Abstract
Many treatments for neuropathic pain activate or augment norepinephrine release in the spinal cord, yet these treatments are less effective against acute nociceptive stimuli. We previously showed in mice that peripheral nerve injury results in sprouting of spinal noradrenergic fibers, possibly reflecting the substrate for this shift in drug efficacy. Here we tested whether such sprouting also occurs in rats after nerve injury and examined one signal for such sprouting. Ligation of L5 and L6 spinal nerves unilaterally in rats resulted in hypersensitivity to tactile stimulation of the ipsilateral paw, and sprouting of noradrenergic fibers in the dorsal horn of the lumbar spinal cord. Brain derived nerve growth factor (BDNF) content increased in L4–L6 dorsal root ganglia ipsilateral to injury and in lumbar spinal cord following nerve injury, and intrathecal infusion of BDNF antiserum prevented spinal noradrenergic sprouting. This treatment also prevented the increased analgesic efficacy of intrathecal clonidine observed after nerve injury. Intraspinal injection of BDNF in non-injured rats mimicked the sprouting of spinal noradrenergic fibers seen after nerve injury. These results suggest that increased BDNF synthesis and release drives spinal noradrenergic sprouting following nerve injury, and that this sprouting may paradoxically increase the capacity for analgesia in the setting of neuropathic pain from drugs which utilize or mimic the noradrenergic pathway.
Keywords: BDNF, Neuropathic pain, Noradrenergic, Spinal cord
1. Introduction
Neuropathic pain, associated with traumatic, metabolic, or oncologic injury to peripheral nerves, is often accompanied by hyperalgesia and allodynia, and often exhibits poor response to traditional analgesics. In rodents, various types of peripheral nerve injury result in hypersensitivity to mechanical and thermal stimuli and are associated with a plethora of changes in both injured primary sensory afferents and their uninjured neighbors, which are responsible for the central sensitization in the spinal cord (for review, see (Woolf and Salter 2000). Plasticity following nerve injury not only drives mechanisms of central sensitization, but also alters analgesic drug efficacy. For example, two approved drugs to treat neuropathic pain, oral duloxetine and gabapentin, exhibit low or no efficacy against acute noxious stimuli, but relieve hypersensitivity in animals after nerve injury and relieve pain in patients with neuropathic pain (Rowbotham et al. 1998;Goldstein et al. 2005). Each of these treatments activates or augments, release of norepinephrine, an inhibitory neurotransmitter in the spinal cord of animals (Iyengar et al. 2004;Tanabe et al. 2005) and humans (Hayashida et al. 2007). Additionally, intrathecal clonidine, which mimics the effects of spinally released norepinephrine, demonstrates increased potency and efficacy in neuropathic pain states (Eisenach et al. 1995). We previously showed in mice that peripheral nerve injury results in sprouting of noradrenergic fibers in the spinal cord (Ma and Eisenach 2003), possibly reflecting a substrate for increased efficacy of these analgesic treatments. The goal of this study was to determine the signal for noradrenergic sprouting after nerve injury and its importance to anti-hypersensitivity from clonidine, an approved treatment for neuropathic pain.
Unlike postganglionic sympathetic noradrenergic neurons, which express tropomyosine receptor kinase A (trkA) and respond to nerve growth factor (NGF), spinal noradrenergic fibers, which descend from locus coeruleus and adjacent nuclei, express trkB and respond to brain-derived neurotrophic factor (BDNF) (King et al. 1999). We therefore focused on this factor as a stimulus to spinal noradrenergic sprouting. Studies quantified the effect of peripheral nerve injury on dorsal root ganglion and spinal cord content of BDNF, blockade of BDNF action in the spinal cord after injury, and effect of exogenous BDNF on spinal noradrenergic fiber density in the absence of injury.
2. Methods
2.1. Animals, surgery, and behavioral assessments
Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN, USA), weighing 200–250 g, were used. All studies confirmed to the Wake Forest University Guidelines on the ethical use of animals, and studies were performed under Animal Care and Use Committee approval. Animals were housed under a 12-h light-dark cycle, with food and water ad libitum.
Spinal nerve ligation (SNL) was performed as previously described (Kim and Chung 1992). In brief, animals were anesthetized with inhalational halothane (2%) in oxygen, the lateral laminae of lower lumbar and upper sacral vertebrae were exposed, the right L6 transverse process was removed and the right L5 and L6 spinal nerves were identified and tightly ligated using 6-0 silk suture. Then the wound was closed. Two types of controls were used in this study: normal rats without surgery and sham operated rats with anesthesia and surgical exposure without ligation of the right L5 and L6 spinal nerves.
Hypersensitivity to light touch following SNL was confirmed using calibrated von Frey filaments (Stoelting, Wood Dale, IL) applied to the plantar surface of the paw. Filaments were applied to the bending point for 5 sec, and a brisk paw withdrawal was considered a positive response. Withdrawal threshold was determined using an up-down statistical method (Chaplan et al. 1994). To determine the effect of intraspinal injection of BDNF on withdrawal threshold, we utilized withdrawal threshold to paw pressure, which is sensitive to demonstrate either hypersensitivity or hyposensitivity (Randall and Selitto 1957). For this the rat hindpaw was placed between a plinth and a conical piece of plastic, with increasing pressure applied until the rat withdrew the paw. A pressure of 250 g was not exceeded in order to avoid tissue injury.
Some animals were anesthetized with halothane (2%) for insertion of an intrathecal catheter as previously described (Yaksh and Rudy 1976). Animals were placed prone in a stereotaxic frame and a small incision was made at the back of the neck. A small puncture was made in the atlanto-occipital membrane of the cisterna magnum and a polyethylene catheter, 8.5 cm, was inserted so that the caudal tip reached the lumbar enlargement of the spinal cord. The rostral end of the catheter was exteriorized at the top of the head and the wound was closed with sutures. After implantation of the intrathecal catheters, rats were housed individually with free access to food and water. Animals were allowed at least 5 days to recover from the surgery. No animal showed signs of motor dysfunction (fore limb or hind limb paralysis) in this study.
2.2. Anti-BDNF treatment
Antibody to BDNF (sheep polyclonal BDNF antibody, 5 µg/day, Chemicon, Temecula, CA; n=6) or control sheep IgG (5 µg/day, Sigma, St. Louis, MO; n=7) were dissolved in sterile saline and injected into osmotic minipumps (0.5µl/h, model 2002, Alzet, Cupertino, CA). One day before SNL surgery, animals were anesthetized and the osmotic minipumps were implanted subcutaneously on the back of the rat and connected to the intrathecal catheter. After 11 days of spinal infusion, both IgG and BDNF antibody infused rats received a single intrathecal (i.t.) injection of clonidine (15 µg). Before and 1h after each intrathecal clonidine injection, the withdrawal threshold of both hindpaws were tested, using von Frey filaments as described above.
2.3. Intraspinal BDNF treatment
To determine the role of exogenous BDNF on noradrenergic fiber density in the spinal cord, 6 rats were randomized to receive either recombinant human BDNF (R&D systems, Minneapolis, MN, 150 ng/300 nL) or vehicle (0.1% BSA-PBS, 300 nL) by intraspinal injection. Animals were anesthetized with halothane and the right dorsal surface of the mid lumbar spinal cord exposed via a small laminotomy. Either BDNF or vehicle was filled in a glass micropipette (20–30 µm tip diameter) and injected at 500 µm from the midline and 1 mm under the surface of the right dorsal spinal cord using a micro injector (Nanojector II, Drummond Scientific Co., Broomall, PA). The surgical site was closed in layers. Five days later, animals were deeply anesthetized, perfused intracardially with fixative and the spinal cord removed for immunohistochemical study. Sections of spinal cord approximately 1.0 mm rostral to the injection site used for analysis.
2.4 Norepinephrine and BDNF assays
Norepinephrine content of spinal cord was determined as previously described (Zhu et al. 2002). Normal (n=7) and SNL animals (n=8 at day 3, n=9 at day 10 after SNL surgery) were killed by deep halothane anesthesia followed by decapitation. The spinal cord was quickly removed and the left and right lumbar enlargement were rapidly dissected on ice, then frozen in dry ice cooled 2-methylbutane. After treatment with 0.1 M perchloric acid, the spinal tissues were homogenized on ice and centrifuged. Supernatants were collected and norepinephrine content was measured by high-pressure liquid chromatography with electrochemical detection (Eisenach et al. 1992).
BDNF content of tissues were determined using an electrochemiluminescent immunoassay (Blackburn et al. 1991) as previously described in detail (Frost et al. 2001). Briefly, dorsal root ganglia (DRG) and spinal cord from the thoracic level (T1–3) and the lumbar enlargement were collected from normal (n=4), sham operated (n=4, 3 days after sham surgery), and SNL animals (n=5 at day 3, n=5 at day 10 after SNL) and then frozen as described above. Spinal cord and DRG tissues were homogenized and centrifuged. Supernatants were collected and BDNF content was measured with an Origen electrochemiluminescent analyzer (Igen, Inc., Gaithersburg , MD).
2.5.1. Immunohistochemistry
Under deep anesthesia with sodium pentobarbital (100 mg/kg), rats were perfused intracardially with cold phosphate buffered saline (PBS) containing 1% sodium nitrite and subsequently with 4% paraformaldehyde in 0.1M phosphate buffer (PB, pH 7.4). The L4–6 spinal cord segments and L4–6 DRGs were removed and postfixed in the same fixative for 3 hours. Then all tissues were transferred to 30% sucrose in 0.1M PB at 4°C for cryoprotection. Tissues were cut on a cryostat at a 40 and 16-µm thickness, respectively for spinal cord and DRG. For dopamine β hydroxylase (DβH) staining in spinal cord, spinal sections were pretreated with 0.3% H202 and 1.5% normal goat serum (NGS, Vector, Burlingame, CA) and then incubated for 24 hours at 4°C with a mouse monoclonal anti-DβH antibody (1:1,000, Chemicon International Inc., Temecula, CA, USA) diluted in PBS containing 0.3% Triton-X 100 (PBS+T) and 1.5% NGS. Subsequently, the sections were incubated in biotinylated goat anti-mouse IgG (Vector) and further processed using Elite Vectastain ABC kit (Vector) according to the instructions of the manufacturer. Finally, the immunoprecipitates were developed by 3,-3’ diaminobenzedine, and the chromogen was enhanced by the glucose oxidase-nickel method.
2.5.2. Quantification
For spinal DβH image analysis, four L4 and L5–6 spinal cord sections randomly selected from each rat were used. Images of both ipsilateral and contralateral dorsal horns of SNL or normal rats were captured using a digital CCD camera. A square with a fixed area (250 µm × 250 µm) covering the region of laminae I to II was positioned in the middle one third of the mediolateral extent of the dorsal horn. Using image analysis software (SigmaScan, Jandel Scientific Inc., San Rafael, CA, USA), the images of DβH-immunoreactive (-IR) axons within this area were quantified based on a constant threshold of optical density. The number of pixels occupied by IR axons was measured automatically.
2.6. Statistical analysis
Data are presented as mean ± SEM. Differences among groups for withdrawal threshold and norepinephrine and BDNF content were determined using un-paired t-test or where appropriate, one-way ANOVA followed by Fisher’s post-hoc test for multiple comparisons. Significance level was set at p< 0.05.
3. Results
SNL resulted in a decreased withdrawal threshold of the ipsilateral paw (1.8 ± 0.9 g) compared to pre-surgery values (20.2 ± 1.6 g), accompanied by guarding of the hindlimb ipsilateral to surgery, as described previously (Kim and Chung 1992). Behavior was otherwise normal in all animals throughout the study, with normal grooming and food intake.
3.1. DβH-IR fibers sprout in the spinal dorsal horn 3–10 days after SNL
In the dorsal horn of L4–L6 spinal cord of both normal and SNL animals, numerous DβH-IR axons were observed distributing throughout the dorsal horn (Figure 1). Numerous axonal varicosities along these axons were easily seen. Ten days after SNL surgery, DβH-IR axons in the dorsal horn were obviously more abundant, both ipsi- and contralateral to nerve lesion, than those of normal rats (Figure 1). Quantitatively, the mean number of pixels in a given area occupied by DβH-IR axons in the ipsilateral and contralateral dorsal horn at the L5–L6 level 10 days after SNL surgery were significantly higher than those of normal rats (Table 1). In contrast, DβH-IR axons were only increased ipsilateral to injury at the L4 level in SNL rats at this time compared to controls (Table 1).
Figure 1.
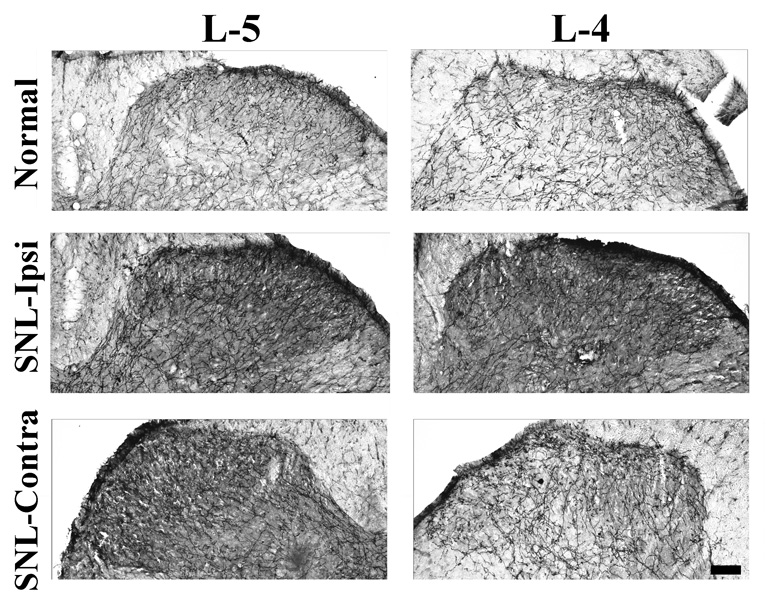
Photomicrographs of DβH-IR axons at the L4 (right panels) and L5 (left panels) spinal dorsal horn of a normal rat (top panels) or 10 days after spinal nerve ligation, ipsilateral to injury (SNL-ipsi, middle panels) and contralateral to injury (SNL-contra, lower panels). Numerous DβH-IR axons are observed throughout the dorsal horn, particularly in laminae I, II and V. Note that abundant axonal varicosities are distributed along DβH-IR axons. Scale bar = 100 µm.
Table 1.
Effect of spinal nerve ligation (SNL) on DβH-IR density in the dorsal horn 10 days after surgery
| Normal (n=4) | SNL-ipsilateral (n=6) | SNL-contralateral (n=6) | |
|---|---|---|---|
| L4 | 18 ± 0.62 | 28 ± 2.1*+ | 22 ± 2.5 |
| L5/L6 | 19 ± 0.26 | 30 ± 2.0+ | 30 ± 1.6+ |
Values are mean ± SEM of pixels/1,000 in a fixed area of 250 µm × 250 µm.
P<0.05 compared to saline
P<0.05 compared to contralateral side
To confirm that DβH immunostaining reflected increased norepinephrine content after SNL, we quantified norepinephrine in spinal cord tissue in normal rats and in those 3 and 10 days after SNL (Table 2). At 10 days after SNL, when there was a bilateral increase in DβH fiber density at L5 and L6, but unilateral at L4, there was an increased norepinephrine content in homogenates from blocks of L4–L6 tissue (p<0.05). At 3 days after SNL, norepinephrine content was increased ipsilateral to injury compared to normal animals (p<0.05).
Table 2.
Effect of spinal nerve ligation (SNL) on norepinephrine content in the dorsal horn
| Ipsilateral | Contralateral | |
|---|---|---|
| Normal (n=8) | 790 ± 22 | 750 ± 27 |
| SNL Day 3 (n=8) | 910 ± 40* | 840 ± 34 |
| SNL Day 10 (n=9) | 1000 ± 40* | 1000 ± 54* |
Values are mean ± SEM of norepinephrine in pg/mg wet tissue weight
P<0.05 compared to normal
3.2. BDNF expression increases in spinal cord dorsal horn and DRG after SNL, coincident with noradrenergic sprouting
BDNF content was greatly increased in L5–L6 (injured) DRGs from SNL animals 3 days after injury compared to normal and sham groups (p<0.05, Figure 2A). BDNF content numerically decreased by a non-significant amount on Day 10 compared to Day 3 after SNL in L5–L6 DRGs. In contrast, BDNF content significantly increased from Day 3 to Day 10 in L4 (uninjured) DRG from SNL animals (p<0.05). In L6 DRGs contralateral to nerve injury 10 days after SNL and DRGs from sham (3 days after sham surgery) animals, smaller but significant increases in BDNF content were also observed (p<0.05, Figure 2A).
Figure 2.
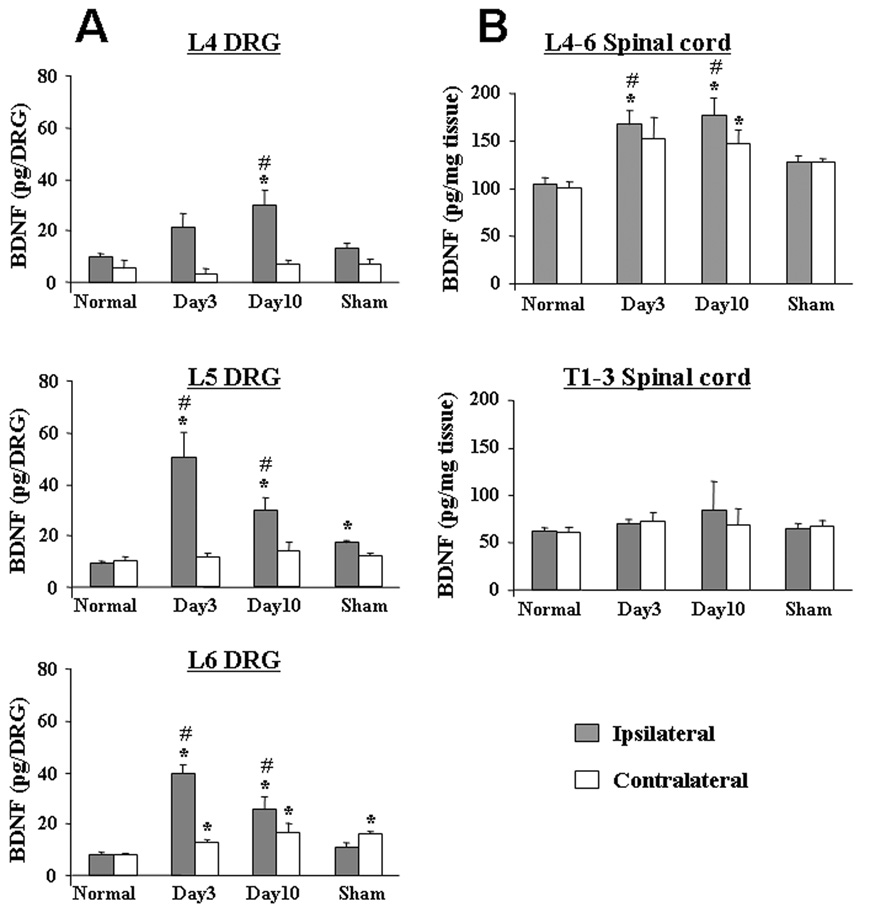
BDNF content in DRG (A) and spinal cord (B) from SNL, normal and sham-operated animals. BDNF content in L4–6 DRG, L4–6 level and T1–3 level of spinal cord were measured from normal (n=4), SNL (3, 10 days after SNL, n=5), and sham-operated (n=4) animals. *p<0.05 vs. normal group, #p<0.05 vs. sham-operated group.
At 10 days after SNL, a time when DβH fiber density (L5 and L6) and norepinephrine content (tissue block of L4–L6) were increased bilaterally, there was also a bilateral increase in BDNF content in tissue blocks of L4–L6 (Figure 2B; p<0.05). Additionally, at 3 days after SNL, a time when norepinephrine content in L4–L6 spinal cord blocks increased only ipsilateral to injury, BDNF content also increased in L4–L6 spinal cord blocks ipsilateral to injury (Figure 2B; p<0.05). No significant change in BDNF content was observed in lumbar spinal cord from sham animals or thoracic spinal cord from any group of animals.
3.3. BDNF is necessary to the development of DβH-IR axon sprouting in the dorsal horn induced by SNL
Animals tolerated intrathecal infusion of the antibody to BDNF with no change in spontaneous behavior. Ten days after SNL, hypersensitivity to von Frey filament testing in nerve ligated animals was not affected by BDNF antibody-infused group compared to IgG-infused group (3.9 ± 0.8 g in IgG-infused group, n=8, 3.8 ± 0.4 g in BDNF antibody-infused group, n=7). However, the efficacy of 15 µg intrathecal clonidine to increase withdrawal threshold was reduced in the BDNF antibody-infused group (12.1 ± 2.3 g) compared to the IgG-infused group, (21.3 ± 0.8 g, p<0.05). BDNF antibody dramatically reduced DβH-IR axons in the spinal cord (n=7) compared to IgG-infused group (n=8) (Figure 3A,B). This effect was observed at both L4 (uninjured) and L5–L6 (injured) levels of the spinal cord (Figure 3C).
Figure 3.
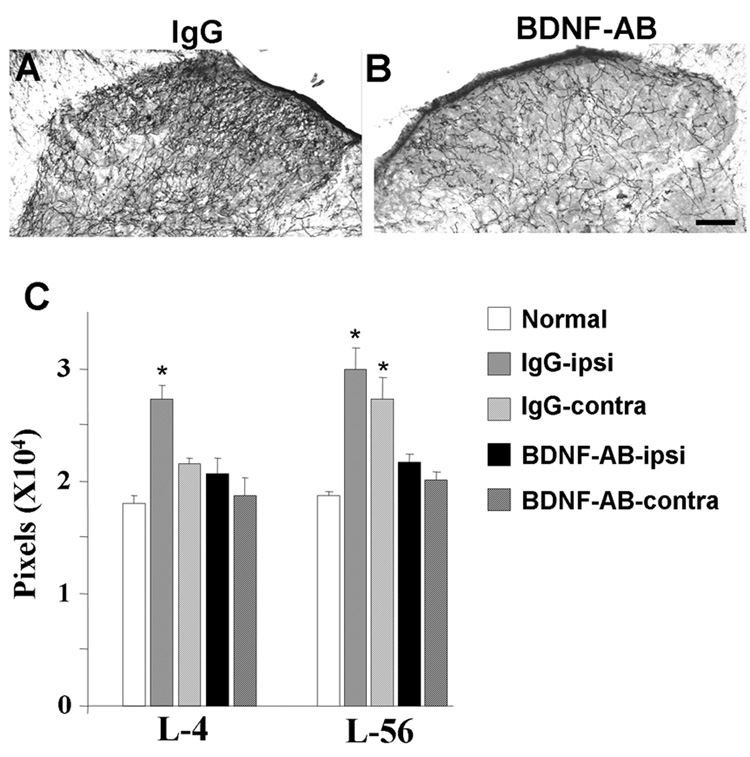
Effect of spinal infusion of BDNF antibody in DβH-IR axons in the spinal dorsal horn 10 days following SNL. (A, B) Photomicrographs of DβH-IR axons in the ipsilateral spinal dorsal horn of IgG-infused (IgG, A) and BDNF antibody-infused (BDNF-AB, B) SNL animals. Scale bar = 100 µm. (C) Quantification (mean±SEM) of DβH-IR axons in normal, in the contralateral (contra) and ipsilateral (ipsi) spinal dorsal horn from normal (n=4), IgG-infused (n=8) and BDNF-AB infused (n=7) animals. *p<0.05 vs. normal.
3.4. Exogenous BDNF increases spinal cord noradrenergic fiber density in normal rats
All animals recovered from intraspinal injection and fed, groomed, and ambulated normally afterwards. Intraspinal injection resulted in hypersensitivity to mechanical pressure on the hindpaw to a similar extent in both vehicle treatment (withdrawal threshold 146 ± 5 g before and 94 ± 12 g 5 days after intraspinal injection) and BDNF treatment (withdrawal threshold 156 ± 13 g before and 92 ± 7 g 5 days after intraspinal injection). DβH fibers were more numerous in the dorsal spinal cord ipsilateral to injection of BDNF compared to vehicle injected animals (Figure 4). Quantification confirmed these observations, with the number of pixels above threshold with DβH immunostaining in a fixed area greater in BDNF injected than vehicle injected animals (14,200 ± 2,600 pixels versus 6,400 ± 180 pixels, respectively; P < 0.01).
Figure 4.
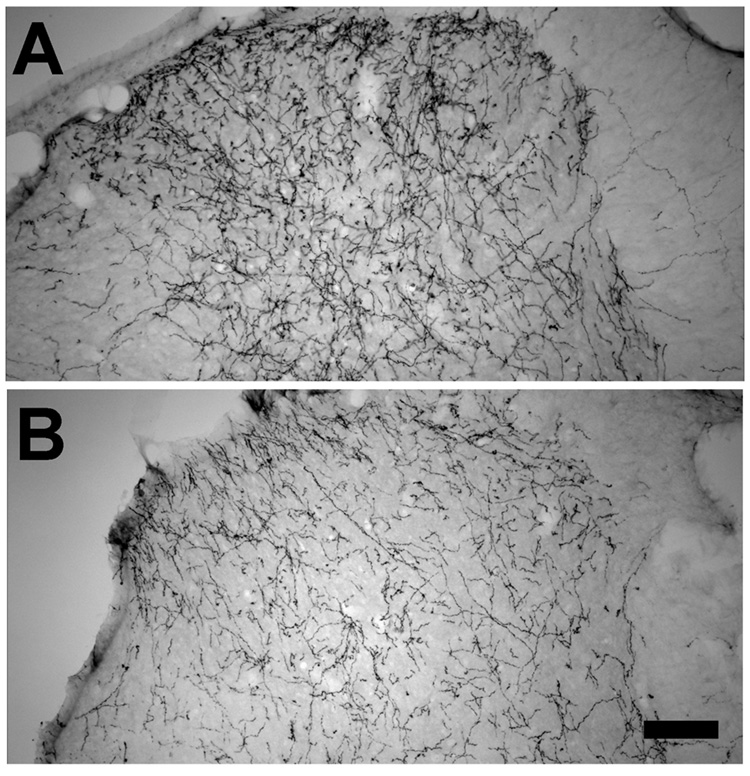
Effect of intraspinal injection of BDNF (A) or vehicle (B) 5 days prior to study on DβH-IR axons in the spinal dorsal horn. Scale bar = 100 µm.
4. Discussion
Neuropathic pain remains an unmet medical need, with approved medications providing meaningful pain relief in only a minority of patients (Finnerup et al. 2005). Interestingly, the most commonly used treatments, including epileptics and antidepressants, as well as neuraxial clonidine, target or mimic noradrenergic systems. Gabapentin can directly reduce responses in injured primary afferents after local application (Yang et al. 2005). In contrast to this peripheral or spinal effect, the anti-hypersensitivity effect of gabapentin after oral administration reflects primarily an action in the brain to activate descending noradrenergic pathways, and its behavioral effect is blocked by systemic injection of adrenoceptor antagonists in rats (Tanabe et al. 2005). Oral gabapentin increases norepinephrine concentrations in cerebrospinal fluid of humans with chronic pain (Hayashida et al. 2007), consistent with a relevance of this mechanism to the clinic. Additionally, antidepressant efficacy against neuropathic pain correlates stronger with their ability to block the norepinephrine transporter than other monoamine transporters (McQuay et al. 1996), and neuraxial clonidine is more potent to treat hypersensitivity states in humans than acute nociceptive stimuli (Eisenach et al. 2000). These results are paradoxical to observations which suggest that descending noradrenergic inhibition is reduced in neuropathic pain (Ren and Dubner 2002). The current study, while not addressing tonic noradrenergic activity, provides an explanation for the increased efficacy of these treatments which activate, augment, or mimic descending noradrenergic inhibition and identifies a signal for this plasticity.
4.1. BDNF is necessary to the SNL-induced noradrenergic fiber sprouting
BDNF regulates survival and differentiation of neurons not only during development but also in adulthood, including plasticity processes related to pain. Noxious stimulation results in release of BDNF and consequent phosphorylation of trkB in the spinal dorsal horn, which enhances NMDA receptor-induced depolarization in the dorsal horn neurons (Kerr et al. 1999). Although there is a consensus that BDNF expression increases in DRG neurons and spinal cord after nerve injury, its role in neuropathic pain is unclear. Thus, blockade of BDNF signaling by intrathecally administered antibodies to BDNF or trkB, a tyrosine kinase inhibitor, or a trkB-neutralizing receptor bodies has been reported to reduce neuropathic hypersensitivity (Yajima et al. 2002). In contrast, conditional BDNF gene knock-out fails to reduce neuropathic hypersensitivity (Zhao et al. 2006), and intrathecal injection of BDNF-producing cells attenuates rather than exacerbates neuropathic hypersensitivity (Cejas et al. 2000). Others have shown that intrathecal injection of BDNF-producing microglia generates hypersensitivity and that established sciatic compression-induced hypersensitivity is acutely reduced by an intrathecal bolus injection of anti-BDNF antibody (Coull et al. 2005). In contrast, we observed no greater effect of hypersensitivity from intraspinal BDNF injection than from vehicle, and chronic anti-BDNF antibody failed to prevent the generation of hypersensitivity from spinal nerve ligation. These varying results likely reflect the highly different experimental conditions of the current study and that focused on microglia (Coull et al. 2005).
On the other hand, the current study strongly supports a role for BDNF in spinal noradrenergic sprouting after nerve injury. Noradrenergic innervation of the spinal cord derives primarily from the locus coeruleus. These cells are known to possess trkB (King et al. 1999), and to depend on BDNF signaling for their innervation of frontal cortex (Matsunaga et al. 2004).
There was a similar bilateral increase in BDNF and norepinephrine content in the spinal cord 10 days after nerve injury in the current study, accompanied by a bilateral increase in noradrenergic fiber density at the L5 and L6 dermatomes. Similarly, there was a parallel unilateral increase in BDNF and norepinephrine content in the spinal cord ipsilateral to injury at an earlier time, 3 days after surgery. Additionally, intrathecal infusion of BDNF antibody beginning just prior to injury prevented noradrenergic sprouting. Taken together, these results support increased spinal release of BDNF after injury to drive noradrenergic sprouting.
BDNF in the spinal cord as a signal for local sprouting of noradrenergic fibers is supported by the current observation that intraspinal injection of BDNF induces sprouting ipsilateral to injection in normal animals. Noradrenergic neurons are found in the spinal cord of mice (Ma and Eisenach 2003), and it is conceivable that local release of BDNF in the spinal cord induces sprouting in this species by direct actions on these cells. In rats, as in humans, noradrenergic innervation of the spinal cord derives exclusively from extrinsic cells, mostly in the locus coeruleus, and one might question whether local action of BDNF in the spinal cord, far from these cells, would cause local sprouting. The current study in normal rats indicates that locally applied BDNF does indeed result in such local sprouting.
4.2. Noradrenergic sprouting is necessary for intrathecal clonidine efficacy after nerve injury
The potency and efficacy of intrathecal clonidine increases in both animals (Puke and Wiesenfeld-Hallin 1993) and humans (Eisenach et al. 2000) to alleviate hypersensitivity as compared to inhibit response to acute nociception. Several potential causes for this plasticity have been demonstrated in animals, including increased expression of inhibitory α2-adrenoceptors on calcitonin gene related peptide expressing afferents (Eisenach et al. 2005), increased α2-adrenoceptor mediated activation of inhibitory cholinergic interneurons (Obata et al. 2005), increased G protein coupling efficiency of spinal α2-adrenoceptors (Bantel et al. 2005), and an increased reliance on α2nonA-adrenoceptor subtypes (Duflo et al. 2002). The current study suggests that these changes in α2-adrenoceptor location or function may reflect injury induced noradrenergic sprouting. As such intrathecally administered BDNF antibody decreased both noradrenergic sprouting and efficacy of intrathecal clonidine. Peptides which are co-released by noradrenergic fibers, especially galanin and neuropeptide Y, are known to alter noradrenergic neuronal function and α2-adrenoceptor expression and function (Slonimsky et al. 2003;Costoli et al. 2005), and altered quantity or location of such peptide release following noradrenergic sprouting could explain the plasticity in response to clonidine after nerve injury. Alternatively, it is conceivable that increased BDNF signaling could alter clonidine responsiveness.
4.3. Source of spinal BDNF
The most likely sources of BDNF in the spinal cord include the terminals of primary afferents and resident glia. In normal animals, BDNF is found mainly in small to medium size DRG neurons (Ha et al. 2001). SNL increases NGF mRNA in injured DRG neurons and increases NGF content in adjacent uninjured DRG neurons in rats (Shen et al. 1999;Fukuoka et al. 2001), and NGF signaling through trkA increases BDNF expression after injury (Karchewski et al. 2002). It is conceivable that this increased BDNF in primary afferents drives noradrenergic sprouting in the spinal cord. Against this argument, however, is the unilateral increase in BDNF content in DRG tissue ipsilateral to injury, yet a bilateral increase in BDNF content in spinal cord. Alternatively, SNL induces bilateral activation of microglia in the spinal cord (Colburn et al. 1999), and activated microglia produce BDNF (Coull et al. 2005). Although the current study did not distinguish the source of BDNF, it demonstrates, by 3 lines of evidence, that increased action of this neurotrophin likely drives noradrenergic sprouting in the spinal cord after nerve injury.
In summary, SNL induces noradrenergic fiber sprouting in the spinal cord in which BDNF plays a pivotal role. Interfering with BDNF signaling blocks not only noradrenergic fiber sprouting, but the anti-hypersensitivity effect of intrathecal clonidine, suggesting that noradrenergic fiber sprouting is important to the novel analgesic properties of drugs approved in the treatment of neuropathic pain which activate, augment, or mimic noradrenergic inhibition.
Acknowledgements
Supported in part by grants GM35523 and NS41386 from the National Institutes of Health (Bethesda, MD). The authors wish to thank Tanishua Bynum and Dr. Xinhui Li for technical work and Dr. Michelle Vincler for advice regarding immunocytochemical studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. Spinal nerve ligation increases α2- adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005;1038:76–82. doi: 10.1016/j.brainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Blackburn GF, Shah HP, Kenten JH, Leland J, Kamin RA, Link J, Peterman J, Powell MJ, Shah A, Talley DB. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin Chem. 1991;37:1534–1539. [PubMed] [Google Scholar]
- Cejas PJ, Martinez M, Karmally S, McKillop M, McKillop J, Plunkett JA, Oudega M, Eaton MJ. Lumbar transplant of neurons genetically modified to secrete brain-derived neurotrophic factor attenuates allodynia and hyperalgesia after sciatic nerve constriction. Pain. 2000;86:195–210. doi: 10.1016/s0304-3959(00)00245-1. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Costoli T, Sgoifo A, Stilli D, Flugge G, Adriani W, Laviola G, Fuchs E, Pedrazzini T, Musso E. Behavioural, neural and cardiovascular adaptations in mice lacking the NPY Y1 receptor. Neurosci Biobehav Rev. 2005;29:113–123. doi: 10.1016/j.neubiorev.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De KY. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;138:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Duflo F, Li X, Bantel C, Pancaro C, Vincler M, Eisenach JC. Peripheral nerve injury alters the α-adrenoceptor subtype activated by clonidine for analgesia. Anesthesiology. 2002;97:636–641. doi: 10.1097/00000542-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D Epidural Clonidine Study Group. Epidural clonidine analgesia for intractable cancer pain. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R. Relative potency of epidural to intrathecal clonidine differs between acute thermal pain and capsaicin-induced allodynia. Pain. 2000;84:87–64. doi: 10.1016/S0304-3959(99)00181-5. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Tong C, Limauro D. Intrathecal clonidine and the response to hemorrhage. Anesthesiology. 1992;77:522–528. doi: 10.1097/00000542-199209000-00018. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Zhang Y, Duflo F. alpha2-adrenoceptors inhibit the intracellular Ca(2+) response to electrical stimulation in normal and injured sensory neurons, with increased inhibition of calcitonin gene-related peptide expressing neurons after injury. Neuroscience. 2005;131:189–197. doi: 10.1016/j.neuroscience.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Frost DO, Ma YT, Hsieh T, Forbes ME, Johnson JE. Developmental changes in BDNF protein levels in the hamster retina and superior colliculus. J Neurobiol. 2001;49:173–187. doi: 10.1002/neu.1073. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Ha SO, Kim JK, Hong HS, Kim DS, Cho HJ. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience. 2001;107:301–309. doi: 10.1016/s0306-4522(01)00353-0. [DOI] [PubMed] [Google Scholar]
- Hayashida KI, Degoes S, Curry R, Eisenach JC. Gabapentin Activates Spinal Noradrenergic Activity in Rats and Humans and Reduces Hypersensitivity after Surgery. Anesthesiology. 2007;106:557–562. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311:576–584. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]
- Karchewski LA, Gratto KA, Wetmore C, Verge VMK. Dynamic patterns of BDNF expression in injured sensory neurons: differential modulation by NGF and NT-3. Eur J Neurosci. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- King VR, Michael GJ, Joshi RK, Priestley JV. trkA, trkB, and trkC messenger RNA expression by bulbospinal cells of the rat. Neuroscience. 1999;92:935–944. doi: 10.1016/s0306-4522(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Ma WY, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003;970:110–118. doi: 10.1016/s0006-8993(03)02293-5. [DOI] [PubMed] [Google Scholar]
- Matsunaga W, Shirokawa T, Isobe K. BDNF is necessary for maintenance of noradrenergic innervations in the aged rat brain. Neurobiol Aging. 2004;25:341–348. doi: 10.1016/S0197-4580(03)00093-9. [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Tramèr M, Nye BA, Carroll D, Wiffen PJ, Moore RA. Systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217–227. doi: 10.1016/s0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- Obata H, Li XH, Eisenach JC. α2-Adrenoceptor activation by clonidine enhances stimulation-evoked acetylcholine release from spinal cord tissue after nerve ligation in rats. Anesthesiology. 2005;102:657–662. doi: 10.1097/00000542-200503000-00027. [DOI] [PubMed] [Google Scholar]
- Puke MJC, Wiesenfeld-Hallin Z. The differential effects of morphine and the α2-adrenoceptor agonists clonidine and dexmedetomidine on the prevention and treatment of experimental neuropathic pain. Anesth Analg. 1993;77:104–109. doi: 10.1213/00000539-199307000-00021. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch int Pharmacodyn. 1957;4:409–419. [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Shen H, Chung JM, Chung K. Expression of neurotrophin mRNAs in the dorsal root ganglion after spinal nerve injury. Brain Research Molecular Brain Research. 1999;64:186–192. doi: 10.1016/s0169-328x(98)00314-3. [DOI] [PubMed] [Google Scholar]
- Slonimsky JD, Yang B, Hinterneder JM, Nokes EB, Birren SJ. BDNF and CNTF regulate cholinergic properties of sympathetic neurons through independent mechanisms. Mol Cell Neurosci. 2003;23:648–660. doi: 10.1016/s1044-7431(03)00102-7. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Takasu K, Kasuya N, Shimizu S, Honda M, Ono H. Role of descending noradrenergic system and spinal α2-adrenergic receptors in the effects of gabapentin on thermal and mechanical nociception after partial nerve injury in the mouse. Br J Pharmacol. 2005;144:703–714. doi: 10.1038/sj.bjp.0706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuroscience - Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Narita M, Matsumoto N, Suzuki T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002;958:338–346. doi: 10.1016/s0006-8993(02)03666-1. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;7:1032–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yang RH, Xing JL, Duan JH, Hu SJ. Effects of gabapentin on spontaneous discharges and subthreshold membrane potential oscillation of type A neurons in injured DRG. Pain. 2005;116:187–193. doi: 10.1016/j.pain.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006;31:539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Zhu X, Li X, Eisenach JC. Spinal norepinephrine release from nitric oxide species is not increased following peripheral nerve injury in rats. Brain Res. 2002;947:199–203. doi: 10.1016/s0006-8993(02)02924-4. [DOI] [PubMed] [Google Scholar]


