Abstract
Vasopressin affects behavior via its two brain receptors, the vasopressin 1a and vasopressin 1b receptors (Avpr1b). Recent work from our lab has shown that disruption of the Avpr1b gene reduces inter-male aggression and reduces social motivation. Here we further characterized the aggressive phenotype in Avpr1b −/− (knockout) mice. We tested maternal aggression and predatory behavior. We also analyzed the extent to which food deprivation and competition over food increases inter-male aggression. We quantified defensive behavior in Avpr1b −/− mice and later tested offensive aggression in these same mice. Our results show that attack behavior toward a conspecific is consistently reduced in Avpr1b −/− mice. Predatory behavior is normal, suggesting that the deficit is not due to a global inability to detect and attack stimuli. Food deprivation, competition for food, and previous experience increase aggression in both Avpr1b +/+ and −/− mice. However, in these circumstances the level of aggression seen in knockout mice is still less than that observed in wild-type mice. Defensive avoidance behaviors, such as boxing and fleeing, are largely intact in knockout mice. Avpr1b −/− mice do not display as many "retaliatory" attacks as the Avpr1b +/+ mice. Interestingly, when territorial aggression was measured following the defensive behavior testing, Avpr1b −/− mice typically show less initial aggressive behavior than wild-type mice, but do show a significant increase in aggression with repeated testing. These studies confirm that deficits in aggression in Avpr1b −/− mice are limited to aggressive behavior involving the attack of a conspecific. We hypothesize that Avpr1b plays an important role in the central processing that couples the detection and perception of social cues (which appears normal) with the appropriate behavioral response.
Keywords: V3, agonistic behavior, social motivation, neuropeptide, predation, maternal aggression
In species ranging from molluscs to mammals, the neuropeptide vasopressin (Avp) or its homologs participate in the regulation of social behavior (Grober and Sunobe, 1996; Albers and Bamshad, 1998; Dantzer, 1998; Godwin et al., 2000; Bester-Meredith and Marler, 2001; Goodson and Bass, 2001; Pitkow et al., 2001; Semsar et al., 2001). In mammals, Avp is involved in the regulation of aggression, affiliative behavior, and social recognition. Of the two Avp receptor subtypes found centrally, early pharmacological (Ferris et al., 1985; Ferris et al., 1988; de Wied et al., 1991; Bluthe and Dantzer, 1992; Engelmann et al., 1992; Alescio-Lautier et al., 1995; Bamshad and Albers, 1996; Dantzer, 1998; Dluzen et al., 1998) and genetic (Bielsky et al., 2004; Egashira et al., 2004) manipulations have implicated the vasopressin 1a receptor (Avpr1a) in the regulation of the aforementioned behaviors.
There is, however, mounting evidence that the vasopressin 1b receptor (Avpr1b) is also an important regulator of social behavior. Analysis of the Avpr1b knockout mouse implicates this receptor in the regulation of aggression and social memory (Wersinger et al., 2002; Wersinger et al., 2004). Pharmacological antagonism of the Avpr1b also has behavioral consequences, producing reduced anxiety and aggression (Griebel et al., 2002; Blanchard et al., 2005; Stemmelin et al., 2005). While Avpr1b distribution has remained elusive in most species, studies in rats suggest that the Avpr1b has a more limited regional distribution and is expressed at lower levels than the Avpr1a (Hernando et al., 2001). Interestingly, Avpr1b-immunoreactivity in rat has been reported in regions implicated in the regulation of social behavior, including the olfactory system and the medial preoptic area (Hernando et al., 2001). To date, rats are the only species in which the distribution of the Avpr1b has been described.
Although the exact definition of aggression is still debated, aggression is traditionally separated into five categories: offensive aggression, defensive aggression, maternal aggression, play fighting, and predatory aggression (Nelson and Chiavegatto, 2000; Blanchard et al., 2003). Unlike predatory aggression, the first four types can also be defined as social aggression, since they involve an interaction with a conspecific animal. Irrespective of theoretical debate as to whether or not these all fit a strict definition of aggression, clearly the motivation behind these behaviors differs. These categories of aggression are also discernable by the neural circuits mediating them. While defensive rage and predatory attack behavior are elicited by electrical stimulation of many brain regions in the cat, stimulation of a given brain region elicits only one of the two, never both (Gregg and Siegel, 2001). Thus, these two classes of behavior are mediated by different neural circuits. Other forms of aggression, such as offensive and maternal, may involve more similar neural circuitry. When male and female rodents are placed in situations that would elicit offensive aggression and maternal aggression, respectively, Fos-like immunoreactive (Fos-ir) neurons are found in similar brain regions in both sexes (Lonstein and Stern, 1997; Stork et al., 1997; Lyons et al., 1999; Bester-Meredith and Marler, 2001; Gammie and Nelson, 2001; Wersinger et al., 2002; Hasen and Gammie, 2005; Lonstein, 2005).
Although offensive aggression and maternal aggression have been traditionally difficult to discern in terms of gross neural circuitry, they appear to be biochemically distinguishable. Offensive aggression is generally enhanced by the presence of circulating testosterone whereas maternal aggression is not, or may even be inhibited by it (Rothballer, 1967; Owen et al., 1974; Saal et al., 1976; Svare, 1980; Albert et al., 1988). Treatment with drugs that alter serotonin reliably affect offensive aggression and predatory behavior (Welch and Welch, 1968; Miczek et al., 1975; Albert et al., 1985; Nikulina, 1991; Holmes et al., 2002; Miczek et al., 2002; Chiavegatto and Nelson, 2003; Garris, 2003) but other drugs, such as fluprazine, affect offensive aggression but not predatory behavior (Parmigiani et al., 1989; Parmigiani and Palanza, 1991). Thus, although the brain regions involved (e.g., the amygdala) may not differ between some forms of aggression, the types of cells involved within a brain region may differ (e.g., androgen receptor- vs. estrogen receptor-containing neurons). Examination of different forms of aggression in Avpr1b −/− mice not only allows us to define further the role of the Avpr1b in aggression, but also to help explain how these various types of aggression can be differentially regulated.
As we have previously observed reduced offensive aggression in male Avpr1b −/− mice (Wersinger et al., 2002; Wersinger et al., 2004), in the present study we asked whether maternal aggression, another form of social aggression, is also disrupted or if only inter-male aggression is. We also assessed predatory behavior to see if a non-social attack behavior is also affected by the absence of the Avpr1b. We also asked whether aggressive behavior could be modulated by non-social cues by food-restricting subjects and by creating competition for a resource. Lastly, based on previous work by Blanchard (Blanchard et al., 2004), Miczek (Miczek, 1983) and Maxson (Maxson, 1996), we attempted to refine our analysis by including a more detailed analysis of both defensive and offensive behaviors.
Materials and Methods
Generation of Subjects
Targeted Disruption of the Vasopressin 1b Receptor Gene
The generation of the Avpr1b −/− mouse line has been previously described (Wersinger et al., 2002). The subjects of these experiments were a mix of the C57BL/6J and 129X1/SvJ strains. All the subjects were littermates of heterozygous crosses.
PCR Analysis
The offspring were genotyped at weaning using PCR analysis of DNA isolated from tail clips. The wild-type allele is identified by a 763bp PCR product using the primers GAAACGGCTACTCTCTCCGATTCCAAAAGAAAG and ACCTGTAGATATTTGACAGCCCGG and the mutant allele by a 461bp PCR product using the primers GAAACGGCTACTCTCTCCGATTCCAAAAGAAAG and ACCCCTTCCCAGCCTCTGAGCCCAGAAGCGAAGG. Both reactions are run in the same tube under the following conditions: 95°C × 4min, (95°C × 1min, 60°C × 1min, 72°C × 1min) × 40 cycles, 72°C × 5min.
Housing
Unless otherwise stated, all animals were initially group-housed in single-sex cages and the subjects were 60-100 days of age. Although this reflects a broad range of ages, there were no significant genotypic differences in the ages of the subjects. Also, since control and KO animals were littermates for each experiment, the subjects in each group were, necessarily, of approximately the same distribution of ages. The subjects were singly housed on a 12L:12D light cycle (for example, if the lights came on at 0600h, the light-phase was defined as 0600-1800 and the dark-phase as 1800-0600) with food and water available ad libitum for at least two weeks prior to testing.
Aggression
All aggression studies except for predatory aggression tests were performed during the time the lights were off. Since mice are nocturnal, they are behaviorally more active during this dark-phase of the light cycle. Predatory behavior was assessed during the time the lights were on for logistical reasons related to animal husbandry. Interactions were videotaped using a Sony NightShot digital video camera (Sony model DRV120) for subsequent analysis because many of the behaviors occur very quickly. Behavior, except predatory aggression, was scored using the Noldus Observer system.
Maternal Aggression
Singly housed female Avpr1b +/+ and −/− mice (final n=10 per group) were paired with male C57BL/6J mice (Jackson Labs, Bar Harbor, ME). Each morning the female was inspected for the presence of a sperm plug. Once a plug was found, the male was removed from the cage. Females were then monitored closely until they gave birth. Gestational length and the number of pups delivered were recorded. Seventy-two hours later, during the dark-phase of the light cycle, an adult, gonad-intact male 129X1/SvJ mouse was added to the cage. The litter was not removed. If no aggression was observed in the first five minutes, a latency of 300s was recorded and the test ended. If the male attacked the pups, the test was immediately ended and the test not included in the statistical analysis (one Avpr1b +/+ and one Avpr1b −/− were excluded). Each mouse was given three tests with one day between each test. Unless specified otherwise, the latency to attack, number of attacks, number of bites, and number of tail rattles were recorded. An attack was scored whenever the subject approached the stimulus animal and engaged in any aggressive behavior. Thus, the number of attacks includes the number of bites and lunges. The latency to attack was defined as the number of seconds of observation time until the subject attacks through, at the most, 3 tests. For example, if a subject first attacked 40s into the second test, the attack latency was scored as 340s.
Competitive Aggression
Mice were singly housed for at least two weeks prior to testing. Avpr1b +/+ and Avpr1b −/− mice were divided into four groups (8 subjects of each genotype in each group): 1. ad libitum food overnight but no food during the test, 2. ad libitum food overnight and food during the test, 3. food-restricted overnight and no food during the test, and 4. food-restricted overnight but food during the test. In the food-restricted groups, all the food was removed from the subject’s home cage at approximately 1800h the evening before. For all groups, water was available ad libitum. Subjects were tested the next day during the dark-phase of the light cycle between 1800-2400h (lights went off at 1800h). For the groups with food present during the test, a piece of lab chow was placed into a clean Plexiglas testing cage (40cm × 40cm × 30cm). The subject was placed in the cage for 60 seconds. The no-food groups were simply placed into an empty testing cage. An adult male Balb/c that had not been food-restricted was then placed in the testing cage. The latency to and number of attacks, tail-rattles, and bites were recorded. The test continued for two minutes after the first attack. If no aggression was observed within five minutes, a latency of 300s was recorded and the test stopped.
Defensive Aggression
The subjects (n= 6 +/+ and 6 −/−) were housed singly for at least two weeks prior to testing. Stimulus Balb/c mice were purchased from NCI. Briefly, the subjects were first tested as the intruder in three tests to assess their defensive behavior (Tests 1-3). They were then tested (six weeks later) three times as the resident to determine the extent to which experience alters their aggressive behavior (Tests 4-6). All resident-intruder tests were one week apart and began 1 hour after lights went out at 1600h. All residents were singly housed for two weeks and the bedding was not changed for at least five days prior to behavioral testing. Behaviors were recorded for 2 minutes after the resident attacked the intruder. The test was stopped at 5 minutes if the resident did not attack. The following behaviors were scored in subjects during the defensive testing (Tests 1-3): flight, boxing, defensive attacks, and investigatory behavior towards the resident. We combined flight and boxing behavior into a category we call “defensive avoidance.” We defined defensive attacks as an attack by the subject that directly followed an attack by the stimulus animal. Offensive aggression in Tests 4-6 was quantified as described above.
Predatory Aggression
Male Avpr1b +/+ and −/− (n=8 per genotype) were singly housed. Each male was tested for its latency to attack a cricket (Fluker Farms, Port Allen, LA) in three different tests, with at least one full day between tests. In the first two tests, the subjects had been food-deprived overnight. In the third test, the mice had food available ad libitum before the test. The tests were conducted during the light-phase of the light cycle due to the logistics of using crickets of unknown pathogen status and the necessity to remove the animals from the animal facility for testing. A subject was moved to a testing room one hour before a cricket was placed into its cage. The subject was observed constantly during the first 15 minutes of the test. After this time, the researcher looked for the cricket every 5 minutes. The latency was then recorded as the five minute bin in which the mouse presumably consumed the cricket. If the cricket was still alive after 60 minutes, that time was recorded as the latency and the test stopped.
Statistics
Due to the relatively skewed distribution of data (subjects tended to display either no aggressive behavior or robust aggression), we used non-parametric statistics for most analyses. Unless otherwise stated, the numbers of behaviors per test and the attack latencies were compared between groups using a Mann-Whitney rank sum test. The percentages of animals in each group attacking were compared using Fisher’s exact test of probability. The predatory aggression data were analyzed using a one-way ANOVA on repeated measures to compare the attack latencies in Tests 1 and 2 between the genotypes. Since the subjects were not food-deprived for Test 3, this trial was treated as independent and was analyzed using a Mann-Whitney rank sum test. The latencies to attack and the numbers of attacks collected during the competitive aggression test were analyzed with a three-way ANOVA followed by an all pairwise multiple comparison procedure (Holm-Sidak method) as the post-hoc analysis. The percentages of subjects in each group attacking in Test 1, Test 2, Test 3, and in any test were calculated and compared using Fisher’s exact test of probability. Defensive behaviors were compared among groups using a one-way ANOVA on repeated measures. Since Tests 1-3 used a different testing paradigm than Tests 4-6, we treated them as independent trials and analyzed them separately. We used a Mann-Whitney rank sum analysis to compare data pooled over Tests 1-3 and pooled over Tests 4-6. The percentages of subjects in each group attacking were calculated and compared using Fisher’s exact test of probability. In all cases, groups were considered significantly different when p<0.05.
RESULTS
Aggression
Maternal Aggression is Reduced in Avpr1b −/− Mice (Table 1)
Table 1.
Maternal aggression is significantly reduced in Avpr1b −/− females compared to their wild-type littermates.
| Vasopressin 1b Receptor Knockout Line
|
+/+
|
−/−
|
|---|---|---|
| Attack Latency (seconds±SEM) | 163±85 | 750±101* |
| Number of attacks/test | 4.7±0.9 | 0.7±0.5* |
| Percentage attacking by Test 1 | 90% | 20%* |
| Percentage attacking by Test 2 | 90% | 20%* |
| Percentage attacking by Test 3 | 90% | 20%* |
=significantly different from +/+ group.
The percentage of Avpr1b −/−females attacking an intruder male is significantly less than the percentage of Avpr1b +/+ females attacking (20% vs. 90%; p<0.05). The attack latency is significantly longer in knockout females compared to wild-type females in the maternal aggression test (749.9±101.0 vs. 163.4±84.5; U=10.0, p<0.05), as is the number of attacks (4.7±0.9 vs. 0.7±0.5; U=10.5, p<0.05). The numbers of tail rattles were not significantly different between the genotypes, but few tail rattles were observed (0.4±0.2 vs. 0.5±0.3, U=1.5, p>0.05).
Avpr1b −/− mice show more aggressive behavior in a competitive paradigm, but are still less aggressive than Avpr1b +/+ mice (Figure 1)
Figure 1.
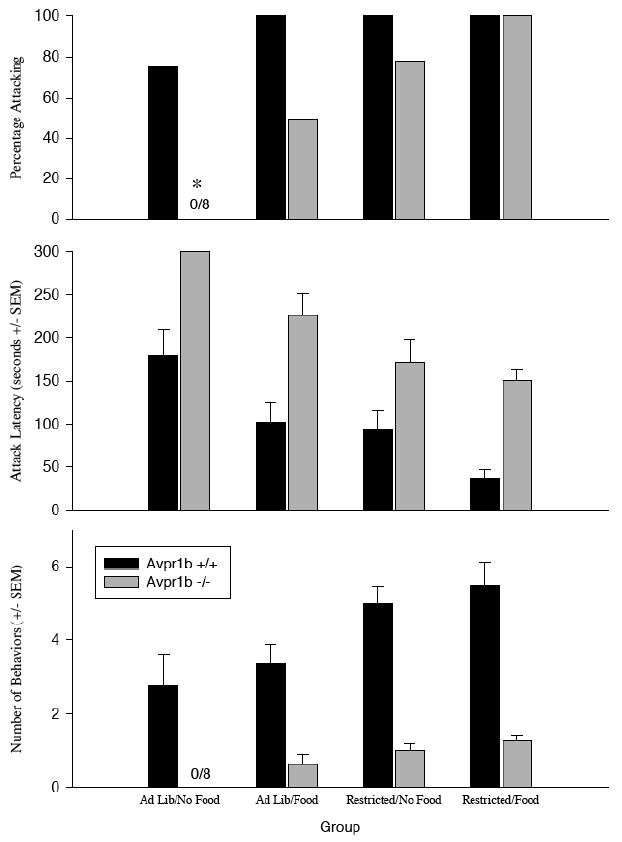
Aggression in Avpr1b +/+ and Avpr1b −/− mice in a competitive aggression paradigm. Ad lib = ad libitum food; Restricted = food deprived overnight before the test; No Food = no food present during aggression test; Food = food present during aggression test. Top Panel. The percentage of subjects attacking the stimulus animal in the single, three-minute test. The percentage of Avpr1b −/− mice in the AdLib/No Food group attacking was significantly lower than the other groups. There were no other group differences. (*=significantly lower than other groups, P<0.05) Center Panel. The mean attack latency (seconds ± SEM). There was a significant effect of genotype (Avpr1b +/+ mice attacked more quickly than Avpr1b −/− mice), restricted condition (restricted animals attacked faster than ad libitum-fed animals), and competition state (animals attacked faster when food was present during the test than when it was absent). There were no interactions among any of the factors. See Results for the statistics. Bottom Panel. The mean number of attack behaviors (± SEM) observed. There was a significant effect of genotype (Avpr1b +/+ mice exhibited more attack behaviors than Avpr1b −/− mice), food restriction (restricted animals showed more attack behavior than ad libitum-fed animals), and competition state (animals attacked more when food was present during the test than when food was absent). There were no interactions among any of the factors. See Results for the statistics.
There are main effects of genotype (F1,56=53.33, p<0.05), food restriction (F1,56= 34.87, p<0.05) and competition (F1,56=14.26, p<0.05) on the latency to attack. There are no interactions among any of the factors (p>0.10 for all). There are main effects of genotype (F1,56=109.4, p<0.05) and food restriction (F1,56= 20.84, p<0.05), but not competition (F1,56=2.32, p>0.10) on the number of attacks per test. There is a significant interaction between genotype and food restriction (F1,56=4.34, p<0.04) but not between any other factors (p>0.10 for all). The presence of food decreases the attack latency in Avpr1b +/+ animals, but not Avpr1b −/− animals. Although the presence of food during the test increases the percentage of knockout animals attacking, their levels of aggression are still significantly less than the levels displayed by their Avpr1b +/+ littermates. Fasting overnight increases aggression in both genotypes.
Avpr1b −/− mice show normal defensive avoidance behavior, but fail to show defensive attack behavior (Figure 2)
Figure 2.
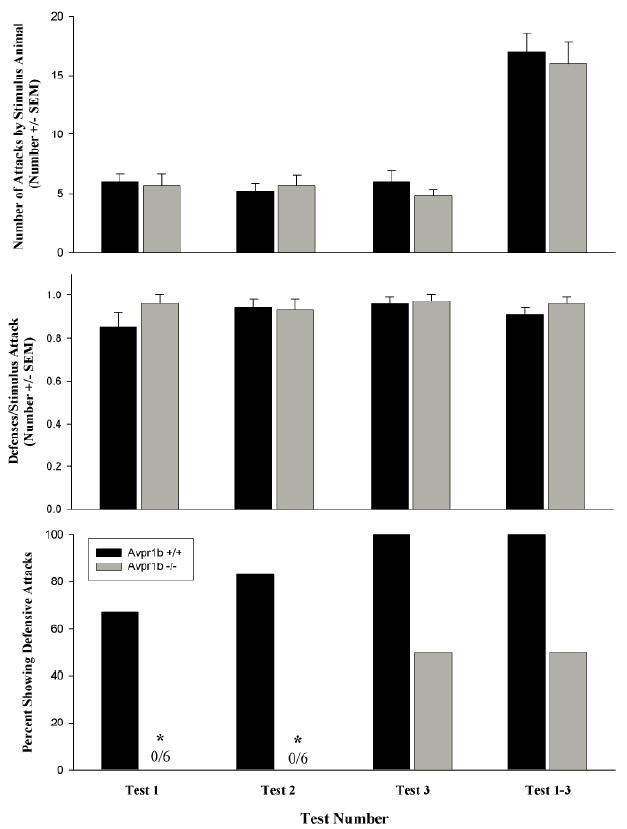
Defensive behavior in Avpr1b +/+ and Avpr1b −/− mice. Top Panel. The mean number (±SEM) of attacks on the subjects observed in each test and in Tests 1-3 combined. The stimulus animals attacked both genotypes equally. There were no significant differences among the groups. Center Panel. The mean number of defenses per stimulus attack (± SEM) observed in each test and in Tests 1-3 combined There is no effect of genotype or test number. Bottom Panel. The percentage of Avpr1b −/− subjects showing defensive attack behavior is significantly lower than the percentage of Avpr1b +/+ mice displaying defensive attacks in Test 1 and Test 2, but not in Test 3 or Tests 1-3 combined. (* is p<0.05).
All the subjects were attacked in each test, and all the subjects exhibited defensive behavior in response to the stimulus attack. There was no effect of genotype (F1,35=0.16, p>0.10) or test number (F1,35=0.23, p>0.10) and no interaction between the two (F1,35=0.68, p>0.10) on the number of attacks made by the stimulus animals on the subjects in each test or in Tests 1-3 combined (T=41.0, p>0.10) (Figure 2, top panel). There was no effect of genotype (F1,35=0.06, p>0.10) or test number (F2,35=0.52, p>0.10) and no interaction between the two (F2,35=1.09, p>0.10) on the number of bites made by the stimulus animals on the subjects in each test or in Tests 1-3 combined (T=38.0, p>0.10) The mean number of bites received (±SEM) was 5.7±0.7, 4.5±0.6, 5.7±0.8 and 15.0±1.7 for Avpr1b +/+ mice in Test 1, Test 2, Test 3, and Test 1-3, respectively. The mean number of bites received (±SEM) was 5.3±0.8, 5.3±0.7, 4.7±0.4 and 15.2±1.6 for Avpr1b −/− mice in Test 1, Test 2, Test 3, and Test 1-3, respectively. As shown in Figure 2 (center panel), there was no significant effect of genotype (F1,35=0.86, p>0.10),test number (F2,35=0.79, p>0.10), or an interaction between the two (F2,35=1.1, p>0.10) on the number of defensive behaviors per test in individual tests or in Tests 1-3 combined (T=30.0, p>0.05). A significantly lower percentage of Avpr1b −/− mice showed defensive attacks during Tests 1 and 2 (p<0.05) but not Test 3 or Tests 1-3 combined (p>0.10; Figure 2, bottom panel). There was a significant main effect of genotype (F1,35=34.1, p<0.01) and test number (F2,35=5.2, p<0.05), on the number of defensive attacks but there was no interaction between the two factors (F2,35=0.4, p>0.10). There was a significant difference in the total number of retaliatory attacks over tests 1-3 between Avpr1b +/+ and Avpr1b −/− mice (T=57.00, p<0.01). The mean number of retaliatory defensive attacks (±SEM) was 0.8±0.3, 1.2±0.3, 1.7±0.2 and 3.7±0.5 for Avpr1b +/+ mice in Test 1, Test 2, Test 3, and Test 1-3, respectively. The mean number of retaliatory attacks (±SEM) was 0.0±0.0, 0.0±0.0, 0.5±0.2 and 0.5±.2 for Avpr1b −/− mice in Test 1, Test 2, Test 3, and Test 1-3, respectively. Knockout mice do not engage in defensive attack behavior as often as wild-type littermates.
Prior experience in an agonistic interaction increases aggression in Avpr1b −/− mice, but does not bring the level of aggression up to that observed in Avpr1b +/+ mice (Figure 3)
Figure 3.
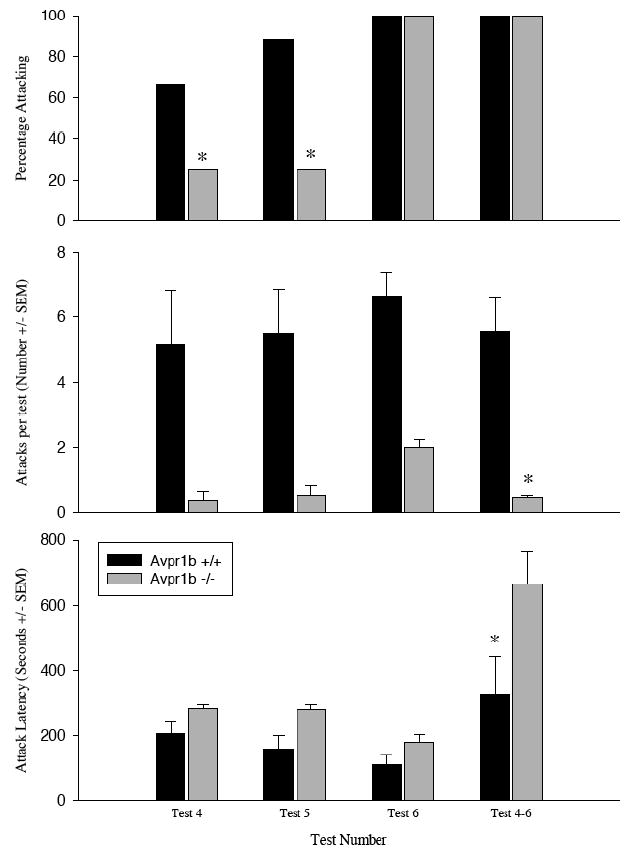
Offensive behavior in Avpr1b +/+ and Avpr1b −/− mice. Top Panel. The percentage of animals displaying offensive aggression. Center Panel. The number of attacks (± SEM) in test 4, 5, and 6 as well as the average number of attacks per test (± SEM) when the data are pooled across tests 4-6 (* = significantly lower than the other genotypes; p<0.05) Bottom Panel. The latency to attack (± SEM). Knockouts took significantly longer to attack than did wild-types. (* = significantly lower than Avpr1b +/+ group; p<0.05).
The percentage of Avpr1b +/+ mice attacking is significantly greater than the percentage of Avpr1b −/− mice in Tests 4 and 5 (p<0.05), but not in Test 6 or Tests 4-6 combined (p>0.10). There is a significant main effect of genotype (F1,36=50.14, p<0.01), but not test number (F2,36=2.05, p>0.10), on the number of behaviors observed. When the data are combined over Test 4-6, there is a significant difference between wild-type and knockout mice (T=69.00, p>0.01). There are significant main effects of genotype (F1,36=17.21, p<0.05) and test number (F2,36=8.23, p<0.01) on the attack latency within each test. There is no interaction between the two (F2,36=0.53, p>0.10). The latencies to first attack (combining all three tests) were significantly different between the genotypes (T=29.0, p<0.05).
Predatory Aggression is Intact in Avpr1b −/− Mice (Table 2)
Table 2.
Predatory aggression is intact in Avpr1b −/−males.
| Predatory Aggression Paradigm
|
+/+
|
−/−
|
|---|---|---|
| Percentage attacking by Test 1 | 75% | 81% |
| Percentage attacking by Test 2 | 100% | 100% |
| Percentage attacking by Test 3 | 100% | 100% |
| Attack Latency (seconds±SEM) Test 1 | 201±62 | 262±77 |
| Attack Latency (seconds±SEM) Test 2 | 118±47 | 101±21* |
| Attack Latency (seconds±SEM) Test 3 | 28±16* | 32±18* |
=significantly lower than attack latency in Test 1.
There are no differences between Avpr1b +/+ and Avpr1b −/− mice in the percentages of mice attacking or in the latencies to attack the crickets (p>0.10). There is no main effect of genotype (F1,20=2.85, p>0.10) on attack latency. However, there is a significant main effect of test number on the attack latency for both genotypes (F2,20=22.5, p<0.05). There is no interaction between the two factors (F2,20=1.10, p>0.10). In Avpr1b +/+ mice, the latency to attack is lower in Test 3 than in Test 1. In Avpr1b −/− mice, the latency to attack is lower in Tests 2 and 3 than in Test 1.
DISCUSSION
Disruption of the Avpr1b gene appears to alter aggression by specifically reducing attack behavior, but only that directed toward conspecifics. This conclusion is based on our current results as well as our previous reports showing that aggression is reduced in mice without a functional Avpr1b (Wersinger et al., 2003; Wersinger et al., 2004). In the current study we found that Avpr1b −/− mice show deficits in offensive aggression and maternal aggression. In the defensive behavior tests, Avpr1b −/− mice displayed normal avoidance behavior, but, unlike their Avpr1b +/+ littermates, fail to retaliate with an attack.
To quantify offensive and maternal aggression we measured the number of lunges, bites (which, comprised attacks when combined), and the number of tail rattles. Offensive attack behavior was significantly lower in Avpr1b −/− mice than Avpr1b +/+ mice. Not only did a lower percentage of Avpr1b −/− then Avpr1b +/+ mice display attack behavior, but those Avpr1b −/− mice that did display attack behavior had a longer latency to first attack and displayed fewer behaviors per test. This suggests that the Avpr1b −/− plays a role in the regulation of attack behavior that is similar in a broad range of interactions with a conspecific. In the offensive, but not maternal aggression test, Avpr1b −/− mice displayed fewer tail rattles than Avpr1b +/+ mice. It is unclear whether or not tail rattles are specifically related to attack behavior given that they are displayed in a variety or interactions, including those with the investigators (personal observation, HKC and SRW). They may be indicative of an animal perceiving a threat.
Although offensive and maternal aggression differ in many ways, there is mounting evidence to suggest that some of the neural circuitry mediating these behaviors is similar (Owen et al., 1974; Saal et al., 1976; DeBold and Miczek, 1981; Gammie et al., 2003), although it is possible these apparent similarities reflect a dearth of knowledge about the neural regulation of aggression. Both forms of aggression rely on olfactory cues, as animals with deficits in olfaction fail to display either form of aggression (Denenberg et al., 1973; Cain, 1974; Neckers et al., 1975). Here we show that both forms of aggression also rely on the presence of the Avpr1b. Recent studies demonstrating that the Avpr1b-specific antagonist, SSR149415, reduces aggression in mice (Griebel et al., 2003) and hamsters (Blanchard et al., 2005) support our findings. In both forms of aggression the offensive and defensive component can be dissociated pharmacologically (Parmigiani et al., 1998). A key question remaining is what is the mechanism by which the Avpr1b affects aggression. We propose that the Avpr1b couples the perception of social stimuli with the appropriate behavioral response. Others suggest that the reduction in aggression after antagonism of the Avpr1b may be secondary to reduced anxiety (Blanchard et al., 2005; Griebel et al., 2005; Serradeil-Le Gal et al., 2005). The two are not necessarily incompatible. However, plasma cortisol levels rise in Avpr1b −/− mice during exposure to a conspecific male as much as they do in Avpr1b +/+ mice (Wersinger et al., 2002). This suggests that Avpr1b −/− may still experience the interaction as threatening and stressful at some level.
The presence of normal predatory behavior in Avpr1b −/− mice is an important observation. This unequivocally demonstrates that Avpr1b −/− mice are capable of perceiving, identifying, and attacking a stimulus. When the stimulus is a conspecific, as is the case with offensive aggression, maternal aggression, and “retaliatory attacks,” Avpr1b −/− mice fail to attack. Although we tested predatory behavior in the light-phase of the light cycle, we observed robust behavior in both genotypes. Mice generally hunt at night, but we are unaware of an influence of the circadian rhythm on the display of predatory behavior.
Consistent with previous reports, our data suggest that defensive behavior is not a unitary phenomenon and that it must be divided into its component behaviors for detailed analysis (Blanchard et al., 1977; Miczek, 1983; Blanchard and Blanchard, 1989). To analyze the behavior in our mice, we split defensive behavior into 2 broad components: defensive avoidance and defensive attack behaviors. Although both are observed in response to an attack from the stimulus animal, we believe they are each mediated by different neural circuitry. Defensive avoidance, which we operationally define as flight or the assumption of a boxing pose, does not rely on the Avpr1b since knockouts continue to display these behaviors. However, defensive attacks, which we define as a bite or a lunge in immediate response to an attack by the stimulus animal (“retaliation”), require the Avpr1b since this behavior is almost completely absent in knockouts.
We are confident that the deficit in aggressive behavior cannot be explained by an inability to detect and respond to internal or external cues. Inputs carrying information about the physiological state of the animal or the environmental state into the neural circuitry underlying aggression are clearly intact. Confrontation with a conspecific elevates Fos-ir in olfactory areas and cortisol levels normally (Wersinger et al, 2002). Aggression can be modulated in knockout mice by food-deprivation, competition and social experience. Food deprivation (Miller et al., 1979; Brady et al., 1980; Kantak et al., 1980) and competition (Albert et al., 1988; Albert et al., 1989) increase aggression by altering general arousal, irritability, and motivation. Although Avpr1b −/− mice are still less aggressive than Avpr1b +/+ mice, these manipulations nevertheless increase aggression in Avpr1b −/− mice. We also observe increases in aggression in Avpr1b −/− mice over repeated testing. Again, this suggests that the subjects are capable of detecting and responding to cues in their environment and that deficits in the response to social stimuli can be overcome to a certain extent by strong physiological drives, such as hunger and mating.
Superficially, sexual behavior is present in Avpr1b −/− mice, suggesting there is not a global deficit in social behavior. We are currently analyzing sexual behavior in much greater detail to determine the extent to which it differs between wild-type and knockout animals. If there is a deficit, it is subtle given that we have no trouble breeding knockout animals (unpublished observations). In the clinical literature there are reports that severely autistic patients exhibit sexual motivation, albeit in an atypical manifestation (Konstantareas and Lunsky, 1997; Van Bourgondien et al., 1997; Realmuto and Ruble, 1999). Thus, sexual motivation and social motivation are dissociable. Perhaps sexual behavior, but not other social behaviors, activates a fundamental reward circuit independent of the Avpr1b (Melis and Argiolas, 1995; Pfaus et al., 2001; Holstege and Georgiadis, 2004; Paredes and Agmo, 2004).
There is no evidence through hybridization histochemistry or RT-PCR for expression of the Avpr1b in the mouse vomeronasal organ, olfactory epithelium or olfactory bulb (Young et al., in press). The determination of the distribution of the Avpr1b will provide an important foundation for understanding the circuitry involved and the role the Avpr1b plays in coupling those brain regions involved in the perception and processing of social cues with those that underlie the appropriate behavioral response.
Acknowledgments
We thank the NINDS and NIMH Animal Facilities in Buildings 36 and Building 14 for their exceptional care and flexibility in accommodating our many special requests. Drs. Judy Davis and James O’Malley provided excellent advice and we are grateful for their efforts in helping us solve logistical challenges (including working around the unavailability of VAF crickets). We also thank Melissa Stephens for technical assistance. This research was supported in part by the NIMH Intramural Research Program (Z01-MH-002498-16)
References
- Albers HE, Bamshad M. Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus) Prog Brain Res. 1998;119:395–408. doi: 10.1016/s0079-6123(08)61583-6. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Walsh ML, White R. Mouse killing induced by para-chlorophenylalanine injections or septal lesions but not olfactory bulb lesions is similar to that of food-deprived spontaneous killers. Behav Neurosci. 1985;99:546–554. doi: 10.1037//0735-7044.99.3.546. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Petrovic DM, Walsh ML. Competitive experience activates testosterone-dependent social aggression toward unfamiliar males. Physiol Behav. 1989;45:723–727. doi: 10.1016/0031-9384(89)90285-0. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Dyson EM, Walsh ML, Wong R. Defensive aggression and testosterone-dependent intermale social aggression are each elicited by food competition. Physiol Behav. 1988;43:21–28. doi: 10.1016/0031-9384(88)90093-5. [DOI] [PubMed] [Google Scholar]
- Alescio-Lautier B, Rao H, Paban V, Devigne C, Soumireu-Mourat B. Inhibition of the vasopressin-enhancing effect on memory retrieval and relearning by a vasopressin V1 receptor antagonist in mice. Eur J Pharmacol. 1995;294:763–770. doi: 10.1016/0014-2999(95)00632-x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Albers HE. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus) J Comp Neurol. 1996;369:252–263. doi: 10.1002/(SICI)1096-9861(19960527)369:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Horm Behav. 2001;40:51–64. doi: 10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(Suppl):S3–14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Takahashi T, Kelley MJ. Attack and defensive behaviour in the albino rat. Anim Behav. 1977;25:622–634. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V(1b) selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R. Chronic intracerebral infusions of vasopressin and vasopressin antagonist modulate social recognition in rat. Brain Res. 1992;572:261–264. doi: 10.1016/0006-8993(92)90480-w. [DOI] [PubMed] [Google Scholar]
- Brady K, Brown JW, Thurmond JB. Behavioral and neurochemical effects of dietary tyrosine in young and aged mice following cold-swim stress. Pharmacol Biochem Behav. 1980;12:667–674. doi: 10.1016/0091-3057(80)90146-x. [DOI] [PubMed] [Google Scholar]
- Cain DP. Olfactory bulbectomy: neural structures involved in irritability and aggression in the male rat. J Comp Physiol Psychol. 1974;86:213–220. doi: 10.1037/h0035933. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Nelson RJ. Interaction of nitric oxide and serotonin in aggressive behavior. Horm Behav. 2003;44:233–241. doi: 10.1016/j.yhbeh.2003.02.002. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 1998;119:409–414. doi: 10.1016/s0079-6123(08)61584-8. [DOI] [PubMed] [Google Scholar]
- de Wied D, Elands J, Kovacs G. Interactive effects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: mediation by a cerebral neurohypophyseal hormone receptor? Proc Natl Acad Sci U S A. 1991;88:1494–1498. doi: 10.1073/pnas.88.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBold JF, Miczek KA. Sexual dimorphism in the hormonal control of aggressive behavior of rats. Pharmacol Biochem Behav. 1981;14(Suppl 1):89–93. doi: 10.1016/s0091-3057(81)80015-9. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Gaulin-Kremer E, Gandelman R, Zarrow MX. The development of standard stimulus animals for mouse (Mus musculus) aggression testing by means of olfactory bulbectomy. Anim Behav. 1973;21:590–598. doi: 10.1016/s0003-3472(73)80021-1. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Higashihara F, Mishima K, Fukue Y, Takano Y, Tsujimoto G, Iwasaki K, Fujiwara M. V1a receptor knockout mice exhibit impairment of spatial memory in an eight-arm radial maze. Neurosci Lett. 2004;356:195–198. doi: 10.1016/j.neulet.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ludwig M, Landgraf R. Microdialysis administration of vasopressin and vasopressin antagonists into the septum during pole-jumping behavior in rats. Behav Neural Biol. 1992;58:51–57. doi: 10.1016/0163-1047(92)90907-l. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Pollock J, Albers HE, Leeman SE. Inhibition of flank-marking behavior in golden hamsters by microinjection of a vasopressin antagonist into the hypothalamus. Neurosci Lett. 1985;55:239–243. doi: 10.1016/0304-3940(85)90027-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Singer EA, Meenan DM, Albers HE. Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. Eur J Pharmacol. 1988;154:153–159. doi: 10.1016/0014-2999(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Rhodes JS, Girard I, Garland T., Jr Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior in mice. Horm Behav. 2003;44:209–221. doi: 10.1016/s0018-506x(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Garris DR. Aggression-associated changes in murine olfactory tubercle bioamines. Brain Res. 2003;963:150–155. doi: 10.1016/s0006-8993(02)03963-x. [DOI] [PubMed] [Google Scholar]
- Godwin J, Sawby R, Warner RR, Crews D, Grober MS. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav Evol. 2000;55:77–84. doi: 10.1159/000006643. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Gal CS, Soubrie P. Non-peptide vasopressin V1b receptor antagonists as potential drugs for the treatment of stress-related disorders. Curr Pharm Des. 2005;11:1549–1559. doi: 10.2174/1381612053764797. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Stemmelin J, Gal CS, Steinberg R. The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr Drug Target CNS Neurol Disord. 2003;2:191–200. doi: 10.2174/1568007033482850. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober MS, Sunobe T. Serial adult sex change involves rapid and reversible changes in forebrain neurochemistry. Neuroreport. 1996;7:2945–2949. doi: 10.1097/00001756-199611250-00029. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav. 2005;84:681–695. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR. The emotional brain: neural correlates of cat sexual behavior and human male ejaculation. Prog Brain Res. 2004;143:39–45. doi: 10.1016/S0079-6123(03)43004-5. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Hegstrand LR, Eichelman B. Dietary tryptophan modulation and aggressive behavior in mice. Pharmacol Biochem Behav. 1980;12:675–679. doi: 10.1016/0091-3057(80)90147-1. [DOI] [PubMed] [Google Scholar]
- Konstantareas MM, Lunsky YJ. Sociosexual knowledge, experience, attitudes, and interests of individuals with autistic disorder and developmental delay. J Autism Dev Disord. 1997;27:397–413. doi: 10.1023/a:1025805405188. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. resolving apparent contradictions concerning the relationships among fear or anxiety and aggression during lactation: theoretical comment on D’Anna, Stevenson, and Gammie (2005) Behav Neurosci. 2005;119:1165–1168. doi: 10.1037/0735-7044.119.4.1165. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson SC. Searching for candidate genes with effects on an agonistic behavior, offense, in mice. Behav Genet. 1996;26:471–476. doi: 10.1007/BF02359751. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Ethological analysis of drug action on aggression and defense. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:519–524. doi: 10.1016/0278-5846(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Altman JL, Appel JB, Boggan WO. Para-chlorophenylalanine, serotonin and killing behavior. Pharmacol Biochem Behav. 1975;3:355–361. doi: 10.1016/0091-3057(75)90043-x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miller BL, Pachter JS, Valzelli L. Brain tryptophan in isolated aggressive mice. Neuropsychobiology. 1979;5:11–15. doi: 10.1159/000117658. [DOI] [PubMed] [Google Scholar]
- Neckers LM, Zarrow MX, Myers MM, Denenberg VH. Influence of olfactory bulbectomy and the serotonergic system upon intermale aggression and maternal behavior in the mouse. Pharmacol Biochem Behav. 1975;3:545–550. doi: 10.1016/0091-3057(75)90170-7. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S. Aggression in knockout mice. Ilar J. 2000;41:153–162. doi: 10.1093/ilar.41.3.153. [DOI] [PubMed] [Google Scholar]
- Nikulina EM. Neural control of predatory aggression in wild and domesticated animals. Neurosci Biobehav Rev. 1991;15:545–547. doi: 10.1016/s0149-7634(05)80146-0. [DOI] [PubMed] [Google Scholar]
- Owen K, Peters PJ, Bronson FH. Effects of intracranial implants of testosterone propionate on intermale aggression in the castrated male mouse. Horm Behav. 1974;5:83–92. doi: 10.1016/0018-506x(74)90009-9. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73:179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P. Fluprazine inhibits intermale attack and infanticide, but not predation, in male mice. Neurosci Biobehav Rev. 1991;15:511–513. doi: 10.1016/s0149-7634(05)80141-1. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Ferrari PF, Palanza P. An evolutionary approach to behavioral pharmacology: using drugs to understand proximate and ultimate mechanisms of different forms of aggression in mice. Neurosci Biobehav Rev. 1998;23:143–153. doi: 10.1016/s0149-7634(98)00016-5. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Rodgers RJ, Palanza P, Mainardi M, Brain PF. The inhibitory effects of fluprazine on parental aggression in female mice are dependent upon intruder sex. Physiol Behav. 1989;46:455–459. doi: 10.1016/0031-9384(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realmuto GM, Ruble LA. Sexual behaviors in autism: problems of definition and management. J Autism Dev Disord. 1999;29:121–127. doi: 10.1023/a:1023088526314. [DOI] [PubMed] [Google Scholar]
- Rothballer AB. Aggression, defense and neurohumors. UCLA Forum Med Sci. 1967;7:135–170. [PubMed] [Google Scholar]
- Saal FS, Gandelman R, Svare B. Aggression in male and female mice: evidence for changed neural sensitivity in response to neonatal but not adult androgen exposure. Physiol Behav. 1976;17:53–57. doi: 10.1016/0031-9384(76)90269-9. [DOI] [PubMed] [Google Scholar]
- Semsar K, Kandel FL, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, 3rd, Tonnerre B, Roux R, Garcia G, Griebel G, Aulombard A. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev. 2005;11:53–68. doi: 10.1111/j.1527-3458.2005.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Lukovic L, Salome N, Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Cremer H, Schachner M. Increased intermale aggression and neuroendocrine response in mice deficient for the neural cell adhesion molecule (NCAM) Eur J Neurosci. 1997;9:1117–1125. doi: 10.1111/j.1460-9568.1997.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Svare B. Testosterone propionate inhibits maternal aggression in mice. Physiol Behav. 1980;24:435–439. doi: 10.1016/0031-9384(80)90232-2. [DOI] [PubMed] [Google Scholar]
- Van Bourgondien ME, Reichle NC, Palmer A. Sexual behavior in adults with autism. J Autism Dev Disord. 1997;27:113–125. doi: 10.1023/a:1025883622452. [DOI] [PubMed] [Google Scholar]
- Welch AS, Welch BL. Effect of stress and para-chlorophenylalanine upon brain serotonin, 5-hydroxyindoleacetic acid and catecholamines in grouped and isolated mice. Biochem Pharmacol. 1968;17:699–708. doi: 10.1016/0006-2952(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Molecular Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Christiansen M, Hu SB, Gold PW, Young WS., 3rd Aggression is reduced in vasopressin 1b receptor null mice but is elevated in vasopressin 1a receptor null mice. Society for Neuroscience Annual Meeting; New Orleans, LA. 2003. [Google Scholar]
- Wersinger SR, K RK, Zufall F, Lolait SJ, O’Carroll AM, Young WS., 3rd Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]


