. Front Biosci. 2005;10:1–11. doi: 10.2741/1500. [DOI] [PubMed] [Google Scholar]
- 26.Bayne K, Novak MA. Behavioral Disorders. In: Bennett BT, Abee CR, Hendrickson R, editors. Nonhuman primates in biomedical research diseases. New York: Academic Press; 1998. pp. 485–500. [Google Scholar]
- 27.Schroeder SR, Mulick JA, Rojahn J. The definition, taxonomy, epidemiology, and ecology of self-injurious behavior. J Autism Develop. 1980;10:417–432. doi: 10.1007/BF02414818. [DOI] [PubMed] [Google Scholar]
- 28.Simeon D, Favazza AR. Self-injurious behaviors: Assessment and treatment. In: Simeon D, Hollander E, editors. Self-injurious behvaiors: Phenomenology and assessment. Washington, DC: American Psychiatric Publishing; 2001. pp. 1–28. [Google Scholar]
- 29.Lutgendorf SK, Reimer TT, Harvey JH, Marks G, Hong SY, Hillis SL, et al. Effects of housing relocation on immunocompetence and psychosocial functioning in older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M97–M105. doi: 10.1093/gerona/56.2.m97. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson N, Freedman VA, Granger DA, Erno A. Biobehavioral correlates of relocation in the frail elderly: salivary cortisol, affect, and cognitive function. J Am Geriatr Soc. 2004;52:1856–1862. doi: 10.1111/j.1532-5415.2004.52505.x. [DOI] [PubMed] [Google Scholar]
- 31.Watson SL, McCoy JG, Stavisky RC, Greer TF, Hanbury D. Cortisol responses to relocation stress in Garnett’s Bushbaby (Otolemur garnetti) Contemp Topics. 2005;44:22–24. [PubMed] [Google Scholar]
- 32.Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. 3rd ed. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 33.Powers SI, Gunlicks M, Laurent H, Balaban S, Bent E, Sayer A. Differential effects of subtypes of trauma symptoms on couples' hypothalamus-pituitary-adrenal (HPA) axis reactivity and recovery in response to interpersonal stress. Ann NY Acad Sci. 2006;1071:430–433. doi: 10.1196/annals.1364.036. [DOI] [PubMed] [Google Scholar]
- 34.Maschke C, Hecht K. Stress hormones and sleep disturbances - electrophysiological and hormonal aspects. Noise Health. 2004;6:49–54. [PubMed] [Google Scholar]
- 35.Lutz CK, Tiefenbacher S, Jorgensen MJ, Meyer JS, Novak MA. Techniques for collecting saliva from awake, unrestrained, adult monkeys for cortisol assay. Am J Primatol. 2000;52:93–99. doi: 10.1002/1098-2345(200010)52:2<93::AID-AJP3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Le Roux CW, Sivakumaran S, Alaghband-Zadeh J, Dhillo W, Kong WM, Wheeler MJ. Free cortisol index as a surrogate marker for serum free cortisol. Ann Clin Biochem. 2002;39:406–408. doi: 10.1258/000456302760042182. [DOI] [PubMed] [Google Scholar]
- 38.Le Roux CW, Chapman GA, Kong WM, Dhillo WS, Jones J, Alaghband-Zadeh J. Free cortisol index is better than serum total cortisol in determining hypothalamic-pituitary-adrenal status in patients undergoing surgery. J Clin Endocrinol Metab. 2003;88:2045–2048. doi: 10.1210/jc.2002-021532. [DOI] [PubMed] [Google Scholar]
- 39.Lutz CK, Well A, Novak MA. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–5. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- 40.Curtis B, Warren R, Sackett GP. Sleep behavior in SIB and non-SIB singly housed long-tailed macaques. Am J Primatol. 2004;62 Suppl. 1:93–94. [Google Scholar]
- 41.Oreilly MF, Lancioni G. Response covariation of escape-maintained aberrant behavior correlated with sleep deprivation. Res Dev Disabil. 2000;21:125–136. doi: 10.1016/s0891-4222(00)00029-9. [DOI] [PubMed] [Google Scholar]
- 42.Reite M, Short RA. Nocturnal sleep in separated monkey infants. Arch Gen Psychiatry. 1978;35:1247–1253. doi: 10.1001/archpsyc.1978.01770340097011. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5:55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- 44.Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- 45.Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology. 1995;136:5336–5342. doi: 10.1210/endo.136.12.7588279. [DOI] [PubMed] [Google Scholar]
- 46.Spencer RL, Miller AH, Moday H, McEwen BS, Blanchard RJ, Blanchard DC, et al. Chronic social stress produces reductions in available splenic type II corticosteroid receptor binding and plasma corticosteroid binding globulin levels. Psychoneuroendocrinology. 1996;21:95–109. doi: 10.1016/0306-4530(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 47.Stefanski V. Social stress in laboratory rats: hormonal responses and immune cell distribution. Psychoneuroendocrinology. 2000;25:389–406. doi: 10.1016/s0306-4530(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 48.Tannenbaum B, Rowe W, Sharma S, Diorio J, Steverman A, Walker M, et al. Dynamic variations in plasma corticosteroid-binding globulin and basal HPA activity following acute stress in adult rats. J Neuroendocrinol. 1997;9:163–168. doi: 10.1046/j.1365-2826.1997.t01-1-00550.x. [DOI] [PubMed] [Google Scholar]
- 49.D’elia M, Patenaude J, Hamelin C, Garrel DR, Bernier J. Corticosterone binding globulin regulation and thymus changes after thermal injury in mice. Am J Physiol Endocrinol Metab. 2005;288:E852–E860. doi: 10.1152/ajpendo.00407.2004. [DOI] [PubMed] [Google Scholar]
- 50.Beishuizen A, Thijs LG, Vermes I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive Care Med. 2001;27:1584–1591. doi: 10.1007/s001340101073. [DOI] [PubMed] [Google Scholar]
- 51.Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, et al. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biol Psychiatry. 2001;50:238–245. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- 52.Lutz CK, Meyer JS. Self-injurious behavior: Nonhuman primate models. In: Burbacher TM, Sackett GP, Grant KS, editors. Nonhuman Primate Models in Research on Developmental Disabilities. New York: Elsevier; 2007. in press. [Google Scholar]
- 53.Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother. 2000;54:135–141. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- 54.Sandman CA, Hetrick W, Taylor DV, Marion SD, Touchette P, Barron JL. Long-term effects of naltrexone on self-injurious behavior. Am J Ment Retard. 2000;105:103–117. doi: 10.1352/0895-8017(2000)105<0103:LEONOS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Gerson J, Stanley B. Suicidal and self-injurious behavior in personality disorder: controversies and treatment directions. Curr Psychiatry Rep. 2002;4:30–38. doi: 10.1007/s11920-002-0009-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.