Abstract
The TGIF homoeodomain protein functions as an important negative regulator in the TGF-β signalling pathway. The inhibitory function of TGIF is executed in part through its ability to sequester the tumour suppressor cytoplasmic promyelocytic leukaemia (cPML) in the nucleus, thereby preventing the phosphorylation of Smad2 by the activated TGF-β type I receptor. Here, we report on the identification of PCTA (PML competitor for TGIF association), a TGIF antagonist that promotes TGF-β-induced transcriptional and cytostatic responses. We provide evidence that PCTA functions in TGF-β signalling by relieving the suppression of Smad2 phosphorylation by TGIF. Furthermore, we demonstrate that PCTA selectively competes with cPML for TGIF association, resulting in the accumulation of cPML in the cytoplasm, where it associates with SARA and coordinates the access of Smad2 for phosphorylation by the activated TGF-β type I receptor. Thus, our findings on the mode of action of PCTA provide new and important insights into the molecular mechanism underlying the antagonistic interplay between TGIF and cPML in the TGF-β signalling network.
Keywords: nuclear retention of cPML, PCTA, Smad2 phosphorylation, TGF-β signalling, TGIF
Introduction
Transforming growth factor beta (TGF-β) is a member of a large family of cytokines that regulates a broad range of biological processes, including cell growth, apoptosis, and differentiation (Heldin et al, 1997; Whitman, 1998; Derynck and Zhang, 2003). TGF-β signals through a heteromeric complex of two cell surface transmembrane serine–threonine kinases, known as type I (TβRI) and type II (TβRII) receptors. TGF-β binding to TβRII induces recruitment and phosphorylation of TβRI, which in turn transduces signals to the downstream intracellular substrates, Smad2 and Smad3 (Massague et al, 2005; Schmierer and Hill, 2007). Receptor-mediated phosphorylation of Smad2 and Smad3 induces their association with Smad4, and these complexes enter the nucleus, where they regulate transcription of specific genes (Derynck and Zhang, 2003; Massague et al, 2005; Schmierer and Hill, 2007).
The recognition of Smad2 by the receptor is facilitated by a variety of auxiliary proteins, the most characterized of which are SARA (Smad anchor for receptor activation) and cPML (cytoplasmic promyelocytic leukaemia) (Tsukazaki et al, 1998; Lin et al, 2004). SARA contains two discrete binding domains for Smad2 and the receptor and a FYVE domain that targets the molecule to the membrane of early endosomes (Tsukazaki et al, 1998). The enrichment of the SARA–Smad2 complex in early endosomes to which the activated receptor complexes are internalized through clathrin-coated pits suggests that SARA may function to recruit Smad2 to TGF-β receptors by controlling the subcellular localization of Smad2 (Di Guglielmo et al, 2003). The role of cPML in Smad2 phosphorylation appears to be mediated through its ability to exert an effect as a bridging factor between SARA and Smad2 and to bring the SARA–Smad2 complex within the proximity of the receptor at the cell surface. Once signalling has commenced, cPML promotes the movement of the receptor complex, SARA and Smad2 to early endosomes in which the phosphorylation of Smad2 takes place. Phosphorylation of Smad2 induces dissociation from SARA and cPML with concomitant formation of the Smad2–Smad4 complex and movement to the nucleus (Tsukazaki et al, 1998; Lin et al, 2004).
TGF-β signalling can be limited by TGIF (TG-interacting factor), an exclusive nuclear protein originally thought to repress Smad2 transcriptional activity by recruiting a corepressor complex containing histone deacetylases (HDAC) (Wotton et al, 1999, 2001). However, this view came under challenge in the past few years due to, among others, the lack of effect on TGF-β signalling by a holoprosencephaly-causing mutant of TGIF that retains its ability to associate with both Smad2 and the HDAC corepressor complex (Gripp et al, 2000; Melhuish and Wotton, 2000). Recently, two additional mechanisms by which TGIF restricts TGF-β signalling were reported, one depends on ubiquitin-dependent degradation of Smad2 through recruitment of the E3 ubiquitin ligase Tiul1–WWP1 (TGIF-interacting ubiquitin ligase 1–WW-containing protein 1), whereas the other relies on suppression of Smad2 phosphorylation (Seo et al, 2004, 2006). TGIF does not seem to be targeted for degradation by Tiul1–WWP1 but rather functions to form with Smad2 and Tiul1–WWP1 a stable complex from which Tiul1–WWP1 then induces ubiquitination of Smad2 with subsequent degradation through the proteasome pathway (Seo et al, 2004). The molecular mechanism that accounts for the inhibition of Smad2 phosphorylation is mediated through the ability of TGIF to sequester cPML in the nucleus, resulting in the disruption of the SARA–cPML complex required for the interaction and phosphorylation of Smad2 by the activated TβRI (Seo et al, 2006). Although these findings suggest that TGIF may exert an effect through multiple biochemical circuits that work together in a cooperative setting to achieve full repression of TGF-β signalling, they do raise fundamental questions about the regulatory mechanisms that govern the coordination of each branch of this molecular network to enable tight regulation of the inhibitory activity of TGIF towards TGF-β signal transduction.
In this study, we describe the identification of PCTA, a novel TGIF binding protein that reverses TGIF inhibition of Smad2 phosphorylation as well as TGF-β-mediated transcriptional and antiproliferative responses. We investigated the basis of this negative crosstalk and found that PCTA can release cPML from TGIF nuclear retention by selectively competing with cPML for binding to TGIF. Thus, PCTA defines a new component of the TGF-β signalling pathway that functions to facilitate Smad2 phosphorylation through controlling the accumulation of cPML into the cytoplasm, and consequently, the assembly of Smad2–receptor complex.
Results
PCTA interacts with TGIF
To discover novel effectors of the TGF-β signalling pathway, we performed a two-hybrid screen with full-length TGIF as bait. By screening 10 × 106 clones of a human placental cDNA library, we isolated several partial PCTA clones displaying a perfect match to a previously identified human gene called IRF2BP1 (Childs and Goodbourn, 2003). At present, the physiological roles of the IRF2BP1 protein have not been elucidated and the only available observation that attributed a transcriptional repressor function for IRF2BP1 was derived from one experiment using an artificial GAL4-based assay (Childs and Goodbourn, 2003). For simplicity, we refer to IRF2BP1 herein as PCTA based on our subsequent analyses.
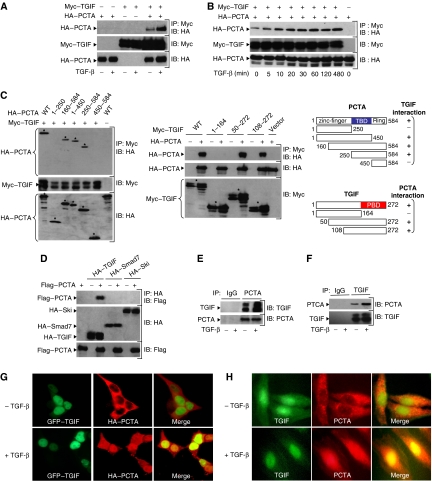
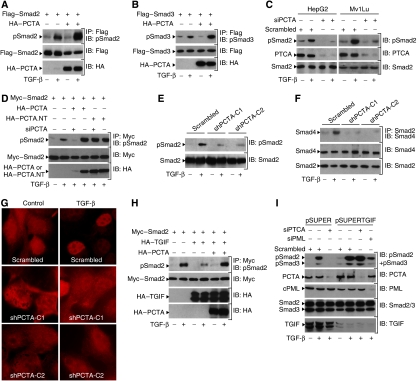
To determine whether PCTA binds to TGIF in mammalian cells, we transfected 293 cells with expression vectors encoding HA-PCTA and Myc-TGIF. As shown in Figure 1A, PCTA was detectable in Myc immunoprecipitates, and this interaction was increased by TGF-β. In a kinetic experiment, we found that the PCTA–TGIF interaction was maximal after 30–60 min of TGF-β treatment (Figure 1B). To analyse this interaction in more detail, we made use of various PCTA and TGIF deletion mutants. We found that the TGIF-binding domain (TBD) mapped to the middle region of PCTA and the PCTA-binding domain (PBD) mapped to the C terminus domain of TGIF (Figure 1C). The interaction of PCTA with TGIF is specific, because we were unable to detect an interaction between PCTA and c-Ski or Smad7 (Figure 1D), two important inhibitors of TGF-β signalling (Itoh and ten Dijke, 2007). Furthermore, the interaction of PCTA with TGIF occurred independently of activation of Smad signalling because expression of a mutant of TβRI, TβRImL45.act, which retains a constitutively active kinase domain but lost its ability to phosphorylate Smad2 and Smad3 (Yu et al, 2002; Seo et al, 2006), induced the association of PCTA with TGIF as potently as the original constitutively active TβRI.act (Supplementary Figure S1A).
Figure 1.
PCTA interacts with TGIF. (A, B) 293 cells were transfected with Myc-TGIF in the absence or presence of HA-PCTA and treated with or without TGF-β for 1 h (A) or various times (B). Cell lysates were subjected to anti-Myc immunoprecipitation (IP) followed by immunoblotting (IB) with anti-HA. In these and all the following experiments, the expression of proteins under investigation was determined by direct immunoblotting. (C) 293 cells were transfected with Myc-TGIF and various HA-PCTA mutants (left) or HA-PCTA and various Myc-TGIF mutants (middle). Cell lysates were immunoprecipitated with anti-Myc and blotted with anti-HA. The TGIF-binding domain of PCTA (TBD, blue) and the PCTA-binding domain of TGIF (PBD, red) are indicated (right). (D) 293 cells were transfected with the indicated combinations of HA-TGIF, HA-Smad7, HA-Ski, and Flag-PCTA. Cell lysates were subjected to anti-HA immunoprecipitation followed by immunoblotting with anti-Flag. (E, F) 293 cells were treated with or without TGF-β for 1 h and cell lysates were immunoprecipitated with either rabbit IgG or anti-PCTA (E), and mouse IgG or anti-TGIF (F). TGIF-bound to PCTA (E) and PCTA-bound to TGIF (F) were detected by immunoblotting with anti-TGIF and anti-PCTA, respectively. (G) 293 cells were transfected with HA-PCTA and GFP–TGIF and treated with or without TGF-β for 1 h. Then, cells were immunostained with anti-HA and the localization of PCTA (red) or TGIF (green) was analysed by a fluorescence microscope. (H) Mv1Lu cells were treated with or without TGF-β for 1 h, immunostained with anti-PCTA and anti-TGIF, and the localization of PCTA (red) or TGIF (green) was visualized by a fluorescence microscope.
To examine whether PCTA interacts with TGIF under physiological conditions, we raised a rabbit polyclonal anti-PCTA antibody. Use of this antibody for immunoblotting analysis identified a specific band of approximately 65 kDa, which is the expected size for PCTA (Supplementary Figure S1B). In coimmunoprecipitation experiments using 293 cell extracts, endogenous PCTA and TGIF could coprecipitate each other and this association was increased by TGF-β (Figure 1E and F). A similar TGF-β-dependent association of endogenous PCTA and TGIF was observed in multiple cell lines, including Mv1Lu, HepG2, HaCat, CaCO2, and mouse embryonic fibroblasts (MEFs) (Figures 4B and 7C–H).
Figure 4.
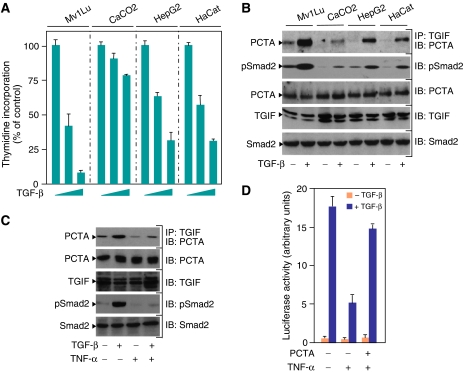
Physiological relevance of the PCTA function in TGF-β signalling. (A) Mv1Lu, CaCO2, HepG2, and HaCat cells were treated with increasing amounts of TGF-β for 48 h and the rate of cell proliferation was determined by the thymidine incorporation method. (B) Mv1Lu, CaCO2, HepG2, and HaCat cells were treated with or without TGF-β for 1 h. PCTA bound to TGIF was detected by blotting anti-TGIF immunoprecipitates with anti-PCTA. To detect Smad2 phosphorylation, cell lysates were blotted with anti-pSmad2. (C) MEFs were treated with TNF-α for 8 h prior to treatment with TGF-β for 1 h. Cell extracts were immunoprecipitated with anti-TGIF followed by blotting with anti-PCTA. To detect Smad2 phosphorylation, cell lysates were blotted with anti-pSmad2. (D) MEFs were transfected with ARE3-Lux together with FAST1 in either the presence or absence of PCTA. After 24 h, cells were treated with the indicated combinations of TGF-β and TNF-α for 16 h and were analysed for luciferase activity.
Figure 7.
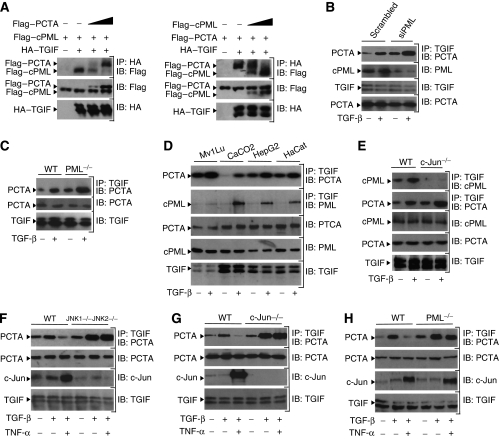
PCTA and cPML competitively share TGIF. (A) 293 cells were transfected with HA-TGIF, Flag-cPML, and increasing amounts of Flag-PCTA (left) or HA-TGIF, Flag-PCTA, and increasing amounts of Flag-cPML (right). Cell lysates were immunoprecipitated with anti-HA and the simultaneous associations of TGIF with PCTA and PML were analysed by immunoblotting with anti-Flag. (B) 293 cells were transfected with Scrambled or PML siRNA and treated with or without TGF-β for 1 h. Then, the association of PCTA with TGIF was analysed by blotting anti-TGIF immunoprecipitates with anti-PCTA. (C) Wild-type or PML−/− MEFs were treated with or without TGF-β for 1 h. To detect the PCTA–TGIF complex, cell lysates were immunoprecipitated with anti-TGIF before being analysed by immunoblotting with anti-PCTA. (D) Mv1Lu, CaCO2, HepG2, and HaCat cells were treated with or without TGF-β for 1 h. PCTA bound and cPML bound to TGIF were detected by blotting anti-TGIF immunoprecipitates with anti-PCTA or anti-PML, respectively. (E) Wild-type or c-Jun−/− MEFs were treated with or without TGF-β for 1 h and the association of TGIF with cPML or PCTA was analysed by blotting anti-TGIF immunoprecipitates with anti-PML or anti-PCTA, respectively. (F–H) JNK1−/−JNK2−/− (F), c-Jun−/− (G) or PML−/− (H) MEFs and their wild-type counterparts were treated with TNF-α for 8 h prior to treatment with TGF-β for 1 h. Cell lysates were immunoprecipitated with anti-TGIF before being analysed by immunoblotting with anti-PCTA. The expression of c-Jun was analysed by immunoblotting with anti-c-Jun.
Next, we performed immunofluorescence experiments to investigate the possibility that TGF-β may induce the association of PCTA with TGIF by influencing their subcellular distribution. In transfected cells, HA-PCTA localized predominantly in the cytoplasm and TGF-β treatment caused a substantial fraction of HA-PCTA to accumulate in the nucleus and to colocalize with TGIF (Figure 1G). A similar TGF-β-dependent colocalization of endogenous PCTA and TGIF was observed in the nucleus of Mv1Lu cells (Figure 1H), suggesting that TGF-β might induce the assembly of PCTA–TGIF complex by promoting the nuclear translocation of PCTA.
PCTA contributes to TGF- signalling
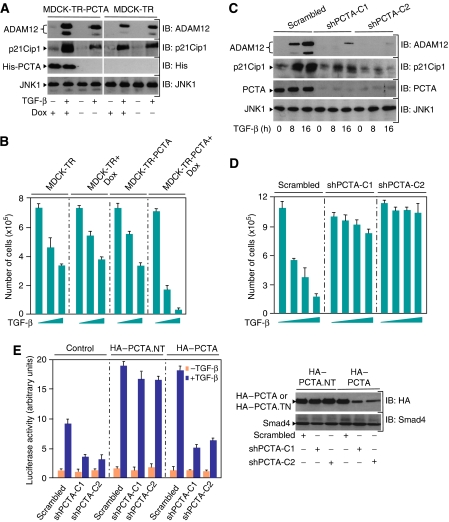
To investigate the functional significance of the interaction between PCTA and TGIF, we examined whether expression of PCTA could influence TGF-β signalling. We first tested the effect of PCTA on the TGF-β responsive reporter ARE3-Lux. As shown in Supplementary Figure S2A, coexpression of PCTA resulted in a significant increase of the sensitivity of cells to TGF-β. To provide further evidence that expression of PCTA enhances TGF-β signalling, we generated a MDCK cell line (MDCK-TR-PCTA) that expresses a doxycylin-inducible PCTA. Consistent with the gene reporter data, induction of PCTA enhanced the ability of TGF-β to induce expression of endogenous ADAM12 and p21Cip1 (Figure 2A), two TGF-β–Smad target genes (Siegel and Massague, 2003; Atfi et al, 2007). More importantly, induction of PCTA also increased the sensitivity of cells to TGF-β-induced growth inhibition (Figure 2B).
Figure 2.
PCTA contributes to TGF-β signalling. MDCK-TR or MDCK-TR-PCTA cells were treated with doxycyclin (Dox) for 24 h and then with or without 2 ng/ml TGF-β for 16 h (A) or with increasing amounts of TGF-β for 72 h (B). (A) Cell lysates were analysed by immunoblotting using antibodies against PCTA, p21Cip1, ADAM12 and JNK1 as a loading control. (B) Cells were counted and the results were expressed as mean±s.d. of triplicate from a representative experiment performed at least three times. (C) MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells were treated with or without 2 ng/ml TGF-β for 8 or 16 h (C) or with increasing amounts of TGF-β for 72 h (D). (C) Cell lysates were analysed by immunoblotting using antibodies against PCTA, p21Cip1, ADAM12, and JNK1. (D) Cells were counted and the results were expressed as mean±s.d. of triplicate from a representative experiment performed at least three times. (E) MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells were transfected with ARE3-Lux together with FAST1 and either HA-PCTA or the non-targetable form HA-PCTA.NT. Cells were treated with or without TGF-β for 16 h and analysed for luciferase activity (left). Cell extracts were analysed by immunoblotting with anti-HA or anti-Smad4 as a loading control (right).
To ascertain the physiological relevance of PCTA in TGF-β signalling, we knocked down the expression of endogenous PCTA by RNA interference. We observed that depletion of endogenous PCTA by a specific siRNA decreased TGF-β-mediated activation of ARE3-Lux (Supplementary Figure S2B). To confirm this result, we established two MDCK cell clones (MDCK-shPCTA) in which the expression of endogenous PCTA was reduced by stable expression of a short-hairpin RNA (shRNA). As expected, PCTA depletion prevented TGF-β-induced expression of endogenous ADAM12 or p21Cip1 (Figure 2C). Depletion of PCTA also blunted the growth inhibitory action of TGF-β (Figure 2D), further supporting the notion that PCTA is crucial for TGF-β responses. As a control, expression of an shRNA-resistant mutant of PCTA (PCTA.NT) in MDCK-shPCTA cells restored TGF-β-induced transcription (Figure 2E).
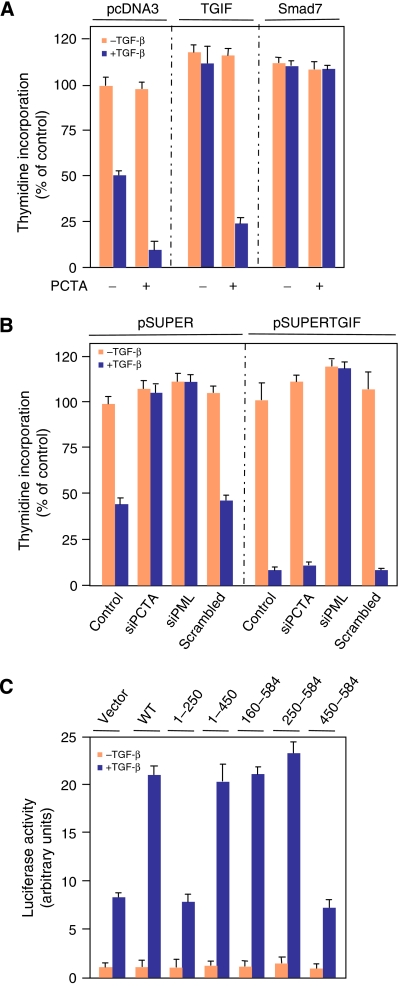
Next, we used two different approaches to investigate the possibility that PCTA contributes to TGF-β signalling through its association with TGIF. In the first approach, we employed a well-characterized MDCK cell line (MDCK-TGIF) stably expressing TGIF (Seo et al, 2006) to examine whether forced expression of PCTA could affect the ability of TGIF to restrict TGF-β signalling. We found that expression of PCTA partially reversed the inhibitory activity of TGIF towards TGF-β-mediated growth arrest and gene expression (Figure 3A and Supplementary Figure S3A). This partial antagonistic effect of PCTA on TGIF is specific, as expression of PCTA had no effect on the ability of Smad7 to restrict TGF-β signalling. The inability of PCTA to achieve complete reversion of TGIF effect was not unexpected, given that TGIF can simultaneously operate through a variety of biochemical routes, including transcriptional repression of Smad2 and its ubiquitin-dependent degradation (Wotton et al, 1999; Seo et al, 2004).
Figure 3.
PCTA functions by a mechanism that is dependent on its association with TGIF. (A) MDCK-pcDNA3, MDCK-TGIF, or MDCK-Smad7 cells were transfected with pEGFP alone or together with PCTA. After 24 h, GFP-transfected cells were sorted by FACS, exposed to TGF-β for 48 h and the rate of cell proliferation was determined by the thymidine incorporation method. Data (mean±s.d.) are expressed as percentages of the radioactivity incorporated by MDCK-pcDNA3 cells in the absence of TGF-β. (B) MDCK-pSUPER or MDCK-pSUPERTGIF cells were transfected with the indicated siRNA, treated with or without TGF-β for 48 h and the rate of cell proliferation was determined as in (A). (C) MDCK cells were transfected with ARE3-Lux together with FAST1 and various PCTA fragments. Cells were treated with or without TGF-β for 16 h and were analysed for luciferase activity.
In the second approach, we compared the effects of PCTA depletion on TGF-β responses in the absence or presence of endogenous TGIF. For this, we used a previously characterized MDCK cell line (MDCK-pSUPERTGIF) in which the expression of endogenous TGIF was reduced by stable expression of a shRNA targeting TGIF (Seo et al, 2004, 2006). As anticipated, TGIF depletion increased the sensitivity of cells to TGF-β-induced growth arrest and transcription (Figure 3B and Supplementary Figure S3B). In control MDCK cells, depletion of PCTA by siRNA blocked TGF-β-mediated responses. In contrast, MDCK-pSUPERTGIF cells did not exhibit a decrease in the TGF-β antiproliferative or transcriptional responses upon expression of PCTA siRNA (Figure 3B and Supplementary Figure S3B). The inability of PCTA siRNA to suppress TGF-β signalling in MDCK-pSUPERTGIF is specific, as depletion of PML by siRNA completely blocked TGF-β-mediated growth inhibition and transcription in these cells.
PCTA contains a N-terminal zinc-finger domain and a C-terminal Ring finger domain. To further demonstrate that PCTA promotes TGF-β signalling by a mechanism that is dependent on its association with TGIF, we mapped the region of PCTA required for upregulation of TGF-β signalling. As shown in Figure 3C, loss of the first 250 amino acids of PCTA, which include the zinc-finger motif, did interfere with the ability of PCTA to enhance TGF-β-induced transcription, whereas further deletions that remove the TBD abolished this effect. A similar analysis of C-terminal truncations, which remove the Ring finger domain leaving TBD intact, did not alter the effect of PCTA on TGF-β-induced transcription. These results not only confirm that PCTA contributes to TGF-β responses through association with TGIF, but also suggest that both the zinc-finger and Ring finger domains are dispensable for PCTA function in TGF-β signalling. As many proteins containing a zinc-finger motif can display DNA-binding activity, we also performed a pull-down experiment with biotinylated SBE (Smad-binding element) oligonucleotides to investigate whether PCTA can also function in TGF-β signalling by directly modulating transcription. Under conditions that enable the SBE oligonucleotides to bring down Smad4, PCTA was not detected (Supplementary Figure S3C). In the course of these analyses, we also explored the possibility that PCTA could function in TGF-β signalling by an ubiquitin-dependent degradation mechanism, as the Ring finger motif is known to fulfill an ubiquitin ligase function. As an experimental approach, we generated a PCTA mutant (PCTA.C503A/C506A referred to as PCTA.CA) in which the two conserved cysteines (in the Ring finger motif) that could participate in the ubiquitin ligase activity are substituted by alanines. We found that expression of PCTA.CA enhanced the TGF-β-dependent transcription with activity similar to that of wild-type PCTA (Supplementary Figure S3D), thereby arguing against the possibility that PCTA may contribute to TGF-β signalling through an ubiquitin-dependent degradation mechanism.
Role of the PCTA–TGIF complex in TGF- signalling
Having shown that PCTA functions by a mechanism that relies on its association with TGIF, we next sought to examine the physiological significance of the PCTA–TGIF interaction in TGF-β signalling. One attractive possibility could be that PCTA interacts with TGIF to set a threshold level for TGF-β signalling. If this scenario is accurate, the prediction is that the accumulation of PCTA–TGIF complex would vary among different cell types that display different sensitivities to TGF-β. To test this hypothesis, we examined the TGF-β-inducible association of endogenous TGIF and PCTA using a panel of cell lines that undergo growth arrest by TGF-β to a variable extent (Figure 4A). Interestingly, we observed a perfect correlation between the assembly of PCTA–TGIF complex and the extent of TGF-β-induced growth arrest, with the cell lines accumulating the highest levels of PCTA–TGIF complex being highly sensitive to TGF-β (Figure 4B). Given the requirement of PCTA–TGIF complex in TGF-β signalling, these results suggest that the association of PCTA with TGIF may exert an effect to set a threshold level for TGF-β-mediated growth arrest depending on cell types. This situation may not be restricted to the cytostatic function of TGF-β as the extent of assembly of endogenous PCTA–TGIF complex also correlates with the sensitivity of cells to TGF-β-activated transcription (Supplementary Figure S4).
In addition to setting a threshold level for TGF-β signalling, the association of PCTA with TGIF might also allow for modulation of the magnitude of TGF-β responses to another signalling input. To test this possibility, we focused our attention on tumour necrosis factor alpha (TNF-α), which is known to antagonize TGF-β responses in several cell systems (Bitzer et al, 2000; Seo et al, 2006). As shown in Figure 4C, a TGF-β-inducible interaction between endogenous PCTA and TGIF could be detected in MEFs and this interaction was decreased by TNF-α stimulation, suggesting that TNF-α may exert its inhibitory effect in part by interfering with the association of PCTA with TGIF. Consistent with this notion, overexpression of PCTA partially suppressed the inhibitory effect of TNF-α on TGF-β-induced transcription (Figure 4D).
PCTA interferes with the ability of TGIF to suppress Smad2 phosphorylation
We have previously reported that TGIF can restrict TGF-β signalling by preventing the phosphorylation of Smad2 (Seo et al, 2006). Our finding that PCTA elicits its actions in TGF-β signalling by a mechanism that is dependent on TGIF prompted us to consider whether PCTA modulates Smad2 phosphorylation. We observed that forced expression of PCTA increased the sensitivity of cells to TGF-β-mediated phosphorylation of Smad2 (Figure 5A). Similarly, overexpression of PCTA increased the sensitivity of cells to TGF-β-mediated phosphorylation of Smad3 (Figure 5B). Confirmation of these results was obtained by an experiment showing that PCTA can also enhance the association of Smad2 with Smad4 (Supplementary Figure S5). We also investigated the effect of PCTA on Smad2 phosphorylation under physiological conditions. Depletion of PCTA in Mv1Lu or HepG2 cells by a specific PCTA siRNA was associated with a decrease in phosphorylation of endogenous Smad2 (Figure 5C). The specificity of PCTA siRNA was evidenced by its inability to interfere with the non-targetable PCTA (PCTA.NT) in enhancing Smad2 phosphorylation (Figure 5D). Studies using MDCK-shPCTA cells confirmed that depletion of PCTA could cause a marked decrease in phosphorylation of endogenous Smad2 (Figure 5E). This drop in Smad2 phosphorylation was accompanied with a reduction in the assembly of endogenous Smad2–Smad4 complex (Figure 5F) and nuclear accumulation of endogenous Smad2 as well (Figure 5G).
Figure 5.
PCTA promotes TGF-β-mediated phosphorylation of Smad2. (A, B) 293 cells were transfected with Flag-Smad2 (A) or Flag-Smad3 (B) in either the presence or absence of HA-PCTA and treated with or without TGF-β for 1 h. The phosphorylation of Smad2 (A) or Smad3 (B) was assessed by blotting anti-Flag immunoprecipitates with anti-pSmad2 or anti-pSmad3, respectively. (C) HepG2 or Mv1Lu cells were transfected with either Scrambled or PCTA siRNA and 48 h later, they were treated with or without TGF-β for 1 h. The phosphorylation of Smad2 was assessed by immunoblotting with anti-pSmad2. (D) 293 cells were transfected with the indicated constructs, treated with or without TGF-β for 1 h and the phosphorylation of Smad2 was assessed by blotting anti-Myc immunoprecipitates with anti-pSmad2. (E–G) MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells were treated with or without TGF-β for 1 h. (E) The phosphorylation of Smad2 was assessed by immunoblotting with anti-pSmad2. (F) The association of Smad2 with Smad4 was analysed by blotting anti-Smad2 immunoprecipitates with anti-Smad4. (G) The localization of Smad2 was revealed by immunofluorescence with anti-Smad2. (H) 293 cells were transfected with the indicated combinations of Myc-Smad2, HA-TGIF, and HA-PCTA and treated with or without TGF-β for 1 h. The phosphorylation of Smad2 was assessed by blotting anti-Myc immunoprecipitates with anti-pSmad2. (I) MDCK-pSUPER or MDCK-pSUPERTGIF cells were transfected with the indicated siRNA and treated with or without TGF-β for 1 h. The phosphorylation of Smad2 and Smad3 was assessed by immunoblotting with anti-pSmad2 and anti-pSmad3.
Finally, we carried out experiments to examine whether PCTA facilitates Smad2 phosphorylation by a mechanism that is dependent on TGIF. In fact, we observed that forced expression of PCTA could relieve suppression of Smad2 phosphorylation by TGIF (Figure 5H). In an alternative experimental approach, we tested the effect of PCTA depletion on Smad2 phosphorylation using MDCK-pSUPERTGIF cells stably depleted from TGIF. Consistent with our previous findings (Seo et al, 2006), we found that knockdown of TGIF in MDCK cells increased the sensitivity of cells to TGF-β-induced phosphorylation of Smad2 (Figure 5I). Notably, expression of PCTA siRNA in control cells almost completely blocked Smad2 phosphorylation. In contrast, depletion of PCTA in MDCK-pSUPERTGIF cells had little or no effect on Smad2 phosphorylation. A similar conclusion could be drawn when the phosphorylation of Smad3 was examined (Figure 5I). Here again, we have obtained evidence arguing against an altered TGF-β responsiveness in these cells, because Smad2 phosphorylation was strongly reduced by expression of PML siRNA. Taken together, these results suggest that PCTA may function to enhance the phosphorylation of Smad2 or Smad3 by a mechanism that is dependent on its association with TGIF. This mechanism is apparently of physiological significance, as we could detect a strong correlation between the abundance of PCTA–TGIF complex and the extent of TGF-β-induced phosphorylation of Smad2 in cell lines exhibiting different sensitivities to TGF-β (Figure 4B). On the other hand, we found that TNF-α, which can suppress the assembly of PCTA–TGIF complex, was also able to inhibit Smad2 phosphorylation (Figure 4C), as previously described (Bitzer et al, 2000; Seo et al, 2006).
PCTA interferes with cPML binding to TGIF
In light of the observation that TGIF suppresses Smad2 phosphorylation by sequestering cPML in the nucleus (Seo et al, 2006), we wondered whether expression of PCTA could affect the association of TGIF with cPML. Experiments using transfected 293 cells demonstrated an association between TGIF and cPML that was significantly increased in response to TGF-β signalling (Figure 6A), similar to previous findings (Seo et al, 2006). Notably, expression of PCTA resulted in a strong decrease in the association of cPML with TGIF. The ability of PCTA to block the cPML–TGIF interaction is specific, as overexpression of PCTA failed to inhibit the association of TGIF with Smad2, Tiul1–WWP1, or HDAC1 (Supplementary Figures S6A–C). To provide further evidence that PCTA can disrupt the TGIF–cPML complex, we examined whether it could release cPML from nuclear retention by TGIF. To approach this question, we took advantage of our previous observation that overexpression of TGIF is sufficient to induce constitutive retention of cPML in the nucleus (Seo et al, 2006). In 293 cells transfected with cPML alone, we could clearly detect cPML in cytoplasmic and nuclear fractions, with cytoplasmic accumulation being stronger than nuclear accumulation (Figure 6B). Interestingly, expression of TGIF caused the majority of cPML to accumulate in the nucleus and this effect was blocked by coexpression of PCTA. We confirmed these results by immunofluorescence experiments showing that expression of PCTA can restrict the ability of TGIF to retain cPML in the nucleus (Figure 6C).
Figure 6.
PCTA inhibits the association of cPML with TGIF. (A–C) 293 cells were transfected with the indicated combinations of Flag-cPML, Myc-TGIF, and HA-PCTA. (A) Cells were treated with or without TGF-β for 1 h and cell lysates were analysed by immunoprecipitation with anti-Myc followed by immunoblotting with anti-Flag. (B) Cell lysates from cytoplasmic and nuclear fractions were subjected to immunoblotting with anti-Flag and LaminB or tubulin as controls for the purity of fractions. (C) Cells were stained with anti-Flag or DAPI and the localization of cPML (red) or the nuclei (blue) was visualized with a fluorescence microscope. (D) MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells were treated with or without TGF-β for 1 h and the association of cPML with TGIF was assessed by blotting anti-TGIF immunoprecipitates with anti-PML. (E) Cytoplasmic and nuclear fractions were prepared from MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells and subjected to immunoblotting analysis with antibodies against PML, LaminB, or tubulin. (F) MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells were immunostained with anti-PML and DAPI and the localization of PML (red) or the nuclei (blue) was visualized with a fluorescence microscope. (G, H) MDCK-shPCTA (clones 1 and 2) or MDCK-Scrambled cells were treated with or without TGF-β for 1 h. Cell lysates were subjected to immunoprecipitation with anti-SARA followed by immunoblotting with anti-PML (G) or anti-Smad2 (H). (I) 293 cells transfected with the indicated combinations of HA-TβRI, HA-TβRI.act, His-PCTA, and Flag-Smad2.3SA. The association of Smad2.3SA with TβRI was analysed by blotting anti-Flag immunoprecipitates with anti-HA. (J) Wild-type or PML−/− MEFs were transfected with the indicated combinations of Myc-Smad2, HA-PCTA, and Flag-cPML and treated with or without TGF-β for 1 h. The phosphorylation of Smad2 was assessed by blotting anti-Myc immunoprecipitates with anti-pSmad2.
Next, we tested whether expression of endogenous PCTA could affect the assembly of endogenous cPML–TGIF complex as well as the subcellular distribution of endogenous cPML. As shown in Figure 6D, depletion of PCTA increased the association of endogenous TGIF and cPML. Further analysis by the cell fractionation assay revealed that knockdown of PCTA also resulted in increased accumulation of endogenous cPML in the nucleus (Figure 6E). Note that two nuclear isoforms of PML could be detected in these cells, but their distribution was not changed by depletion of PCTA, attesting to specificity of this assay. In immunofluorescence experiments, we detected distinctive PML nuclear bodies as well as punctuate cytoplasmic staining of PML in control cells (Figure 6F), similar to previous observations (Lin et al, 2004; Seo et al, 2006). In cells depleted from PCTA, we observed a marked decrease in cytoplasmic staining of PML (Figure 6F), suggesting that expression of PCTA may induce cPML to dissociate from TGIF and to accumulate in the cytoplasm.
Previous studies have shown that cPML functions in TGF-β signalling by facilitating the access of Smad2 to SARA and TβRI (Lin et al, 2004). To provide further evidence that PCTA can release cPML from nuclear retention by TGIF, we investigated whether depletion of PCTA could affect the association of cPML with SARA. In agreement with previous findings (Lin et al, 2004; Seo et al, 2006), we found that the interaction of endogenous SARA and cPML was disrupted by TGF-β (Figure 6G). As this event was associated with a concomitant interaction of cPML with TGIF (Figure 6D and Seo et al, 2006), these results suggest that SARA and TGIF may have an important function in controlling the subcellular distribution of cPML. Consistent with this, overexpression of SARA induced the majority of cPML to redistribute from the nucleus to the cytoplasm (Supplementary Figure S6D), whereas overexpression of TGIF had the opposite effect (Figure 6B and C and Seo et al, 2006). Interestingly, PCTA depletion resulted in a marked decrease in the association of endogenous SARA and cPML (Figure 6G), reinforcing the idea that PCTA can release cPML from nuclear retention by TGIF. Further support to this concept was obtained by an experiment demonstrating that depletion of PCTA also decreased the assembly of endogenous SARA–Smad2 complex (Figure 6H). In a similar experimental approach, we were unable to see an interaction between endogenous Smad2 and TβRI, presumably due to the transient character of this interaction (Macias-Silva et al, 1996; Prunier et al, 2001). To resolve this issue, we made use of an unphosphorylable mutant of Smad2 (Smad2.3SA) that has been shown to exhibit strong affinity for the activated TβRI (Macias-Silva et al, 1996; Seo et al, 2006). We found that expression of PCTA enhanced the association of Smad2.3SA with the activated TβRI (Figure 6I). All together, these results suggest that a physiological function of PCTA is to enforce the accumulation of cPML in the cytoplasm, where it can coordinate the assembly of Smad2 with SARA and receptor. Crucially, the release of cPML from TGIF-induced nuclear retention by PTCA appears to have an important function in TGF-β signalling, as expression of PCTA was unable to increase Smad2 phosphorylation and transcription in MEFs PML−/−, when compared to wild-type cells (Figure 6J and Supplementary Figure S6E). As a control, add back of cPML into PML−/− MEFs increased the ability of TGF-β to induce Smad2 phosphorylation and transcription to the levels detected in wild-type MEFs.
PCTA and cPML form mutually exclusive complexes with TGIF
To unravel the mechanism by which PCTA releases cPML from nuclear retention by TGIF, we investigated whether it could compete with cPML for binding to TGIF. To test this possibility, we examined the formation of PCTA–TGIF and cPML–TGIF complexes in the same transfectants. Concomitant with the formation of PCTA–TGIF complex, the interaction of cPML with TGIF was decreased (Figure 7A, left). Conversely, concomitant with the formation of cPML–TGIF complex, the interaction of PCTA with TGIF was decreased (Figure 7A, right). Further evidence that PCTA and cPML competitively share TGIF was obtained by experiments showing that depletion of endogenous PCTA enhanced the assembly of endogenous cPML–TGIF complex (Figure 6D). On the other hand, depletion of cPML by siRNA was associated with increased formation of the endogenous PCTA–TGIF complex (Figure 7B). We also made use of wild-type and PML−/− MEFs and found that PML deficiency caused increased association of PCTA with TGIF (Figure 7C and H). Thus, we suggest that PCTA and cPML may competitively share TGIF. Further support to this view was obtained by comparative studies using the panel of cell lines that exhibit variable degrees of responsiveness to TGF-β. In fact, we observed an inverse correlation between the abundance of PCTA–TGIF and cPML–TGIF complexes, with cells that form low levels of endogenous PCTA–TGIF complex being able to display high levels of endogenous cPML–TGIF complex and vice versa (Figures 4B and 7D).
To further corroborate the concept that PCTA and cPML compete for binding to TGIF, we investigated the possibility that expression of c-Jun could modulate the association of PCTA with TGIF, as c-Jun is known to facilitate the TGIF–cPML interaction (Seo et al, 2006). We anticipated that expression of c-Jun would promote the assembly of cPML–TGIF complex at the expense of PCTA–TGIF complex. Studies employing wild-type and c-Jun−/− MEFs showed that c-Jun deficiency led to almost complete loss in the association of endogenous cPML and TGIF (Figure 7E), consistent with our previous findings (Seo et al, 2006). Strikingly, c-Jun deficiency also caused increased association of endogenous PCTA and TGIF. In the course of these studies, we also investigated whether increased expression of endogenous c-Jun in response to another signalling input would disrupt the PCTA–TGIF interaction. In this context, TNF-α was an ideal cue based on its ability to interfere with the association of PCTA with TGIF shown earlier in this study (Figure 4C) and to elicit strong induction of c-Jun (Davis, 2000). To directly attribute the effects of TNF-α to c-Jun, we conducted comparative studies with wild-type and JNK1−/−JNK2−/− MEFs, because JNK is crucial for TNF-α-induced expression of c-Jun (Ventura et al, 2003). In contrast to the strong decrease in the PCTA–TGIF complex seen in wild-type cells, exposure of JNK1−/−JNK2−/− MEFs to TNF-α was void of any effect on that complex (Figure 7F). A similar result was obtained with c-Jun−/− MEFs (Figure 7G). In a control experiment, TNF-α failed to restrict the assembly of PCTA–TGIF complex in PML−/− MEFs despite the strong induction of c-Jun expression (Figure 7H), suggesting that the inhibitory effect of TNF-α on the PCTA–TGIF complex may operate through stabilization of the cPML–TGIF complex by c-Jun. Taken together, these results not only confirm that PCTA and cPML can form mutually exclusive complexes with TGIF but also provide a mechanistic explanation for the antagonistic action of TNF-α on TGF-β-induced association of PCTA with TGIF.
Discussion
In the present study, we report on the identification of PCTA as a novel component of the TGF-β signalling pathway. We presented evidence that PCTA exerts an effect through its ability to relieve the inhibitory activity of TGIF towards TGF-β signal transduction. We proposed a model in which PCTA competes with cPML for binding to TGIF, thereby enforcing the accumulation of cPML in the cytoplasm, where it forms a stable complex with SARA and Smad2 and promotes phosphorylation of Smad2 by the activated TβRI. Thus, the identification of PCTA may define a novel TGIF antagonist that functions in partnership with the tumour suppressor cPML to integrate TGF-β transcriptional and cytostatic responses.
There is now a growing body of evidence that TGIF functions as an important negative regulator of the TGF-β signal transduction. In several mammalian cell types, the expression level of TGIF inversely correlates with the extent of TGF-β-induced gene expression and growth arrest, and knocking down of TGIF enhances TGF-β signalling (Wotton et al, 1999; Lo et al, 2001; Seo et al, 2004, 2006). Homozygous inactivation of TGIF in mice resulted in laterality defects and growth retardation, and TGIF-null MEFs displayed delayed progression through the G1 cell cycle phase (Mar and Hoodless, 2006). Although these effects of TGIF knockout would be consistent with increases in TGF-β signalling activity, MEFs derived from TGIF mutant embryos paradoxically exhibited no alteration in the growth inhibitory response to TGF-β, raising a debate on the relevance of TGIF as a physiological inhibitor of TGF-β signalling. For instance, the plausible possibility that has been proposed to explain this inconsistency is based on a compensatory mechanism involving TGIF2, a TGIF paralogue that possesses many biological proprieties similar to those of TGIF, including repression of Smad signalling (Mar and Hoodless, 2006). By reporting here that PCTA promotes TGF-β-induced transcriptional and cytostatic responses through a mechanism that is dependent on its association with TGIF, the findings outlined in the present work provide a powerful reinforcement for improving the role of TGIF as a physiological negative modulator of TGF-β signalling in mammalian cells.
Despite the importance of TGIF in the negative regulation of TGF-β signalling, its biochemical functions and regulations are not well understood. Yet one important mechanism through which TGIF restricts TGF-β signalling is linked to its ability to sequester cPML in the nucleus, thereby causing the inhibition of TGF-β-mediated Smad2 phosphorylation and an attendant loss of TGF-β signalling to the nucleus (Seo et al, 2006). However, alternative mechanisms may exist, because TGIF was also shown to inhibit TGF-β signalling by recruiting to Smad2 a nuclear corepressor complex containing HDAC1 or by targeting Smad2 for degradation through association with the ubiquitin ligase Tiul1–WWP1 (Wotton et al, 1999; Seo et al, 2004). In several attempts, we have not been able to obtain evidence that PCTA can interfere with the association of TGIF with Smad2, HDAC1, or Tiul1–WWP1. Under the same experimental conditions, we show that PCTA can promote the phosphorylation of Smad2 by a mechanism that relies on its association with TGIF. As TGIF localizes exclusively into the nucleus (Wotton et al, 1999; Seo et al, 2006), it is reasonable to suggest that PCTA might function to restrain TGIF from retaining a component of the TGF-β pathway that facilitates Smad2 phosphorylation at the cell membrane. Our data suggest that cPML represents a strong candidate for such a component, because expression of PCTA can disrupt the association of TGIF with cPML, leading to the accumulation of cPML in the cytoplasm, where it assists Smad2 for phosphorylation. Thus, our results provide a mechanistic explanation for the antagonistic effect of PCTA on TGIF function and establish PCTA as an important component of the TGF-β pathway that is crucial to the initiation of Smad signalling.
How does PCTA disrupt the association of cPML with TGIF? On the basis of our findings that forced expression of PCTA or cPML inhibits binding of TGIF to the other, and depletion of one augments the binding of TGIF to the other, an attractive explanation would be that PCTA and cPML competitively share the pool of TGIF. In support to this notion, we show an interaction between endogenous PCTA and TGIF together with the endogenous cPML–TGIF interaction in different cell types and the abundance of PCTA–TGIF complex inversely correlates with the abundance of cPML–TGIF complex. We propose that upon activation of TGF-β signalling, TGIF would face the challenge to interact with PCTA or cPML and the competitive balance between PCTA–TGIF and cPML–TGIF complexes may allow precise regulation of the extent of TGF-β responses depending on the cellular context. An extracellular signal that opposes TGF-β signalling, such as TNF-α, would also affect the outcome of the competition between PCTA and cPML for binding to TGIF. This mechanism may normally function to balance the activity of these divergent pathways to provide a suitable level of stimulation under physiological conditions that ensure the maintenance of normal cell function and tissue homoeostasis.
Understanding the mechanism of TGF-β cytostatic responses has become an area of intense research, because its loss contributes to tumour development (Derynck et al, 2001). For example, mutational inactivation of the TGF-β type II receptor, Smad4 or Smad2 occurs in various human cancers (Derynck et al, 2001). Important support for a more general role of TGF-β signalling in tumour development came with the recent findings that inactivation of cPML function in acute promyelocytic leukaemia caused a profound impairment in TGF-β-dependent growth suppressive activities (Lin et al, 2004). Our demonstration that cPML and PCTA functions are interconnected in a tightly regulated network that integrates TGF-β cytostatic signals raises the interesting possibility that PCTA may have an important function in suppression of tumorigenesis. In preliminary experiments using several human liver and breast cancer samples, we did not detect any major perturbations in the expression levels of PCTA mRNA (data not shown). Thus, the principal challenge for the future is to establish the existence of more subtle alterations in PCTA (localization, protein levels, interaction with TGIF) that could potentially disable TGF-β cytostatic responses during tumour progression.
In summary, our work defines a functional network between PCTA, TGIF, and cPML. We attributed a function to PCTA in TGF-β-mediated transcriptional and cytostatic responses through its ability to compete with cPML for binding to TGIF. Once released from the TGIF-dependent nuclear sequestration, cPML functions in partnership with SARA to coordinate the phosphorylation of Smad2. Thus, by functioning to ensure the redistribution of cPML into the cytoplasm, PCTA functions in an important regulatory position in the TGF-β signalling pathway. A comprehensive examination of PCTA in the context of different biological settings, beyond the transcriptional and growth inhibitory responses to TGF-β studied here, may offer new directions towards improving our understanding of the rich regulatory network that controls TGF-β signalling in developmental and physiopathological processes.
Materials and methods
Yeast two-hybrid screen
cDNA encoding full-length human TGIF was cloned into the GAL4 DNA-binding domain vector derived from pBTM116 and used as bait in yeast two-hybrid screens of a human placental cDNA library as previously described (Colland et al, 2004). By screening 10 × 106 colonies of a human placental cDNA library, we obtained 34 different cDNA species, one of which encodes PCTA.
Cell culture and transfection
All cell lines were maintained in DMEM supplemented with 10% fetal calf serum (FCS). To establish doxycyclin-inducible PCTA MDCK cells, cells were first transfected with pcDNA6/TR encoding the tetracyclin–doxycyclin repressor and selected with blastidin. Then, cells that express high levels of the repressor were transfected with pcDNA5/TO-His-PCTA and selected with hygromycin. To establish MDCK cells stably expressing shPCTA, cells were transfected with pBLOCKiT-shPCTA or vector and selected with neomycin.
For experiments, cells were cultured in DMEM containing 0.5% FCS for 16 h before being treated with 2 ng/ml TGF-β1 (Sigma) or 50 ng/ml TNF-α (Roche) as indicated.
Immunofluorescence analysis
Cells were fixed in 4% paraformaldehyde for 30 min and permeabilized in 0.1% Triton X-100. Then, they were incubated with the primary antibody for 60 min at room temperature followed by the secondary antibody conjugated to Texas Red or FITC. The cover slips were mounted in PBS containing 50% glycerol and viewed on a fluorescence microscope.
Immunoprecipitation and immunoblotting
Cell extracts were prepared using TNMG buffer and subjected to immunoprecipitation with the appropriate antibody for 2 h, followed by adsorption to sepharose-coupled protein G for 1 h (Prunier et al, 2001). Immunoprecipitates were separated by SDS–PAGE and analysed by immunoblotting. For determination of total protein levels, aliquots of lysates were subjected to immunoblotting with the indicated antibodies.
Transcriptional reporter assay
Cells were transfected by LipofectAMINE and 30 h later they were exposed to TGF-β and/or TNF-α for 16 h. Cells were then lysed and assayed for luciferase activity with the Promega dual luciferase assay system, normalized and expressed as mean±s.d. of triplicate from a representative experiment performed at least three times.
Thymidine incorporation
Cells were labelled with 1 μCi/ml [3H]thymidine for 2 h, washed with PBS and fixed with 10% trichloroacetic acid for 30 min at 4°C. Cells were then extracted with 0.5 NaOH for 30 min and samples were counted in a liquid scintillation spectrometer.
Subcellular fractionation
Cells were scarped off the plate, resuspended in lysis buffer and homogenized by 20 strokes in a tight fitting Dounce homogenizer. Then, nuclei were pelleted by a 30 min centrifugation at 3000 g, washed three times in lysis buffer and resuspended in SDS buffer (Seo et al, 2006).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr P Pandolfi for providing wild-type and PML−/− MEFs and Hybrigenics staff for their contribution. This study was supported by INSERM, CNRS, ARC and La Ligue contre le Cancer comité de Paris.
References
- Atfi A, Dumont E, Colland F, Bonnier D, L'Helgoualc'h A, Prunier C, Ferrand N, Clement B, Wewer UM, Theret N (2007) The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor. J Cell Biol 178: 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Bottinger EP (2000) A mechanism of suppression of TGF-β/SMAD signaling by NF-kappa B/RelA. Genes Dev 14: 187–197 [PMC free article] [PubMed] [Google Scholar]
- Childs KS, Goodbourn S (2003) Identification of novel co-repressor molecules for interferon regulatory factor-2. Nucleic Acids Res 31: 3016–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colland F, Jacq X, Trouplin V, Mougin C, Groizeleau C, Hamburger A, Meil A, Wojcik J, Legrain P, Gauthier JM (2004) Functional proteomics mapping of a human signaling pathway. Genome Res 14: 1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A (2001) TGF-β signaling in tumor suppression and cancer progression. Nat Genet 29: 117–129 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584 [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL (2003) Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol 5: 410–421 [DOI] [PubMed] [Google Scholar]
- Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, Richieri-Costa A, Zackai EH, Massague J, Muenke M, Elledge SJ (2000) Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat Genet 25: 205–208 [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471 [DOI] [PubMed] [Google Scholar]
- Itoh S, ten Dijke P (2007) Negative regulation of TGF-β receptor/Smad signal transduction. Curr Opin Cell Biol 19: 176–184 [DOI] [PubMed] [Google Scholar]
- Lin HK, Bergmann S, Pandolfi PP (2004) Cytoplasmic PML function in TGF-β signalling. Nature 431: 205–211 [DOI] [PubMed] [Google Scholar]
- Lo RS, Wotton D, Massague J (2001) Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J 20: 128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL (1996) MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell 87: 1215–1224 [DOI] [PubMed] [Google Scholar]
- Mar L, Hoodless PA (2006) Embryonic fibroblasts from mice lacking Tgif were defective in cell cycling. Mol Cell Biol 26: 4302–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D (2005) Smad transcription factors. Genes Dev 19: 2783–2810 [DOI] [PubMed] [Google Scholar]
- Melhuish TA, Wotton D (2000) The interaction of the carboxyl terminus-binding protein with the Smad corepressor TGIF is disrupted by a holoprosencephaly mutation in TGIF. J Biol Chem 275: 39762–39766 [DOI] [PubMed] [Google Scholar]
- Prunier C, Ferrand N, Frottier B, Pessah M, Atfi A (2001) Mechanism for mutational inactivation of the tumor suppressor Smad2. Mol Cell Biol 21: 3302–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B, Hill CS (2007) TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 12: 970–982 [DOI] [PubMed] [Google Scholar]
- Seo SR, Ferrand N, Faresse N, Prunier C, Abecassis L, Pessah M, Bourgeade MF, Atfi A (2006) Nuclear retention of the tumor suppressor cPML by the homeodomain protein TGIF restricts TGF-β signaling. Mol Cell 23: 547–559 [DOI] [PubMed] [Google Scholar]
- Seo SR, Lallemand F, Ferrand N, Pessah M, L'Hoste S, Camonis J, Atfi A (2004) The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J 23: 3780–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel PM, Massague J (2003) Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer 3: 807–821 [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell 95: 779–791 [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ (2003) c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol 23: 2871–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M (1998) Smads and early developmental signaling by the TGFβ superfamily. Genes Dev 12: 2445–2462 [DOI] [PubMed] [Google Scholar]
- Wotton D, Knoepfler PS, Laherty CD, Eisenman RN, Massague J (2001) The Smad transcriptional corepressor TGIF recruits mSin3. Cell Growth Differ 12: 457–463 [PubMed] [Google Scholar]
- Wotton D, Lo RS, Lee S, Massague J (1999) A Smad transcriptional corepressor. Cell 97: 29–39 [DOI] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE (2002) TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J 21: 3749–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information