Abstract
White matter is the brain region underlying the gray matter cortex, composed of neuronal fibers coated with electrical insulation called myelin. Previously of interest in demyelinating diseases such as multiple sclerosis, myelin is attracting new interest as an unexpected contributor to a wide range of psychiatric disorders, including depression and schizophrenia. This is stimulating research into myelin involvement in normal cognitive function, learning and IQ. Myelination continues for decades in the human brain; it is modifiable by experience, and it affects information processing by regulating the velocity and synchrony of impulse conduction between distant cortical regions. Cell-culture studies have identified molecular mechanisms regulating myelination by electrical activity, and myelin also limits the critical period for learning through inhibitory proteins that suppress axon sprouting and synaptogenesis.
Introduction
New findings together with experiments spanning 40 years are forcing a pivotal shift in views of white matter in the brain. White matter comprises over half the human brain, a far greater proportion than in other animals [1]. Only vertebrates have myelin (Figure 1), which greatly increases the speed and power of nervous system function [2]. Recently, unanticipated changes in myelin genes and alterations in white matter structure have been observed in a wide range of psychiatric disorders. Together with new data showing that white matter structure is dynamic and myelin can be regulated by impulse activity, these new findings implicate myelin in cognitive function beyond pathology, and illuminate an underappreciated role of myelin in information processing and learning.
Figure 1.
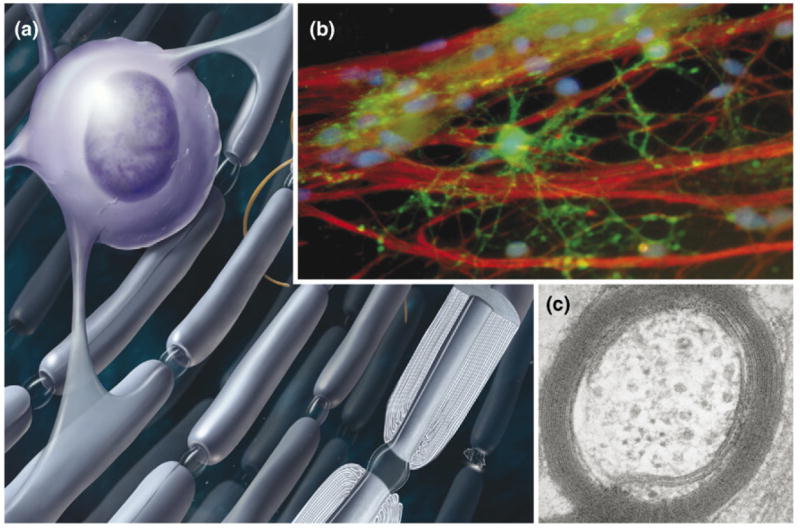
Myelin is the multilayered compacted cell membrane wrapped around axons by glial cells to form electrical insulation that speeds conduction of nerve impulses. (a) In the brain, myelin is wrapped around axons by oligodendrocytes, which have 20 or more cellular processes to insulate multiple axons. (b) An oligodendrocyte (green) is shown at the initial stage of wrapping myelin membrane around several axons (red), in cell cultures equipped with electrodes to stimulate axons for investigations of the role of impulse activity in regulating myelination [87]. (c) An electron micrograph of an axon from the corpus callosum of rat brain is shown in cross-section to reveal the multiple layers of myelin membrane surrounding the axon. Up to 150 layers of myelin are formed on large-diameter axons. Image in (b) courtesy of Varsha Shukla, NICHD and in (c) courtesy of Andrea Nans, NYU School of Medicine. Myelin basic protein (green); neurofilament protein (red). Figure modified from Fields [1].
This article considers evidence that white matter is involved in learning, information-processing, neurological and psychological disorders. It examines historical evidence and information from new techniques indicating that white matter changes with functional experience, and it explores the molecular mechanisms. It presents possible mechanisms for white matter effects on synaptic function and cognition, and it outlines unanswered questions and directions for future research.
White matter in cognition and mental illness
A surprisingly diverse range of psychiatric and nervous system disorders are accompanied by changes in white matter structure or abnormalities in myelin genes (see Box 1). Polymorphisms for several myelin genes have emerged as unexpected risk factors for schizophrenia [3,4], depression [4] and obsessive-compulsive disorder [5]. Post mortem examination of brain tissue from patients suffering schizophrenia [4,6], major depression [7] and bipolar disorder [4] reveals reduced abundance of several mRNA transcripts of myelin genes or genes regulating differentiation and survival of myelin-forming cells (oligodendrocytes).
Box 1. White matter in neurological disease and mental illness.
Many neurological disorders result from damage or disease affecting the myelin sheath on nerve fibers, but recently, white matter defects have also been associated with a wide range of psychiatric and neurological disorders.
Myelin disorders
Conduction failure resulting from myelin damage can cause paralysis, sensory-motor dysfunction, cognitive impairment, mental retardation and death. Myelin can be damaged by autoimmune disease, as in Guillain-Barré syndrome in the peripheral nervous system and multiple sclerosis in the central nervous system. Inherited disorders affecting structural genes in myelin are the cause of such diseases as Charcot-Marie-Tooth disease, Dejerine-Sottas syndrome and Pelizaeus-Marzbacher disease. Myelin is damaged by many metabolic disorders, including Canavan, Menke’s, Krabbe’s and Refsum’s disease, and by infection, trauma, toxins (including alcohol), hormonal imbalance and asphyxia. Oligodendrocytes are especially vulnerable to perinatal asphyxia, resulting in cerebral palsy. Some disorders affecting astrocytes, which provide factors promoting oligodendrocyte development and myelination [87], can impair myelin. For example, Alexander disease is caused by a genetic defect in astrocytes and this results in severe hypomyelination, mental retardation and death at a young age.
Psychiatric disorders
A wide range of psychiatric disorders, including schizophrenia, chronic depression, bipolar disorder, obsessive-compulsive disorder and posttraumatic stress disorder, have recently been associated with white matter defects, as have neurodevelopmental cognitive and emotional disorders including autism, dyslexia and attention-deficit hyperactivity disorder (see table in supplementary material). The evidence for white matter involvement consists of gene expression studies, at least five different types of brain imaging methods and histological analysis of post mortem tissue.
In an analysis of 6000 genes in prefrontal cortex of schizophrenic brains, 89 genes were abnormally regulated; remarkably, of these 35 were genes involved in myelination [3]. From multiple studies, this includes genes encoding myelin MAG, MAL, MBP, PLP, MOG and CNP PMP22; growth factors and receptors ErbB3, NRG1 and BDNF; transcription factors SOX10, Olig 1 and Olig 2; and other genes associated with oligodendroctye development and myelination, including transferrin, QKI and CLDN11. Polymorphisms of several other genes coding proteins in myelin or regulating oligodendrocyte development are indicators of susceptibility to schizophrenia, including MAG, CNP, MOG, NRG1, ERRB4, Olig 2 and Nogo.
Post mortem studies show abnormalities in white matter tracts of schizophrenic brains, including corpus callosum, anterior commissure and fornix. Decreased number of oligodendrocytes is reported in cortex and thalamic nucleus, and myelin abnormalities or apoptotic oligodendrocytes are seen in prefrontal regions.
Several brain imaging methods show volumetric and microstructural white matter differences in patients with schizophrenia, as well as differences in functional connectivity, and biochemical changes in white matter (MRI spectroscopy). Decreased fractional anisotropy or magnetization transfer have been reported in prefrontal white matter, ventromedial prefrontal white matter, frontal white matter, inferior frontal white matter, anterior cingulate, temporal regions, uncinate fasciculus, corpus callosum and internal capsule.
Several other disorders with pronounced cognitive impairments involve alterations in white matter tracts or myelin genes. This includes major depressive and bipolar disorder, Alzheimer’s disease and autism, supporting the concept that myelin abnormalities affect information processing and cognition. Autism, a neurodevelopmental disorder of heterogeneous origins and symptoms, is interesting in this regard, as EEG measurements show increased coherence in the autistic brain. The brain of autistic children is enlarged, and the majority of this enlargement is due to increased white matter volume, particularly in cortico-cortical connections. Hyperconnectivity between particular cortical regions in autism might relate to savant abilities for specific types of knowledge, and early environmental factors are thought to be involved in autism. Similarly, dyslexia is a developmental disorder associated with abnormal temporal processing and EEG coherence in parieto-occipital EEG recording, and microstructural differences in white matter have been reported. (For references to the studies above, see supplementary table online.)
Noninvasive brain imaging is revealing structural differences in appropriate white matter tracts in association with a wide range of neurological and psychiatric illnesses, including dyslexia, ADHD, depression, bipolar disorder, language disorders, stuttering, autism, obsessive-compulsive disorder, posttraumatic stress disorder, cognitive decline in aging, Alzheimer’s disease, Tourette’s disorder, schizophrenia and such idiosyncratic disorders as tone deafness and pathological lying. (See bibliography of white matter abnormalities in psychiatric and neurological disorders in supplementary material.)
An important issue is whether these changes in myelin gene expression or white matter structure are a direct cause of the psychiatric disorder or alternatively, a secondary consequence of abnormal brain function on white matter. Medications or drug abuse can also affect white matter genes or white matter structure in some psychiatric patients. However, the genetic risk factors involving myelin genes, and changes in levels of mRNA transcripts of myelin genes in the absence of changes in neuronal genes in several psychiatric disorders, suggest that white matter is a contributing cause of many disorders affecting mood or cognition. Moreover, as will be described below, experimental manipulation of genes selectively in oligodendrocytes that regulate glial development and myelination can cause behavioral changes mimicking schizophrenia.
Although synaptic dysfunction is the cellular basis for most mental illnesses, disruptions in functional connectivity between distant brain regions can impair information processing in association with a range of neurological processes. Defects in myelin insulation can lead to impaired cognitive function in 40% of multiple sclerosis patients, for example [8]. Cognitive decline in aging also parallels subtle changes in the integrity of white matter [9]. This suggests that impaired cognitive ability, disorganized thinking, mood disorders or hallucinations, accompanying psychiatric illness, might result from slowed or desynchronized impulse conduction between distant cortical regions.
Correlative evidence suggests involvement of myelin in cognition, learning, development of skills and memory. Myelin genes change during REM sleep, for example [10], suggesting myelin remodeling outside the context of disease and in parallel with states of activity in the brain. Changes in gene expression are especially intriguing in the context of sleep-dependent memory consolidation. Myelination of appropriate brain regions coincides with the development of specific cognitive functions [11-13], such as reading [14], development of vocabulary [15] and proficiency in executive decision making [16,17]. Incomplete myelination of the forebrain until the early twenties has been suggested as a neurological basis for weaker decision-making skills in adolescence [17].
Individual differences in normal cognitive development [16,18,19], IQ [20,21], normal variation in reading skill [22-24], working memory [22,25] and musical proficiency [26,27] are correlated with differences in white matter structure in specific brain regions mediating these tasks.
Learning complex skills, such as playing the piano, are accompanied by increased organization of white matter structure in appropriate brain tracts involved in musical performance [26]. Importantly, the level of white matter structure increased proportionately to the number of hours each subject had practiced the instrument, indicating white matter changes in acquiring the skill rather than performance being predetermined by a limitation on white matter development. Myelin plasticity might provide another cellular mechanism of learning complementing the well-studied mechanisms of synaptic plasticity.
Experience changes white matter
Myelination is a developmental process, but it has been known for decades that myelination of the human brain continues into the third decade of life [11], and this can now be tracked by noninvasive brain imaging [17]. However, the significance of this was not fully appreciated. If myelin is simply insulation, why is the process not completed by birth?
Myelination is nearly completed by birth in animals, such as horses [28] or wild mice (Acomys) [29], which are precocial and can walk and feed independently soon after birth, but in humans, myelination is delayed and the process extends at least through the first 20 years of life. The prolonged period of myelination in humans coincides with the same period when the human cerebral cortex undergoes massive remodeling of synaptic connections, which are understood to modify the brain according to experience. This raises the possibility that myelin might participate in optimizing information processing through experience.
New imaging techniques (Figure 2) are reinforcing evidence in the literature for decades indicating that myelin can change according to environmental experience. Myelination in the brain of Alaskan meadow voles is regulated by seasonal changes in day length [30]. Stress during late pregnancy causes hypermyelination in the offspring of laboratory rats [31]. The number of myelin-forming oligodendrocytes increases 27–33% in the visual cortex of rats raised in environments that are enriched by additional play objects and social interaction [32-34].
Figure 2.
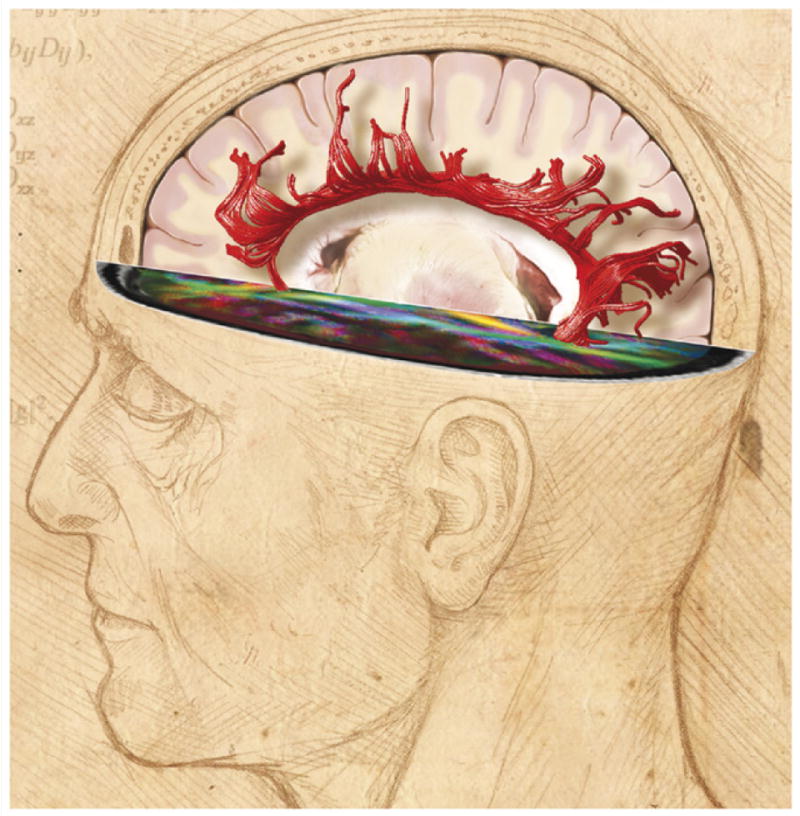
Neuroimaging reveals changes in white matter structure in the human brain. White matter (white) comprises half of the human brain and consists of bundles of myelinated axons connecting neurons in different brain regions. Gray matter (pink) is composed of neuronal cell bodies and dendrites concentrated in the outer layers of the cortex. Microstructural changes in white matter can be revealed by specialized MRI brain imaging techniques such as diffusion tensor imaging (DTI). This method analyzes the fractional anisotropy (FA) of proton diffusion in tissue, which is more restricted in white matter than in gray matter. The anisotropy increases with increased myelination, fiber diameter and axon compaction. The degree of anisotropy is represented on a pseudo color scale as shown in the human brain scan in the horizontal plane of the image above, where the major white matter tracts are revealed against a black background of low fractional anisotropy [107]. These data can be used to calculate the probable anatomy of white matter fiber bundles in living brain, a process called tractography. An example from human brain imaging is shown above as red filamentous bundles radiating out from the corpus callosum. Fiber orientation is calculated from the eigenvectors defining proton diffusion in three dimensions in each voxel. Using algorithms, the principal eigenvalue vector is connected to the next voxel to trace the fiber structure and orientation in white matter tracts [108]. Changes in white matter structure are seen by DTI in association with many neuropsychiatric disorders, cognitive function and during learning. FA image courtesy of Carlo Pierpaoli, NICHD, NIH, and DTI tractography, courtesy of Derek K. Jones, School of Psychiatry, Cardiff University. Illustration by Lydia Kibiuk, Medical Arts, NIH.
The response of myelinating glia to environmental experience is not limited to the visual system or to rats. Enriched environments increase the number of myelinated axons in the corpus callosum connecting the two cerebral hemispheres of rats [35,36], and the corpus callosum increases in size in rhesus monkeys raised in enriched environments in parallel with improved performance in cognitive tests [37].
Environmental effects on white matter extend beyond animal studies. Early experience increases white matter structure in internal capsule and frontal lobes in newborn human infants in parallel with improved performance in behavioral tests [38]. Conversely, children suffering severe childhood neglect have a 17% reduction in corpus callosum area [39]. A specialized MRI technique reveals that the water fraction between the hydrophobic bilayers of myelin sheath steadily increase between ages 20 and 55, suggesting continual remodeling of myelin throughout life [40]. Moreover, the same study reported that water fraction correlates with the number of years of formal education, but not in people with schizophrenia. Abnormalities in white matter structure or biochemistry are detected in schizophrenia with several different types of brain imaging techniques, including MRI image averaging, magnetization transfer imaging, diffusion tensor imaging, measurement of transverse relaxation time, magnetic resonance spectroscopy and functional magnetic imaging.
Myelin in information processing
White matter plasticity in response to environmental experience is puzzling when viewed from the older perspective of myelin. Why should insulation on transmission lines change after neural computation that is carried out in gray matter? The emerging answer is that myelin controls the speed of impulse conduction through axons, and the synchrony of impulse traffic between distant cortical regions is critical for optimal mental performance and learning.
A central concept in synaptic plasticity during learning is the importance of temporal coincidence of firing among multiple synaptic inputs onto a neuron with respect to firing of the postsynaptic neuron (‘neurons that fire together, wire together’). In theory, synaptic inputs that are coincidently active with postsynaptic neuronal firing are functionally important connections that should be retained or strengthened, but inputs that fire noncoincidently should be eliminated. Progress has been made in identifying the molecular mechanisms for synaptic plasticity according to coincident firing, but the conduction time through axons from presynaptic neurons is rarely considered.
If two presynaptic neurons are located at different distances from the postsynaptic neuron, the two synaptic signals will not arrive simultaneously. To arrive simultaneously, the conduction velocity must be delayed through axons from the proximal neuron and/or accelerated in axons from the more distant neuron. If the signals do arrive simultaneously, the voltage changes produced by each input will add together, creating a larger voltage response. A response reaching a critical threshold voltage triggers the recipient neuron to fire impulses and initiate molecular events to reinforce those synapses. Millisecond precision is necessary for the coincident arrival and summation of synaptic signals, because the voltage change produced when a synapse fires is only 2–4 ms in duration (see animation online). Thus, axonal conduction time is a critical variable in information processing and synaptic function. Considering the many variables affecting conduction delays, genetic instruction alone would seem inadequate to specify the optimal conduction velocity in every axon.
Consistent with this, conduction velocity varies widely among different axons (over 100-fold), and many axons are slowly conducting and unmyelinated. The human corpus callosum is unmyelinated at birth, for example, and in adults 30% of the fibers remain unmyelinated. The conduction time between the left and right hemispheres is 30 ms through myelinated callosal fibers and 150–300 ms through unmyelinated fibers. Synaptic integration will be affected profoundly by whether an intercallosal axon becomes myelinated or remains unmyelinated. In certain circuits, the speed of transmission is adjusted to produce synchronous arrival of synaptic inputs from multiple axons that must travel over different distances to reach the same target [41]. Synaptic signals must arrive simultaneously through axons of widely varying path lengths to fire the electric organ of electric fish, for example. This is achieved by higher conduction velocity in axons from motorneurons located farther from the organ, and slower conduction through shorter axons innervating the organ [42]. Similarly, axons from peripheral regions of the retina conduct faster than axons from neurons at the center of the retina to assure simultaneous arrival of impulses in the brain [43]. Experiments in myelin-deficient rats show that myelination is the primary factor producing uniform conduction latency between inferior olive and cerebellar cortex, despite wide variation in axon length [44].
Electrophysiological measurements show that conduction velocity through axons can change. The conduction velocity of 1/3 of callosal axons changes (increases or decreases) over 1 year of chronic recordings in rabbit [45]. As the human body grows, somatosensory and motor conduction delays in CNS pathways remain constant after 2 years of age despite substantial increases in axon length with body growth, reflecting a compensatory increase in conduction velocity in CNS axons [46]. Interestingly, this compensation does not occur in the peripheral nervous system, and conduction delays increase in proportion to the length of a growing limb. Conduction velocity and neural synchrony increase in auditory circuits 1 year after congenitally deaf children receive cochlear implants to restore hearing [47]. Four weeks after dystonia patients were treated with intramuscular botulinum toxin injections to depress motor afferent feedback to the brain, rapid changes in white matter microstructure were evident, indicating experience-dependent white matter plasticity in the adult human brain [48]. Thus, conduction velocity and myelin can change with experience and this correlates with requirements for action potential synchrony in neural circuits to achieve optimal function. This suggests a need to examine the various mechanisms regulating conduction velocity in axons.
Myelin regulation of conduction velocity
Myelin can influence conduction velocity by regulating axon diameter, thickness of the myelin sheath, the number and spacing of nodes of Ranvier, and nodal structure and molecular composition of ion channels in the node and paranodal region.
Larger-caliber fibers conduct impulses at higher speeds because the resistance to electrical current is reduced as the caliber of the fibers increases. However, myelin can also regulate axon diameter. This is obvious at nodes of Ranvier, where the axon often shows marked changes in diameter in the unmyelinated nodal regions (Figure 3). Axon diameter is reduced in myelin-deficient mutants [49], and signaling from myelin proteins, such as myelin-associated glycoprotein (MAG), has been implicated in the signaling cascade controlling neurofilament phosphorylation, which in turn affects axon caliber and axoplasmic transport [50-52].
Figure 3.
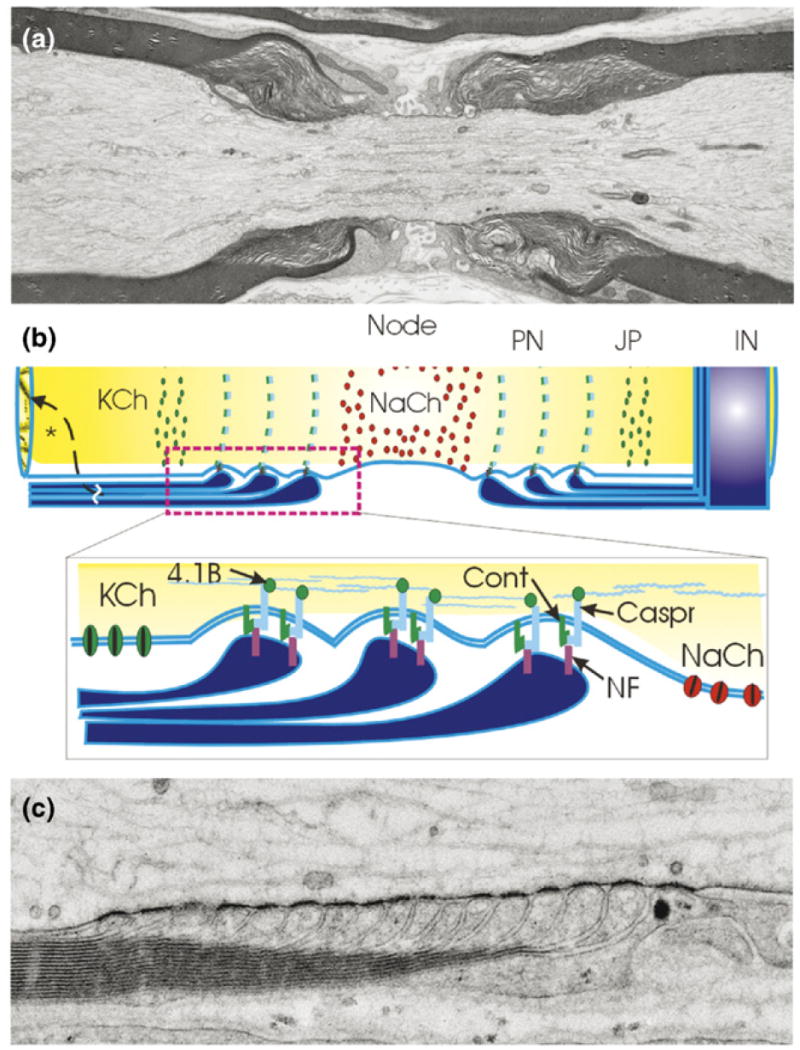
Myelin speeds impulse conduction velocity through nerve fibers by fundamentally changing the way impulses are propagated. Rather than a continuous wave of depolarization, the nerve impulse is generated by sodium and potassium currents at isolated points on the axon called nodes of Ranvier, and each node acts as a repeater. (a) An electron micrograph of a node of Ranvier in long section from spinal dorsal root nerve of rat showing the node of Ranvier flanked by intermodal segments insulated by layers of compact myelin. Each layer of myelin terminates in a series of loops adjacent to the node of Ranvier (the paranodal loops shown in [b] and [c]). (b) Three axonal domains are defined by axon interactions with myelinating glia: the Na+ channel-enriched node of Ranvier, the adjacent paranode (PN) where the loops of myelin adhere to the axon through cell-adhesion molecules linked to the axon cytoskeleton, the juxtaparanodal region (JP) which contains delayed rectifier K+ channels and the internode (IN) sealed by compacted layers of myelin membrane to restrict transmembrane ion currents to the nodal region. These domains are formed and maintained by adhesive interactions and soluble signals from myelinating glia. KCh = K+ channel; NaCh = Na+ channel; Cont = contactin; Caspr = contactin-associated protein; NF = neurofascin 155, 4.1B protein. (c) High-magnification electron micrograph of paranodal loops in a node of Ranvier from mouse spinal root nerve preserved by high-pressure freezing. Note the dense adhesive junctions between each paranodal loop and the axon. (a,c) Courtesy of Gina Sosinsky, Thomas Deerinck, Ying Jones and Mark Ellisman, UCSD, National Center for Microscopy and Imaging Research, San Diego. (b) Modified from Fields and Stevens-Graham [103].
The thickness of the myelin sheath can have a dominant influence on conduction velocity. Optic nerve axons are uniformly small-diameter fibers, but five modes of conduction velocity are observed that are thought to reflect differences in myelination [53]. Myelin sheaths thicker or thinner than the theoretical optimal g-ratio of 0.65 will reduce conduction velocity [54]. (The g-ratio is the axon diameter divided by the total fiber diameter including the myelin sheath.) General agreement between the theoretical optimal g-ratio is widely observed, but this overlooks the large variation in g-ratio around the mean. Considerable differences are seen between fibers [55], and even along the same fiber, and conduction velocity is not always constant along the length of an axon [56,57].
The number of wraps of myelin increases as animals grow and their axons lengthen, showing that myelin production is a perpetual process and that the g-ratio can change [55]. Increased myelin thickness can compensate for increased internodal distance during body growth, which would otherwise reduce conduction velocity [58-60]. Experimentally increasing nodal distance, by lengthening the femur of a rat, increases myelin protein expression 160% [60], suggesting a feedback signaling between changes in internodal distance and myelin thickness, to regulate conduction velocity. Mice lacking the gene encoding periaxin have shorter internodes and slower conduction velocity [61]. Disrupting this gene in Schwann cells prevents the normal remodeling of the Schwann cell myelin sheath during development into adulthood.
The structure of the nodes of Ranvier and the number of nodes along a fiber markedly influence conduction velocity. In the electric fish Sternarchus, abnormally large and electrically passive nodes add capacitance to delay the propagation of the impulse through the axon and modify the waveform of the voltage spike [62].
Little is known of how the dimensions of the node or the morphology of the paranodal loops of myelin flanking the node affect conduction velocity, or how these parameters might change as a result of functional activity. However, swelling of the paranodal region after repetitive activation has been observed [63], suggesting the possibility of activity-dependent structural dynamics at the node. Recently, paired electrophysiological recordings in rat hippocampus revealed that action potentials cause depolarization of the ensheathing oligodendrocytes [64], in part by stimulation of glutamate receptors in oligodendrocytes [65]. Depolarizing the oligodendrocyte with an electrode in turn rapidly increased the conduction velocity through axons myelinated by the oligodendrocyte [64]. Rapid structural changes in the myelin insulation are suspected to account for the rapid changes in conduction velocity. Because one oligodendroglial cell can myelinate multiple axons simultaneously, neural synchrony would be affected by sets of axons under the domain of an individual oligodendrocyte. This would have profound effects on neural synchrony, and might suggest broader transfer effects of white matter plasticity than traditional forms of synaptic plasticity.
Myelinating glia have a major influence on localization of ion channels into concentrated domains at the node during development through contact and diffusible factors [66] (Figure 3). The types of ion channels and the spatial localization of channels in the axon – most prominently, concentration of sodium channels in the node and potassium channels clustered in the paranodal region – affect the excitability, frequency of impulse firing, refractory period and velocity of impulse conduction. In addition to affecting conduction velocity, myelin also decreases the refractory period, thus enabling transmission of spike trains at a higher frequency. The shape and amplitude of the nerve impulse, the firing frequency and the speed of impulse propagation are critical properties in neural coding, information processing and synaptic function.
There is an optimal spacing of nodes of Ranvier, not only for reliable transmission but also for the optimal speed of impulse propagation, because the nodes act as repeaters in propagating the impulse along the axon. Axons in the barn owl auditory system operate as delay lines retarding the speed of action potentials to provide a spatial/temporal map of time delays between sounds reaching the two ears, to enable localization of sounds in space. The conduction velocity is reduced by nodes spaced at unusually close intervals (60 μm in the nucleus laminaris) [67], illustrating again that myelin can regulate conduction velocity up or down. In summary, the primary mechanisms regulating conduction velocity in axons can be influenced by myelinating glia.
Myelin and synaptic plasticity
In addition to controlling conduction velocity, myelin proteins directly control synapse formation by inhibiting axon sprouting, and this limits the critical period for synaptic plasticity and learning. Several proteins in myelin, Nogo-A [68,69], MAG [70] and OMgp [71,72], cause the tips of growing axons to collapse and stop growth toward its target [73,74]. Originally studied in the context of axon regeneration after injury, the normal function of growth-inhibiting proteins in myelin is now appreciated as proteins suppressing axon sprouting after formation of appropriate connections in development. Myelination can have a dominant effect on the critical period for resiliency in recovering from injury. The North American opossum, a marsupial where late fetal development proceeds outside the womb, can recover from spinal cord injury if the cord is severed before 30 days of age when myelination of the animal’s spinal cord is completed [75]. Genetic knockouts and antibody interference with myelin proteins such as Nogo [76] and MAG [70] can improve regeneration of CNS axons and restore functional connections. Axon–glial signaling through these receptors also regulates oligodendrocyte differentiation and myelination, as shown by disrupting LINGO-1, a protein that interacts with the Nogo receptor to inhibit myelination [77]. Intriguingly, mRNA for Nogo is overexpressed in post mortem samples of frontal cerebral cortices from individuals with schizophrenia, and a polymorphism containing a CAA insert in the 3′-untranslated region is more prevalent [78]. In laboratory animals in which the gene for the Nogo-66 receptor is eliminated, the critical period for ocular dominance plasticity, normally 20–33 days postnatal in mice, is extended well into adulthood (45–120 days) [79]. Thus, myelin proteins directly inhibit axon sprouting and synaptogenesis and constrain nervous system plasticity.
Recent findings implicate oligodendrocytes in modulating neurotransmitter function, with possible relevance to schizophrenia. In this study, dopamine transporters and D1 receptors were altered as a consequence of loss of the receptor for NRG1 signaling (erbB) in oligodendrocytes. This reduced the number of branch points on cellular processes of oligodendrocytes, reduced myelin thickness and slowed conduction velocity by 18% in the corpus callosum [80]. These mice exhibit behavioral alterations consistent with schizophrenia and bipolar disorder, but it is not known how dopamine function is disrupted by erbB-dependent interactions between neurons and oligodendrocytes. Thus, by regulating conduction velocity and constraining synaptic plasticity to critical periods, myelin could influence information processing and plasticity of the brain. In contrast to activity-dependent synaptic plasticity, activity-dependent regulation of myelin has received far less attention.
Activity-dependent myelination: cellular and molecular mechanisms
Evidence that impulse activity can affect myelination has been in the literature since the 1960s, from experiments rearing mice in the dark [81] or opening the eye of neonatal rabbits prematurely [82]. Rearing animals in the dark reduces the number of myelinated axons in optic nerve, and premature eye opening increases myelin protein expression. Electrical activity also promotes proliferation of oligodendrocyte progenitor cells in optic nerve [83] and, in cell culture, stimulating firing of neurons electrically [84-87] or modulating it with drugs [88] affects myelination.
Glia are not electrically excitable, but electrophysiological recordings in the 1960s revealed that optic nerve glia could respond to impulse activity in axons evoked by light stimulation of the retina [89]. Glial cell membrane potential was reduced by the elevated concentration of potassium ions liberated by axons firing impulses. In the 1980s, studies using voltage-sensitive and calcium-sensitive fluorescent indicators revealed responses in the myelin sheath of CNS axons firing action potentials [90] and in paranodal loops of myelinating Schwann cells in the peripheral nervous system [91]. Clusters of synaptic vesicles are seen by electron microscopy accumulating at some nodes of Ranvier [92], and functional synapses have been detected between axons and oligodendrocyte progenitor cells [93]. Electrophysiological recordings show rapid depolarization of oligodendrocyte progenitor cells, called NG2 cells, mediated by the neurotransmitters glutamate and GABA. However, the functional significance of these forms of axon–glial communication are unclear, apart from a role in clearing extracellular potassium from the axon environment [89] or involvement in responses to pathological conditions [94,65]. In addition to release from vesicles [95], ATP [84,96] or neurotransmitters [97] can be released from axons through membrane transporters and ion channels.
Three mechanisms by which impulses regulate myelination have been identified from research using cell cultures equipped with electrodes to stimulate action potentials in axons of mouse DRG neurons. First, electrical activity in neurons can alter expression of specific neuronal genes, depending on the frequency and pattern of neural impulse firing [98,99]. Firing axons at the appropriate frequency to lower expression of the cell-adhesion molecule L1-CAM [98], which is necessary for induction of myelin by oligodendrocytes [100] and Schwann cells [85], inhibits myelin formation. Firing axons at a different frequency did not affect the gene, and had no effect on myelination, suggesting that myelination can be regulated by the pattern of impulse activity in developing axons.
Second, diffusible substances released from axons firing bursts of action potentials have been identified that are detected by myelinating glia [84], with subsequent effects on myelination. Studies in cell culture show that at an early stage in development, release of ATP from axons firing impulses is followed by its degradation to adenosine. Adenosine then activates P1 purinergic receptors on oligodendrocyte progenitor cells, stimulating their differentiation and increasing the number of myelinated axons [86].
Third, after the progenitors have differentiated into oligodendrocytes, action potentials increase myelination through a different signaling process involving another glial cell, astrocytes [87]. ATP released by axons firing impulses activates membrane receptors for ATP on astrocytes (P2 receptors), causing release of the cytokine LIF (leukemia inhibitory factor). This in turn stimulates the formation of myelin by oligodendrocytes. These three mechanisms identified in cell culture show a variety of ways impulse activity can affect glia in forming myelin; there are likely to be many more.
Future directions
The signals mediating activity-dependent communication between axons and oligodendrocytes, the developmental time course of activity-dependent effects and the cellular mechanisms that would regulate myelin to optimize conduction velocity are only beginning to be explored.
Is myelin plasticity strictly a change in the number of axons that become myelinated, or a change in the myelin sheath that would regulate impulse conduction speed? Current evidence best supports changes in the number of oligodendrocytes and number of myelinated axons with electrical stimulation or functional activity, but these are easier to detect than more subtle changes in morphology or composition of the myelin sheath that could have substantial effects on conduction velocity.
It remains to be determined whether activity-dependent myelination is restricted to early life, and participates in sculpting the brain for optimal performance in the environment experienced during rearing, or whether it extends throughout life. In the study of white matter structure in pianists, the effects were only detected in regions of brain that had not yet fully myelinated [26]. Similarly, the effects of enriched environments on oligodendrocytes and myelin [36], the effects of modulating visual input on optic nerve myelination [81] and the increased myelin in cultures stimulated to fire action potentials, chemically [88] or with electrical stimulation [86,87], are only observed during narrow developmental windows. Interestingly, new research reveals a class of oligodendrocyte progenitor glia in white matter that receive synaptic inputs and fire sodium-dependent action potentials [101]. The authors speculate that sensitivity to glutamate could enable these cells to myelinate electrically active axons preferentially. Further research is necessary to determine whether activity-dependent changes in white matter structure might continue into adulthood.
Two general molecular mechanisms have been identified for activity-dependent regulation of myelination: changes in cell-adhesion molecules on the axon [85] and purinergic signaling mediated by ATP release from axons [86,87], but other potential channels of communication between axons and myelinating glia, including neurotransmitters, ions, neuromodulators, growth factors, nitric oxide and axon–glial synapses, might inform glia of electrical activity in axons and regulate myelination accordingly.
The critical aspects of impulse activity in regulating myelination are poorly known. Research on gene expression shows that the frequency of impulse activity can be a crucial factor affecting myelination [85], but the duration of stimulation, frequency, phase with respect to firing in other axons and the stimulus burst train parameters are poorly characterized in terms of communication between axon and glia and regulation of myelination.
If conduction velocity is regulated by myelin to optimize performance through synchronization of impulse transmission rather than simply maximizing conduction velocity, how is optimal synchrony evaluated by myelinating glia? An integrator and comparator would be necessary to monitor the degree of synchrony in arrival of inputs converging on the same postsynaptic target, and act on oligodendrocytes over long distances from the synapse. No specific mechanism has been proposed, but some astrocytes and NG2 cells have anatomical features to sense activity at the node of Ranvier and synapses [102], and astrocytes regulate myelination by releasing LIF [87].
Physical therapy, functional activity and electrical stimulation can benefit recovery from nervous system injury and demyelinating disease. The extent to which activity-dependent myelination might contribute to recovery from spinal cord and brain injury is unknown. Interventions to promote remyelination might provide another avenue for improving recovery after axon damage.
Conclusions
Research from new techniques and recent insights into neuron–glial interactions [103] are providing a new perspective on studies of myelin plasticity that have been in the literature for decades. Myelin is not simply a developmental process; it continues for decades in humans; it is modifiable, and it is an important contributor to psychiatric disorders and other diseases affecting cognition.
White matter changes are associated with learning in people, but the extent to which these changes detected by diffusion tensor imaging (DTI) reflect myelin or other structural changes in white matter that influence anisotropy of water diffusion is not certain. Experiments on animals with demyelinating disorders show that myelination affects DTI, but changes in axon diameter or axon packing density can also affect the fractional anisotropy of water diffusion, which is the basis for DTI. Changes in myelin can develop secondary to changes in axon diameter, but myelinating glia can also regulate axon diameter [51]. Moreover, survival of axons and neurons is dependent on trophic support and signaling from myelinating glia, as is seen in multiple sclerosis where chronically demyelinated axons are lost. Thus, myelinating glia can influence axon connectivity and neuronal survival under pathological conditions, raising the possibility that survival of individual axons could be influenced by myelinating glia under normal conditions. Clearly, the close interrelationship between myelinating glia and neurons enables them to act together to alter white matter structure and alter functional connectivity critical for information processing. This new realm of nervous system plasticity operates outside the context of changes in neurotransmitter inputs to neurons at the synapse, by regulating the output of neurons. Spike timing is critical in determining whether a synapse is strengthened or weakened [104], and recent studies reveal that information is coded in spike latency [105], not only in action potential firing rates.
Despite the unresolved issues from the human imaging studies of white matter plasticity in learning and psychiatric disorders, animal and cell-culture studies show that myelin can be affected by functional activity in axons, and the molecular/cellular mechanisms are being identified. Experiments on animals raised in enriched environments support the possibility that myelin can contribute to cognitive development and learning. Considering the possible involvement of myelin in cognitive function and psychiatric illness expands the field of learning research to include the acquisition of complex skills and abilities that require prolonged practice, involving integration of information flow between multiple cortical regions. This broader perspective in plasticity research extends the scope of study well beyond reflexes and instantaneous changes in synaptic strength – indeed beyond neurons [106].
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tins. 2008.04.001.
Acknowledgments
Supported by funds for intramural research, NIH, NICHD.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Fields RD. White matter matters. Sci Am. 2008;298:42–49. [PubMed] [Google Scholar]
- 2.Bullock TH, et al. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- 3.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelinatin-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tkachev D, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 5.Stewart SE, et al. A genetic family-based association study of OLIG2 in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:209–214. doi: 10.1001/archpsyc.64.2.209. [DOI] [PubMed] [Google Scholar]
- 6.Georgieva L, et al. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2006;103:12469–12474. doi: 10.1073/pnas.0603029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aston C, et al. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- 8.Kujala P, et al. The progress of cognitive decline in multiple sclerosis. A controlled 3-year follow-up. Brain. 1997;120:289–297. doi: 10.1093/brain/120.2.289. [DOI] [PubMed] [Google Scholar]
- 9.Gootjes L, et al. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dement Geriatr Cogn Disord. 2004;18:180–188. doi: 10.1159/000079199. [DOI] [PubMed] [Google Scholar]
- 10.Cirelli C, et al. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 11.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Blackwell; 1967. pp. 3–70. [Google Scholar]
- 12.Mabbott DJ, et al. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Nagy Z, et al. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 14.Kraft RH, et al. Hemispheric asymmetries during six- to eight-year-olds performance of Piagetian conservation and reading tasks. Neuropsychologia. 1980;18:637–643. doi: 10.1016/0028-3932(80)90103-7. [DOI] [PubMed] [Google Scholar]
- 15.Pujol J, et al. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- 16.Liston C, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 17.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 18.Casey BJ, et al. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 19.Walhovd KB, Fjell AM. White matter volume predicts reaction time instability. Neuropsychologia. 2007;45:2277–2284. doi: 10.1016/j.neuropsychologia.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Schmithorst VJ, et al. Cognitive functions correlate with white matter architecture in normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller EM. Intelligence and brain myelination: a hypothesis. Pers Indiv Differ. 1994;17:803–832. [Google Scholar]
- 22.Gold BT, et al. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Klingberg T, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 25.Nestor PG, et al. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res. 2007;155:21–28. doi: 10.1016/j.pscychresns.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 27.Hyde KL, et al. Morphometry of the amusic brain: a two-site study. Brain. 2006;129:2562–2570. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
- 28.Szalay F. Development of the equine brain motor system. Neurobiology (Bp) 2001;9:107–135. doi: 10.1556/neurob.9.2001.2.4. [DOI] [PubMed] [Google Scholar]
- 29.Tessitore C, Brunjes PC. A comparative study of myelination in precocial and altricial murid rodents. Brain Res. 1988;471:139–147. doi: 10.1016/0165-3806(88)90159-9. [DOI] [PubMed] [Google Scholar]
- 30.Spears N, et al. Long day lengths enhance myelination of midbrain and hindbrain regions of developing meadow voles. Brain Res Dev Brain Res. 1990;55:103–108. doi: 10.1016/0165-3806(90)90110-k. [DOI] [PubMed] [Google Scholar]
- 31.Wiggins RC, Gottesfeld Z. Restraint stress during late pregnancy in rats elicits early hypermyelination in the offspring. Metab Brain Dis. 1986;1:197–203. doi: 10.1007/BF01001781. [DOI] [PubMed] [Google Scholar]
- 32.Bennett EL, et al. Chemical and anatomical plasticity brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 33.Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- 34.Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- 35.Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- 36.Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–364. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez I, et al. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Als H, et al. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 39.Teicher MH, et al. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Flynn SW. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- 41.Sugihara I, et al. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–271. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett MV. Comparative physiology: electric organs. Annu Rev Physiol. 1970;32:471–528. doi: 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- 43.Stanford LR. Conduction velocity variations minimize conduction time differences among retinal ganglion cell axons. Science. 1987;238:358–360. doi: 10.1126/science.3659918. [DOI] [PubMed] [Google Scholar]
- 44.Lang EJ, Rosenbluth J. Role of myelination in the development of a uniform olivocerebellar conduction time. J Neurophysiol. 2003;89:2259–2270. doi: 10.1152/jn.00922.2002. [DOI] [PubMed] [Google Scholar]
- 45.Swadlow HA. Physiological properties of individual cerebral axons studied in vivo for as long as one year. J Neurophysiol. 1985;54:1346–1362. doi: 10.1152/jn.1985.54.5.1346. [DOI] [PubMed] [Google Scholar]
- 46.Eyre JA, et al. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;343:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon KA, et al. Activity-dependent developmental plasticity of the auditory brain stem in children who use cochlear implants. Ear Hear. 2003;24:485–500. doi: 10.1097/01.AUD.0000100203.65990.D4. [DOI] [PubMed] [Google Scholar]
- 48.Blood AJ, et al. White matter abnormalities in dystonia normalize after botulinum toxin treatment. Neuroreport. 2006;17:1251–1255. doi: 10.1097/01.wnr.0000230500.03330.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole JS, et al. Modulation of axon diameter and neurofilaments by hypomyelinating Schwann cells in transgenic mice. J Neurosci. 1994;14:6956–6966. doi: 10.1523/JNEUROSCI.14-11-06956.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lunn MPT, et al. Anti-myelin-associated glycoprotein antibodies alter neurofilament spacing. Brain. 2002;125:904–911. doi: 10.1093/brain/awf072. [DOI] [PubMed] [Google Scholar]
- 51.Yin X, et al. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci. 1998;18:1953–1962. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh S-T, et al. Regional modulation of neurofilament organization by myelination in normal axons. J Neurosci. 1994;14:6392–6401. doi: 10.1523/JNEUROSCI.14-11-06392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman B. Myelin sheath thickness and conduction latency groups in the cat optic nerve. J Comp Neurol. 1978;181:183–196. doi: 10.1002/cne.901810110. [DOI] [PubMed] [Google Scholar]
- 54.Rushton WA. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951;115:101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berthold CH, et al. Axon diameter and myelin sheath thickness in nerve fibres of the ventral spinal root of the seventh lumbar nerve of the adult and developing cat. J Anat. 1983;136:483–509. [PMC free article] [PubMed] [Google Scholar]
- 56.Baker GE, Stryker MP. Retinofugal fibres change conduction velocity and diameter between the optic nerve and tract in ferrets. Nature. 1990;344:342–345. doi: 10.1038/344342a0. [DOI] [PubMed] [Google Scholar]
- 57.Traub RJ, Mendell LM. The spinal projection of individual identified A-δ- and C-fibres. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- 58.Schröder JM, et al. Changes of the ratio between myelin thickness and axon diameter in the human developing sural nerve. Acta Neuropathol. 1978;43:169–178. doi: 10.1007/BF00685012. [DOI] [PubMed] [Google Scholar]
- 59.Friede RL, et al. Changes in myelin sheath thickness and internode geometry in rabbit phrenic nerve during growth. J Anat. 1985;143:103–113. [PMC free article] [PubMed] [Google Scholar]
- 60.Hara Y, et al. P0 mRNA expression increases during gradual nerve elongation in adult rats. Exp Neurol. 2003;184:428–435. doi: 10.1016/s0014-4886(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 61.Court FA, et al. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431:191–195. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- 62.Waxman SG, et al. Morphological correlates of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Sternarchus. J Cell Biol. 1972;53:210–224. doi: 10.1083/jcb.53.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wurtz CC, Ellisman MH. Alterations in the ultrastructure of peripheral nodes of Ranvier associated with repetitive action potential propagation. J Neurosci. 1986;6:3133–3145. doi: 10.1523/JNEUROSCI.06-11-03133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamazaki Y, et al. Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol. 2008 doi: 10.1017/S1740925X08000070. www.journals.cambridge.org/ngb. [DOI] [PubMed]
- 65.Káradóttir R, et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dupree JL, et al. Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol. 2004;1:1–14. doi: 10.1017/S1740925X04000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen MS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 69.GrandPré T, et al. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 70.McKerracher L, et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 71.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 72.Huang JK, et al. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- 73.Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawe G, et al. Ensheathing the node of Ranvier? Neuron Glia Biol. 2006;2:149–150. [Google Scholar]
- 75.Ghooray GT, Martin GF. The development of myelin in the spinal cord of the North American opossum and its possible role in loss of rubrospinal plasticity. Brain Res Dev Brain Res. 1993;72:67–74. doi: 10.1016/0165-3806(93)90160-c. [DOI] [PubMed] [Google Scholar]
- 76.Bregman BS, et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 77.Mi S, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 78.Novak G, et al. Schizophrenia and Nogo; elevated mRNA in cortex, and high prevalence of a homozygous CAA insert. Brain Res Mol Brain Res. 2002;107:183–189. doi: 10.1016/s0169-328x(02)00492-8. [DOI] [PubMed] [Google Scholar]
- 79.McGee AW, et al. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy K, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function – a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–256. [PubMed] [Google Scholar]
- 82.Tauber H, et al. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–238. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- 83.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 84.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 85.Stevens B, et al. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevens B, et al. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishibashi T, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Demerens C, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orkand RK, et al. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 90.Lev-Ram V, Grinvald A. Ca2+- and K+-dependent communication between central nervous system myelinated axons and oligodendrocytes revealed by voltage-sensitive dyes. Proc Natl Acad Sci U S A. 1986;83:6651–6655. doi: 10.1073/pnas.83.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lev-Ram V, Ellisman MH. Axonal activation-induced calcium transients in myelinating Schwann cells, sources, and mechanisms. J Neurosci. 1995;15:2628–2637. doi: 10.1523/JNEUROSCI.15-04-02628.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waxman SG. Regional differentiation of the axon: a review with special reference to the concept of the multiplex neuron. Brain Res. 1972;47:269–288. doi: 10.1016/0006-8993(72)90639-7. [DOI] [PubMed] [Google Scholar]
- 93.Bergles DE, et al. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 94.Micu I, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 95.Kukley M, et al. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 96.Fields RD, Burnstock G. Purinergic signaling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Itoh K, et al. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- 99.Itoh K, et al. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 100.Barbin G, et al. Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol. 2004;1:65–72. doi: 10.1017/S1740925X04000092. [DOI] [PubMed] [Google Scholar]
- 101.Káradóttir R, et al. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butt AM, et al. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fields RD, Stevens-Graham B. New views of neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 105.Gollisch T, Meister M. Rapid neural coding in the retina with relative spike latencies. Science. 2008;319:1108–1111. doi: 10.1126/science.1149639. [DOI] [PubMed] [Google Scholar]
- 106.Bullock TH, et al. The neuron doctrine, redux. Science. 2005;310:791–793. doi: 10.1126/science.1114394. [DOI] [PubMed] [Google Scholar]
- 107.Basser PJ, et al. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones DK, et al. Isotropic resolution diffusion tensor imaging with whole brain acquisition in clinically acceptable time. Hum Brain Mapp. 2002;15:216–230. doi: 10.1002/hbm.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tins. 2008.04.001.


