Abstract
Neurons in the cerebral cortex originate predominantly from asymmetrical divisions of polarized radial glial or neuroepithelial cells. Fate control of neural progenitors through regulating cell division asymmetry determines the final cortical neuronal number and organization. Haploinsufficiency of human LIS1 results in type I lissencephaly (smooth brain) with severely reduced surface area and laminar organization of the cerebral cortex. Here we show that LIS1 and its binding protein Nde1 (mNudE) regulate the fate of radial glial progenitors collaboratively. Mice with an allelic series of Lis1 and Nde1 double mutations displayed a striking dose-dependent size reduction and de-lamination of the cerebral cortex. The neocortex of the Lis1–Nde1 double mutant mice showed over 80% reduction in surface area and inverted neuronal layers. Dramatically increased neuronal differentiation at the onset of corticogenesis in the mutant led to overproduction and abnormal development of earliest-born preplate neurons and Cajal–Retzius cells at the expense of progenitors. While both Lis1 and Nde1 are known to regulate the mitotic spindle orientation, only a moderate alteration in mitotic cleavage orientation was detected in the Lis1–Nde1 double deficient progenitors. Instead, a striking change in the morphology of metaphase progenitors with reduced apical attachment to the ventricular surface and weakened lateral contacts to neighboring cells appear to hinder the accurate control of cell division asymmetry and underlie the dramatically increased neuronal differentiation. Our data suggest that maintaining the shape and cell–cell interactions of radial glial neuroepithelial progenitors by the Lis1–Nde1 complex is essential for their self renewal during the early phase of corticogenesis.
INTRODUCTION
The cerebral cortex in mammals consists of six highly organized neuronal layers formed by tightly coupled neurogenesis and neuronal migration during embryonic development. The majority of neurons in the cortex are generated by radial glial or neuroepithelial cells (1–5). These polarized cells line the lateral ventricles of the developing telencephalon, form a pseudostratified layer by oscillating their nuclei apical-basally at different phases of the cell cycle and extend their basal end-feet into the cortical margin (6–10). As polarized progenitors, radial glial neuroepithelial cells predominantly undergo symmetrical proliferative cell divisions to self-renew during the early phase of development. Neurogenesis commences when some progenitor cells divide asymmetrically, generating postmitotic neurons that migrate away from the ventricular zone (VZ) along the basal fiber of the radial glial cells towards the outer edge of the cortical wall (11,12). The earliest generated postmitotic cells emigrate from the VZ to form initially the preplate (PP) (13,14), which soon divides into the superficial cell sparse marginal zone (MZ) and a deeper subplate (SP) by the invasion of successive waves of neurons produced from the VZ. The MZ also contains Cajal–Retzius (C–R) cells, another class of oldest cortical neurons that secrete the glycoprotein Reelin, which is known to be essential for guiding the incoming young neurons to migrate past the SP and their earlier arrived predecessors and for the formation of the characteristic ‘inside first, outside last’ cortical laminar structure (15,16). While the normal cortical neuronal migration and lamination rely on the proper formation and function of PP and C–R cells produced in the early phase of cortical neurogenesis, little is known on the mechanisms controlling the generation of these earliest neurons in the cortex.
As the gene mutated in the Miller–Dieker syndrome or type I lissencephaly, LIS1 appears to be essential for regulating both neural progenitor division and cortical neuronal migration. Haploinsufficiency of LIS1 results in a severely malformed smooth cerebral cortex and the disorganization of the normal six-layered cortical neurons (17,18). LIS1 encodes an evolutionarily conserved widely expressed cytoplasmic protein (19,20), but phenotypes of heterozygous LIS1 mutations are largely restricted in the cerebral cortex (21,22). LIS1 is known to interact with multiple proteins in the cytoplasm, but molecular and cellular mechanism underling LIS1's function in cortical development has been controversial. Although LIS1 was originally found as a regulatory subunit of the PAF acetylhydrolase (23), loss of functional mutation of the catalytic subunits of this enzyme did not show CNS phenotype (Yan et al., 2003). LIS1 also interacts with the cytoplasmic dynein. The dynein association is important for both nuclear translocation in migrating neurons and mitotic spindle regulation in neural progenitors (24–30), but it is unclear how the impaired dynein motor function only selectively affects the cell division and migration in the developing cortex and confers a cortical specific phenotype in human lissencephaly. Moreover, a role of Lis1 in regulating the morphology of subventricular zone (SVZ) intermediate neural progenitors was postulated through siRNA study (31). In mice, homozygous mutations of Lis1 resulted in peri-implantation lethality, but Lis1 heterozygous mutants only showed subtle defects in cortical development (32). A compound heterozygous mutation that expresses 30% of wild-type levels of Lis1 protein displayed reduced cortical neurons and delayed neuronal migration (32,33). While it is clear that LIS1 is essential for multiple cellular and developmental events, the cerebral cortical-specific mechanism that is distinctive from the housekeeping functions of LIS1 is yet to be defined.
We previously identified Nde1 as a critical binding partner of LIS1 in CNS development (34). Nde1 is highly expressed in radial glial neuroepithelial cells where its paralogue Ndel1 showed very expression. The Nde1−/− mutant mouse displayed a specific size reduction of the cerebral cortex by ∼33% due primarily to abnormal mitotic spindle function in neural progenitors during mid-phase of cortical neurogenesis (35). Although Nde1 and Lis1 share a common function in regulating the mitotic spindle orientation of neural progenitors, inconsistent results have been obtained from studying the direct correlation between mitotic cleavage angle and neurogenic cell fate in the developing cortex (36–38), suggesting that the cell division asymmetry of neural progenitors may also be controlled by factors other than the mitotic cleavage orientation. To further understand the cerebral-specific function of LIS1 and its relationship with Nde1, we analyzed an allelic series of Nde1 and Lis1 double mutations in mice. Our data have shown that the Lis1–Nde1 protein complex is specifically required by the radial glial/neuroepithelial progenitor cells in CNS development. Besides mitotic spindle regulation, LIS1 and Nde1 are critically required for maintaining morphology and lateral cell–cell contacts of progenitors in the cortical VZ. Such cell shape and organization control appeared necessary for the equal partition of the cytoplasm and cell fate determinants into daughter cells. Thus, the dual regulation of mitotic cleavage orientation and progenitor morphology by Lis1–Nde1 is essential for symmetrical division and self-renewal of neural progenitors during the early phase of corticogenesis. Loss of Lis1–Nde1 function led to dramatically increased neuronal differentiation at the onset of cortical neurogenesis, leading to the overproduction of the earliest-born PP and C–R neurons with a consequent loss of the laminar pattern and over 80% mass and surface area of the cerebral cortex.
RESULTS
Nde1 functions together with Lis1 in cerebral cortical development
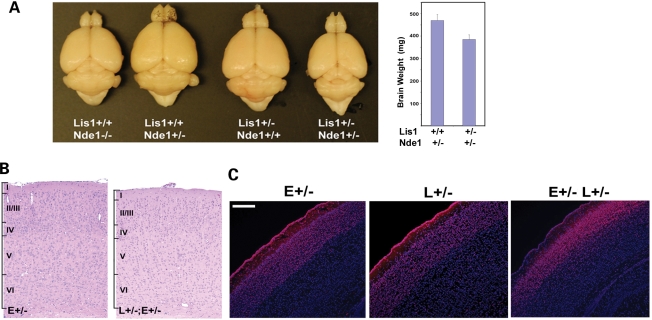
To understand the mechanism and relationship of Lis1 and Nde1 in cerebral cortical development, we generated and analyzed an allelic series of Nde1–Lis1 double mutant mice. First, we found that the brain of the Nde1–Lis1 double heterozygous (Nde1+/−Lis1+/−) mutant was about 20–25% smaller than that of Nde1+/− or Lis1+/− mice (Fig. 1A), and the cerebral cortex of the Nde1+/−Lis1+/− mutant showed preferential thinning of cortical layers II/III similar to what was observed in the Nde1−/− mutant (Fig. 1B and C). The close phenotypic resemblance of Nde1+/−Lis1+/− and Nde1−/− mutants suggested a dose-dependent genetic interaction of Nde1 with Lis1, and that the two genes function in a common molecular pathway regulating cerebral cortical development.
Figure 1.
Lis1 and Nde1 function together in cerebral cortical development. (A) Brains of adult mice with Nde1+/−, Lis1+/−, Nde1+/−Lis1+/− and Nde1−/− mutations. Compared to Nde1 and Lis1 single heterozygous mutants, the brains of double heterozygous mutants (Nde1+/−Lis1+/−) showed an approximately 20–25% reduction by weight (n=13, P < 0.001), resembling that of Nde1−/− mutants. (B, C) Histological and Immunohistological analyses suggested that the brain of the Nde1+/−Lis1+/− mutant was grossly normal, except for a significant thinning of the cortical layers II/III indicated by Cux1 immunoreactivity and blurred cortical layer boundaries. E, Nde1; L, Lis1. Bar: 200 µm.
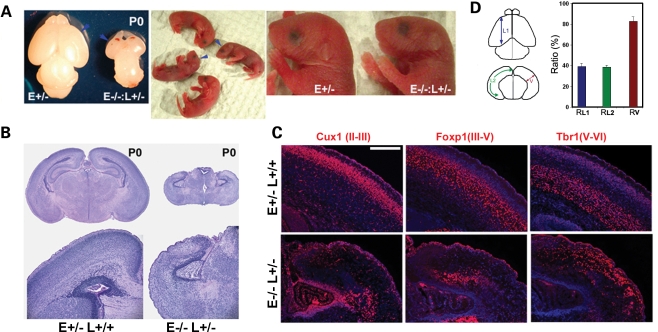
Further crossing of Nde1+/− and Nde1+/−Lis1+/− mice generated Nde1 homozygous and Lis1 heterozygous double mutants (Nde1−/− Lis1+/−), which died within a few hours of birth. Although the body size of these mutants varied from smaller to near normal, their heads were significantly reduced in size and their brains showed severe hypoplasia of cerebral hemispheres (Fig. 2A). While the olfactory bulbs, diencephalons as well as the spinal cords of the mutant were also smaller (Supplementary Material, Fig. S1), the size reduction in the cerebrum was most pronounced.
Figure 2.
The Lis1–Nde1 complex is essential for determining cerebral cortical size, shape and lamina structures. (A) Dying at birth, the brains of Nde1−/−Lis1+/− mutant mice were dramatically reduced (arrows). A more pronounced size reduction of the cerebral hemispheres was observed. (B) Histological analysis showed that the neocortex of the Nde1−/−Lis1+/− mutant was severely disorganized, lacked the normal MZ and any other cortical layers. (C) Immunohistological analyses with layer-specific markers showed that in the Nde1−/−Lis1+/− cortex, cells belonging to superficial and middle cortical layers (marked by Cux1 and Foxp1 immunoreactivity, respectively) were greatly reduced, positioned randomly and often formed heterotopia in deeper cortical regions. In contrast, deep layer maker Tbr1 highlighted cells in the superficial cortex, suggesting grossly inverted cortical layers in the mutant. E, Nde1; L, Lis1. Bar: 200 µm (D) Rostral to caudal length (L1), medial to lateral length (L2) and cortical thickness (V) of Nde1−/−Lis1+/− cortex were measure and compared with those of the Nde1+/− control at P0. The mutant cortex was less than 40% of the controls in L1 and L2, but only reduced by 20% in thickness (V).
The histological analysis demonstrated that the neocortex of the Nde1−/−Lis1+/− mutant lacked all of the distinct cellular layers seen in the normal littermate controls at birth. The most superficial layer I under the cortical pial surface, which normally forms the cell sparse MZ, was cell dense in the mutant. The entire cortex of the mutant was filled with disorganized, haphazardly positioned cells (Fig. 2B). Immunohistological analyses with cortical layer-specific markers further demonstrated the severe lamina disorganization of the Nde1−/−Lis1+/− mutant. The earlier born deep layer V–VI neurons marked by Tbr1 were spread throughout superficial cortical regions; whereas normally more superficially localized later born neurons, marked by Foxp1 (layers III–V) and Cux1 (layers II/III) immunoreactivity, were mostly seen in the deep cortex where they formed focal clusters in the mutant (Fig. 2C). Moreover, very few late-born Cux1 and Foxp1 positive neurons were detected relative to the earlier-born Tbr1 positive cells in the Nde1−/−Lis1+/− cortex (Fig. 2C), suggesting precocious neural progenitor exhaustion during corticogenesis in the mutant.
An additional distinctive feature of the severely malformed Nde1−/−Lis1+/− cortex was that its surface area was notably reduced more strikingly than the cortical thickness. While the mutant cortex was ∼80% of the control cortex in thickness, it measured less than 40% of the control's both in rostral to caudal (L1) and medial to lateral (L2) dimensions (Fig. 2D). This constituted a more than 80% reduction in surface area, and suggested that the mutant cortex had reduced number of radial neuronal units resulting from loss of progenitors in early ages (39,40).
Loss of neural progenitors in the Nde1−/−Lis1+/− cortex
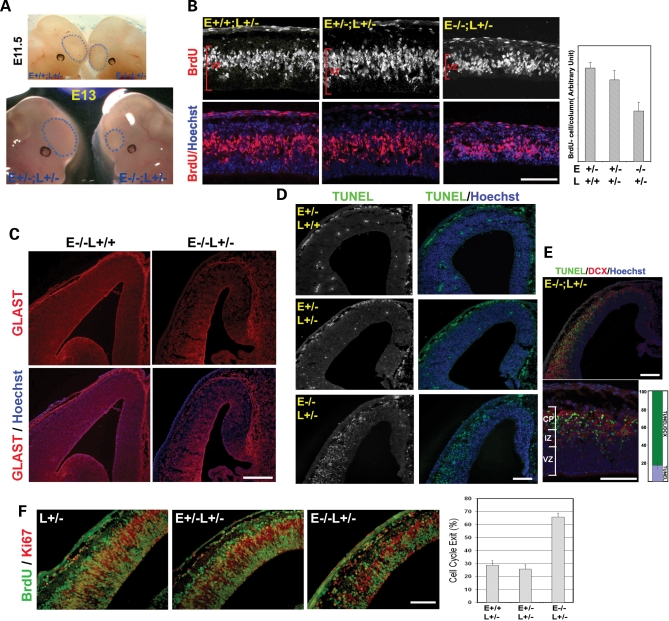
The reduction of neural progenitors during early stages of corticogenesis in the Nde1−/− Lis1+/− mutant was confirmed by a series of developmental studies. Although not obviously recognizable before E11.5, the Nde1−/−Lis1+/− mutants could be identified easily by their distinctively smaller telencephalic vesicles by E12.5–E13.5 (Fig. 3A). Histological and immunohistological analysis also demonstrated a grossly normal anatomical structure of the mutant embryos before E11.5, along with insignificant alteration of dorsal forebrain markers, Pax6 and Pax3 (Supplementary Material, Fig. S2). These suggested that the dorsal telencephalon of the mutant was patterned before E11.5, but failed to expand normally after the onset of corticogenesis at E11.5–E12.
Figure 3.
Reduction of VZ progenitors at the onset of corticogenesis by Lis1–Nde1 double deficiency. (A) The size of telencephalic vesicles (indicated by dashed blue circles) of the Nde1−/−Lis1+/− mutant appeared close to normal before E11, but was significantly smaller by E13 compared to that of their littermates. (B) Correlated with the thinning of cerebral cortex at E12.5, the thickness of cortical VZ and the number of S-phase neural progenitors examined by BrdU transient labeling (in red), was decreased significantly in the Nde1−/−Lis1+/− mutant. P < 0.001. E, Nde1; L, Lis1. Bar: 100 µm. (C) Reduced neural progenitors in the Nde1−/−Lis1+/− mutant cortex was indicated by reduced immunostaining of the glutamate transporter GLAST (in red), a marker of radial glial progenitors. Bar: 100 µm. (D) Substantial cell death was detected in the cerebral cortex of the Nde1−/−Lis1+/− mutant by TUNEL staining (in green). In contrast, very few TUNEL positive cells were detected in the Nde1+/− and Nde1+/−Lis1+/− controls. Bar: 100 µm. (E) Majority (over 80%) of TUNEL positive cells (in green) in the Nde1−/−Lis1+/− mutant were newborn post mitotic neurons in the intermediate zone (IZ) and the cortical plate (CP) and expressed DCX (in red). Bar: 100 µm. (F) Cell-cycle exit profiles of Nde1−/−Lis1+/−progenitors. Pregnant females were given single does of BrdU at E12 and were analyzed 18 h later. Brain sections were stained with antibody to BrdU (in green) and to Ki67 (in red). Cells that exit the cell cycle are counted as those that are positive for BrdU but negative for Ki67, and presented as the percentage of total BrdU positive cells. Approximately 67% of cells labeled by BrdU at E12 became non-progenitor cells in the Nde1−/−Lis1+/− cortex by E13, while only 25–28% of the Nde1+/−Lis1+/− or Lis1+/− progenitors left the cell cycle (P<0.001). Bar: 100 µm.
The failure in telencephalon growth was correlated with a reduction in the number of cortical progenitors. A thinner cortical VZ, fewer BrdU pulse labeled S-phase cells as well as reduced immunosignals of the radial glial progenitor marker GLAST were observed in the Nde1−/−Lis1+/− mutant cortex at E12.5 (Fig. 3B and C). Concurrent with decreased progenitors, TUNEL labeling also indicated substantial cell death in the developing neocortex of the Nde1−/−Lis1+/− mutant (Fig. 3D). However, over 80% of the cell death occurred outside of the VZ, overlapping with young neurons marked by DCX immunoreactivity (Fig. 3E). Thus, the increased cell death contributes to the sharp reduction of the telencephalic vesicles, but does not fully account for progenitor loss in the mutant.
To determine the cause of the severe progenitor loss in the Nde1−/−Lis1+/− mutant during early corticogenesis, we studied the cell-cycle dynamics of Nde1−/−Lis1+/− progenitors. After the progenitors were labeled by BrdU, they were examined in 4 h for mitotic entrance and in 18 h for cell-cycle exit. Approximately 30% of BrdU-labeled Nde1−/−Lis1+/− progenitors were found in the M-phase 4 h after DNA synthesis, as indicated by double immunolabeling with antibodies to BrdU and the mitotic chromatin phospho-Histone H3 (PH3). This percentage was significantly higher than what was observed in control littermates, suggesting that Nde1−/−Lis1+/− progenitors had a normal speed in entering the M-phase from the S-phase, but delayed in M-phase progression (Supplementary Material, Fig. S3). The mitotic arrest of Nde1−/−Lis1+/− progenitors was followed by a drastically increased cell-cycle exit. Eighteen hours after BrdU labeling, 67% of the BrdU-labeled Nde1−/−Lis1+/− progenitors were found outside of the progenitor pool and stained negatively by the progenitor marker Ki67 (Fig. 3F). This cell-cycle exit fraction was two to three times higher than the progenitors in the Nde1+/−Lis1+/− and Lis1+/− controls, indicating that Nde1−/−Lis1+/− mutation resulted in significantly decreased self-renewal and accelerated cell-cycle exit of cortical progenitors.
Overproduction and abnormal development of preplate neurons
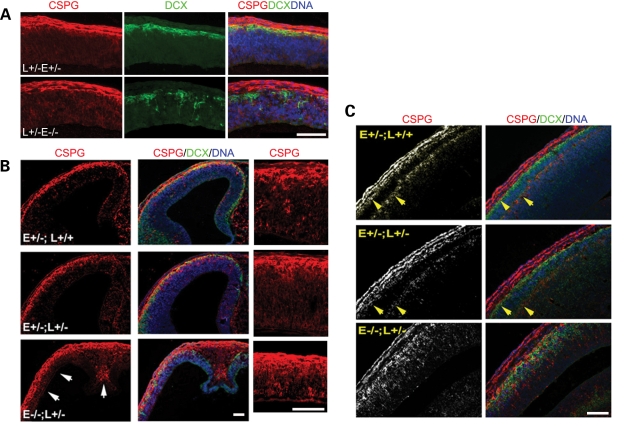
The increased cell-cycle exit and decreased number of cortical progenitors in the Nde1−/−Lis1+/− mutant during the onset of cortical neurogenesis coincided with a massive over-production of the earliest-born PP and C–R cells at this stage. Detected by chondroitin sulfate proteoglycan (CSPG) immunostaining, PP neurons in the Nde1−/−Lis1+/− mutant were significantly increased relative to control littermates. From E11.5 to E12.5, CSPG, secreted by PP neurons, infused almost the entire Nde1−/−Lis1+/− cortex (Fig. 4A and B). The increased production of PP neurons persisted into E13.5 and the abnormally thickened PP failed splitting to form the superficial layer I (MZ) and the deep SP layer in the Nde1−/−Lis1+/− mutant. As demonstrated in Fig. 4C, the Nde1+/− and Nde1+/−Lis1+/− mutants have all shown clear separation of the MZ and the SP by the migration and invasion of the first cohort of cortical plate neurons by E13.5 (14,41). But no such separation was detected in the Nde1−/−Lis1+/− mutant though a large number of DCX positive neurons migrated out of the VZ and mixed with PP neurons underneath the cortical pial surface (Fig. 4C). Hence, the PP neurons in the Nde1−/−Lis1+/− mutant failed to develop properly, instead forming a ‘superplate’-like structure somewhat similar to that seen in the reeler mice with deficiency of Reelin (42).
Figure 4.
Overproduction of PP neurons and lack of preplate splitting in the Nde1−/− Lis1+/− cortex. (A) Preplate neurons were labeled by CSPG antibody in red and cortical plate neurons were labeled with DCX antibody in green. A significant increase in CSPG was detected throughout the entire Nde1+/−Lis1+/− cortex before its size was reduced at E11.5. (B) The cortex of Nde1−/−Lis1+/− mutant was thinner at E12.5, but their CSPG positive zone was broadened (arrows). (C) At E13.5, while Nde1+/−, Nde1−/− and Nde1+/−Lis1+/− embryos all showed well separated preplate (indicated by yellow arrows), no splitting of the preplate could be detected in the Nde1−/−Lis1+/− cortex. In addition to greatly increased CSPG positive preplate neurons (in red), increases of DCX positive young cortical plate neurons (in green) was also detectable. E, Nde1; L, Lis1. Bar: 100 µm.
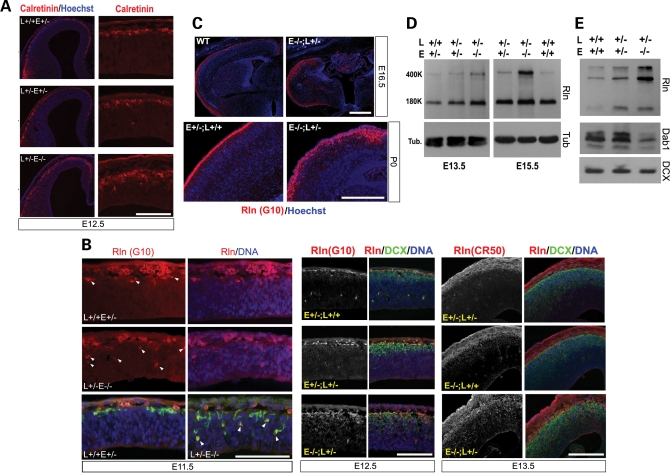
Overproduction and ectopic localization of C–R cells
Paradoxically, the failure of PP splitting and the ‘superplate’-like structure in the Nde1−/−Lis1+/− mutant coexisted with the overproduction of Reelin and Reelin-secreting C–R cells, as opposed to the lack of Reelin in the reeler mutant (15). This was manifested by significantly increased Calretinin immunoreactivity, one of the markers for MZ C–R cells, in the Nde1−/−Lis1+/− mutants (Fig. 5A). Further examination of C–R cells with two monoclonal antibodies to Reelin also showed dramatic overproduction of these earliest generated neurons destined for layer I. Not only was the number of Reelin positive C–R cells increased throughout the entire course of cortical development in the Nde1−/−Lis1+/− mutant (Fig. 5A–C), but many Reelin expressing cells were also delocalized from the layer I, and were observed ectopically in deeper cortical regions in the Nde1−/−Lis1+/− mutant (Fig. 5B and C, arrows). The abnormally localized Reelin secreting cells could be detected as early as E11.5. They were also disorganized, forming clusters in deeper cortical layers in later stages (Fig. 5C). The Reelin protein concentration in the cortex was increased by at least 5-fold in the mutant over wild-type controls as detected by immunoblotting (Fig. 5D). The overproduced Reelin in the Nde1−/−Lis1+/−mutant appeared active, as they elicited the dramatic degradation of Dab1 protein, which indicates elevated activity in the Reelin signaling pathway (43)(Fig. 5D). Together, these data suggested that Nde1–Lis1 double deficiency resulted in a severe cell-fate defect in the cortical neural progenitors, leading to precocious overproduction of earliest-born PP and C–R neurons in the mutant cortex at the expense of progenitor cells.
Figure 5.
Overproduction of C–R cells and enhanced Reelin signaling in the Nde1−/−Lis1+/− cortex. (A) Over production of Calretinin positive C–R cells (in red) was seen in the Nde1−/−Lis1+/− mutant. Significantly increased Calretinin positive cells were observed in the Nde1−/−Lis1+/− cortex at E12.5. E, Nde1; L, Lis1. Bar: 200 µm. (B and C) Over-production and abnormal positioning of Reelin secreting C–R cells in the Nde1−/−Lis1+/− mutant. Cortical sections were immunostained with two monoclonal antibodies to Reelin in red (CR50 and G10), and co-stained with an antibody to DCX in green and Hoechst in blue. The Nde1−/−Lis1+/− mutant showed dramatic increase and mis-localization of Reelin positive C–R cells throughout the entire course of corticogenesis starting from E11.5. Bar: 200 µm. (D) Immunoblotting analysis of Reelin protein levels in developing cerebral cortex. The cerebral cortices of E13.5 and E15.5 embryos were dissected and their total proteins extracts were analyzed on a 7.5% SDS–PAGE, followed by immunoblotting with an antibody to Reelin (G10). Loading is normalized by total protein amount and by immunoblotting with an antibody to tubulin. Bands on immunoblots were analyzed using Quantify One. Over 5-fold increase in Reelin protein (both 400 and 180 kDa bands) was detected in the Nde1−/−Lis1+/− mutant cortex over wild-type controls. (E) Down-regulation of Dab1 by increased Reelin signaling was observed in the Nde1−/−Lis1+/− mutant. Immunoblotting analysis was performed with protein extracts from the cortex of E15.5 embryos with antibodies to Dab1 and DCX. Compared to wild-type and Nde1+/−Lis1+/− mutant, a significant decrease in the Dab1 protein level was detected in the Nde1−/−Lis1+/−cortex, suggesting that the overproduced Reelin in the mutant were active and could elicited Dab1 degradation.
Although C–R cells are believed to be largely generated in extra-cortical origins such as the cortical hem, from where they migrate tangentially to the cortical MZ (44,45), the overproduced C–R cells in the Nde1−/−Lis1+/− mutant appeared to be at least partially generated in situ by cortical VZ progenitors. This conclusion was based on the observation that some C–R cells were observed deep inside of the VZ before the appearance of cortical dysmorphogenesis at E11.5 (Fig. 5B), while the mutant did not show any sign of cortical hem expansion (Supplementary Material, Fig. S4). Along with the massive over-population of PP and C–R neurons, DCX marked CP neurons were also increased and abnormally distributed in the mutant (Figs 4 and 5), suggesting that Lis1–Nde1 deficiency led to an overall increased neuronal production from cortical progenitors.
Reduced requirement of the Nde1–Lis1 complex in SVZ progenitors
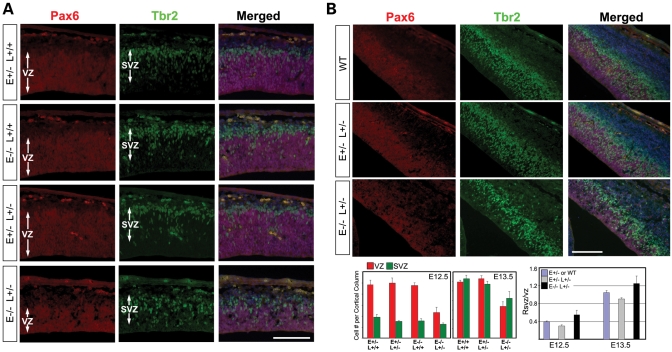
The loss of cortical neural progenitors coupled with the increase in neuronal differentiation suggested an essential role of Lis1–Nde1 in progenitor fate regulation. To understand the mechanism of the Lis1–Nde1 complex in controlling the self-renewal and differentiation of neural progenitors, we compared the defect caused by Lis1–Nde1 double deficiency in different progenitor populations. The CNS-specific phenotype shown by the Nde1−/−Lis1+/− mutant suggests that the mutation affects specifically the neural progenitors. There are at least two distinct neural progenitor populations in the developing cerebral cortex. In addition to the polarized radial glial progenitors that comprise the cortical VZ, the SVZ progenitors that divide away from the ventricular surface also contribute substantial numbers of neurons to most or all layers of the cortex (46,47). Double immunostaining with VZ progenitor marker Pax6 and SVZ progenitor marker Tbr2 was performed to analyze both VZ and SVZ progenitors. In contrast to the significant reduction of the Pax6 zone in the Nde1−/−Lis1+/− mutant, a relatively subtle thinning of the Tbr2 zone and decrease in SVZ cell numbers were observed from E12.5 to E13.5 (Fig. 6A and B), which suggested that the Nde1−/−Lis1+/− mutation had a much stronger impairment in the VZ progenitors than the SVZ progenitors. Thus, the Lis1–Nde1 complex is specifically required by the progenitors in the cortical VZ.
Figure 6.
Moderate changes in SVZ progenitor population by Nde1−/−Lis1+/− mutation. Double immunostaining of VZ progenitors with Pax6 (in red) and SVZ progenitors with Tbr2 (in green) at E12.5 (A) and E13.5 (B) demonstrated that the Nde1−/−Lis1+/− mutation lead to a relatively milder change in the number and density of SVZ progenitors than the VZ progenitors. Although the reduction of SVZ progenitor pool in the Nde1−/−Lis1+/− mutant became more detectable at E13.5, it is less significant compared to the dramatically diminished VZ progenitor pool. Numbers of VZ and SVZ cells per unit length of ventricular surface (Cell # per cortical column) were graphed. Ratios of SVZ/VZ progenitors at E12.5 and E13.5 were also presented. Bar: 100 µm.
Moderate mitotic spindle defects caused by Lis1–Nde1 double deficiency
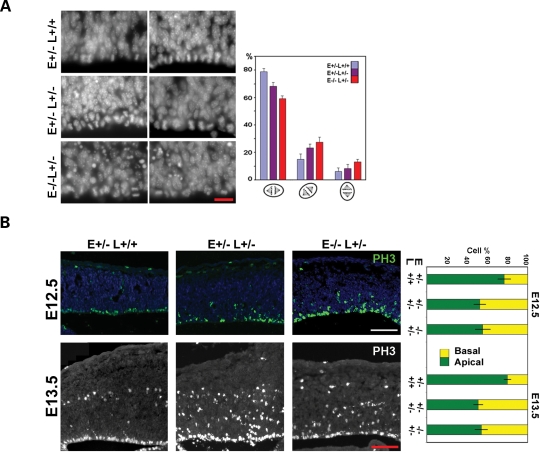
Neural progenitors in the cortical VZ are polarized columnar-shaped neuroepithelial or elongated radial glial cells. The fate of these progenitors may be regulated by the mode of cell division, which presumably controls the segregation of cytoplasmic fate determinants into daughter cells (1,47–49). Many fate determinants might be apically localized, so that they also provide cues for general cell polarity and mitotic cleavage orientation (38,50). The differential effect of Lis1–Nde1 mutation on VZ and SVZ progenitors may reflect a molecular function that associates to the polarized nature of VZ radial glial progenitors. To understand this cell-type specific function of the Lis1–Nde1 complex, we first examined the mitotic orientation of Nde1−/−Lis1+/− progenitors, since both Nde1 and Lis1 are known to play roles in mitotic spindle regulation (26,28,29,35). An increase in horizontal mitosis with the cleavage plane parallel to the ventricular surface were observed in the Nde1−/−Lis1+/− progenitors in comparison to the Nde1+/− and Nde1+/−Lis1+/− progenitors at E12.5 (Fig. 7A). Although the increased horizontal mitotic cleavage is in favor of asymmetrical progenitor division, and consistent with increased neuronal differentiation in the mutant, the alteration in mitotic cleavage orientation by Nde1−/−Lis1+/− mutation was relatively moderate and was not in proportion to the dramatic fate change in the mutant. This suggested that the Lis1–Nde1 complex had functions in addition to determining mitotic orientation.
Figure 7.
Mitotic defect in the Nde1−/−Lis1+/− mutant. (A) Abnormal mitotic orientation of Nde1−/−Lis1+/− cortical progenitors at E12.5. Anaphase progenitor cells were classified into three groups according to the angle of mitotic cleavage plane to the ventricular surface (vertical: 75–90°; horizontal: 0–25°; and diagonal: 25–75°). Approximately 300 anaphase cells were scored for each genotype. Percentage of each class of mitotic cleavage orientation from three litters with Nde1+/−, Nde1+/−Lis1+/− and Nde1−/−Lis1+/− embryos were summarized. A moderate reduction in vertical cell cleavages and a corresponding increase in horizontal cell cleavages in the Nde1−/−Lis1+/−neural progenitors were detected (P < 0.01). Bar: 20 µm. (B) Phospho-Histone H3 (PH3) immunoreactivity demonstrated that a large fraction of mitotic progenitors failed to position metaphase chromosomes apically along the ventricular surface in Nde1+/−Lis1+/− and Nde1−/−Lis1+/− mutants at both E12.5 and E13.5 (P<0.001). However, the degree of abnormal mitotic nuclei positioning was equally severe in Nde1+/−Lis1+/− and Nde1−/−Lis1+/− mutants (P > 0.5). Bar: 100 µm.
Nde1−/−Lis1+/− progenitors were also found to be defective in positioning the metaphase chromosomes, another phenotype of failed mitotic spindle function. At both E12.5 and E13.5, immunolabeling with PH3 antibody displayed that a significant fraction of Nde1−/−Lis1+/− progenitors failed to localize their nuclei/chromosomes apically next to the ventricular lumen during metaphase (Fig. 7B). However, this defect in mitotic chromosome positioning was shared by the phenotypically milder Nde1+/−Lis1+/− and Nde1−/− progenitors (Fig. 7B). Therefore, it could not explain the striking difference between of Nde1−/−Lis1+/− and Nde1+/−Lis1+/− and Nde1−/− progenitors. This further indicated that the Lis1–Nde1 complex might also engage in activities other than the previously described mitotic spindle control.
Severe metaphase architectural defects by Nde1–Lis1 double deficiency
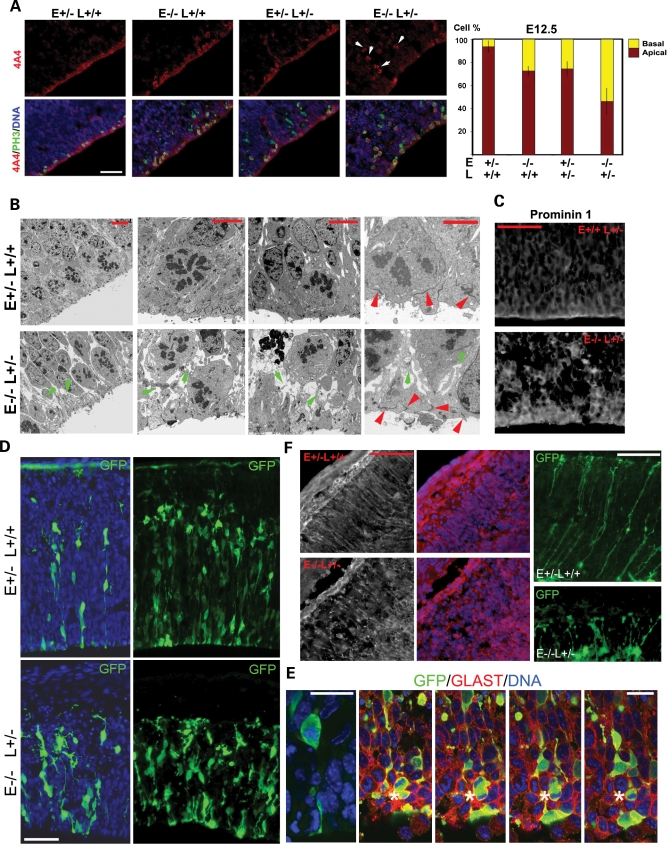
To explore the previously unrecognized function of the Lis1–Nde1 complex in radial glial or neuroepithelial progenitor mitotic fate control, we performed further analyses on the structures of the Nde1−/−Lis1+/− progenitors. Both Lis1 and Nde1 are cytoplasmic scaffold proteins. They were both known as adaptors that control the organization of microtubule cytoskeleton and microtubule-associated motors (26,29,51–54). Besides mitotic spindle assembly and regulation, microtubules are required for controlling the cell shape and polarity. We, therefore, examined the morphology of metaphase progenitors by immunolabeling their cell body with the phospho-vimentin monoclonal antibody 4A4 (55). In contrast to the bright 4A4 signal enriched on the apical side of Nde1+/− progenitors, 4A4 immunosignal along the ventricular surface was found moderately reduced in Nde1+/−Lis1+/− and Nde1−/− mutants, but substantially reduced in the Nde1−/−Lis1+/− mutant at E12.5 (Fig. 8A). Correlated with the severely reduced apical 4A4 signal, enhanced basal 4A4 immunosignal was observed, suggesting the retraction and reduction of apical cell processes of mutant progenitors during mitosis. In contrast to the striking alteration in 4A4 immunosignals during metaphase, many markers for the apical region of radial glial neuroepithelial progenitors showed very little change in the Nde1−/−Lis1+/− mutant. These included the apical adherens junction protein β-catenin, the centrosomal protein pericentrin and F-actin, all of which were indistinguishable in their distribution between the Nde1−/−Lis1+/− progenitors and normal controls (Supplementary Material, Fig. S5). These data suggested that the mutant radial glial progenitors were able to establish apical junctions and overall polarity, but fail to maintain the apical cell shape and intimate association with apical ventricular surface during mitosis.
Figure 8.
Morphology and cytoarchitectural defects of Nde1−/−Lis1+/ progenitors underlie the abnormal fate control. (A) Immunostaining the cell body of metaphase progenitors with a phosphorylated vimentin antibody 4A4 showed moderately reduced apical staining of VZ progenitors in the Nde1−/−, Nde1+/−Lis1+/− mutants and a severe decrease in 4A4 immunoreactivity along the ventricular surface in the Nde1−/−Lis1+/− mutant (P<0.003), suggesting the destabilization and detachment of apical cell structures from the ventricular surface during metaphase. The abnormal 4A4 immunosignals in the VZ were indicated by arrows. Bar: 50 µm. (B) Electron micrograph of metaphase progenitors along ventricular surface, showing the reduced apical cell and membrane anchorage to ventricular surface, and weakened lateral cell–cell contacts of metaphase cells in the Nde1−/−Lis1+/−mutant. Red arrows indicate the electron dense adherens junctions formed between the apical end-feet of radial glial progenitors; green arrows indicate the membrane-like structures between the shrunken apical cell body. Shown are representative images of samples from three independent litters. Bars: 5 µm and 2 µm, respectively. (C) Immunohistological analysis of the apical membrane protein Prominin 1 showed that the space between the apical cell body of Nde1−/−Lis1+/− progenitors was occupied by the retracted apical membrane, further demonstrating the apical instability of the mutant progenitors. Bar: 50 µm. (D) Morphological and organizational abnormalities of Nde1−/−Lis1+/− progenitors were also viewed by labeling them with GFP through in utero electroporation. Although most of the Nde1−/−Lis1+/− progenitors showed well formed apical and basal end-feet, they frequently showed abnormal morphology of the cell body and processes. Two selected fields with different densities of GFP labeled progenitors were presented. Bar: 50 µm. (E) Abnormal metaphase cells with retracted thin apical processes and the formation of rosette-like mitotic cell clusters (indicated by asterisk) were also commonly seen in the Nde1−/−Lis1+/− mutant by co-immunolabeling with the radial glial marker GLAST. Bar: 20 µm. (F) Immunohistological analysis of radial glial marker GLAST and GFP labeling by in uteroelectroporation showed severe morphological defects of radial glial fibers near the MZ in the Nde1−/−Lis1+/− mutant, and suggested a role of Lis1–Nde1 in maintaining neural progenitor morphology at both apical and basal sides. Bars: 50 µm (GLAST) and 25 µm (GFP), respectively.
Ultrastructural analyses confirmed the abnormal apical morphology of metaphase progenitors in the Nde1−/−Lis1+/− mutant. Although electron-dense adherens junctions appeared intact between the apical end-feet, the normal apical structure of metaphase progenitors was disrupted by the Nde1−/−Lis1+/− mutation, resulting in the retraction of the apical membrane from the ventricular surface and reduction in apical cell attachment (Fig. 8B). Altered apical cell morphology also manifested as weakened lateral contacts of metaphase Nde1−/−Lis1+/− progenitors. Spaces between the shrunken apical cell body appeared to contain retracted apical membrane raffles, highlighted by immunoreactivity to the apical membrane marker Prominin 1 (Fig. 8C).
The abnormal cell morphology of Nde1−/−Lis1+/− progenitors was also visualized by labeling a subpopulation of cortical progenitors with the green fluorescence protein through in utero electroporation. In contrast to the wild-type control cortex where neural progenitors in the VZ were tightly packed, arranged radially perpendicular to the ventricular surface with bipolar morphology, progenitors in the cortical VZ of Nde1−/−Lis1+/−mutant were disorganized both between each other and in relative to the ventricular surface (Fig. 8D). The mutant neural progenitors frequently showed irregular cell body contours and long kinky apical processes, which were consistent with reduced cell surface tension, unstable apical processes and lost lateral cell–cell adhesions. These defects in cell morphology may prevent the normal symmetrical mitotic division and result in the disorganization of metaphase progenitors, which often forms rosette-like clusters near the ventricular surface (Fig. 8E, asterisk). Correlated with the striking structural alteration near the ventricular surface, the Nde1−/−Lis1+/− mutant radial glial cells also displayed abnormal morphology on the basal processes near the cortical pial surface, where they appeared less straight, truncated, tangled and branched (Fig. 8F).
Together, these data suggest that the Lis1–Nde1 complex is essential in maintaining the morphology and the lateral cell–cell contacts of the bi-polar radial glial or neuroepithelial progenitors. Loss of such function of Lis1–Nde1 appeared to preferentially affect the metaphase cell and prevented them from going through accurate symmetrical divisions.
DISCUSSION
In this study, we have shown a CNS specific functional interaction between Lis1 with its interacting protein Nde1 in controlling the cell fate of neural progenitors. The Nde1–Lis1 double deficiency affects the polarized neuroepithelial/radial glial progenitors in the VZ preferentially during their mitosis. The principal new finding is that the Lis1–Nde1 complex is critically required for maintaining the morphology of neural progenitors and their lateral contacts with neighboring cells additional to their role in mitotic spindle regulation. The control of the cytoarchitecture and morphological integrity of radial glial progenitors by the Lis1–Nde1 complex is essential for regulating progenitor self-renewal and differentiation during the initial phases of cortical development. Loss of such control and the change of mitotic orientation in radial glial progenitors may collaboratively impair the precise control of their cell division asymmetry, and lead to increased neurogenic divisions with dramatic over-production and abnormal development of the earliest-born PP and C–R neurons. As a result of these severe defects in early cortical development, the mutant mice displayed a striking size reduction of the cerebral cortex and a complete disorganization of cortical layers.
A dual mechanism of progenitor fate control by Lis1–Nde1
While it is generally believed that the self-renewal and differentiation of radial glial or neuroepithelial progenitors may be controlled by means of cell division asymmetry, it is less clear how such pattern of cell division is achieved during cortical development. A number of studies have suggested that symmetrical proliferative divisions may be regulated by mitotic cleavage orientation by genes that control either cell polarity or the mitotic spindles. However, different results have been obtained by examining the direct correlation between mitotic cleavages axis and progenitor fate (36–38), suggesting that mitotic spindle regulation is not a sufficient fate determinate, but rather one of the many tools used by neural progenitors to control mitotic asymmetry. The Lis1–Nde1 complex is clearly required for the proliferative symmetrical divisions that expand the progenitor pool during the early stage of cortical development. By analyzing and comparing the phenotypes of an allelic series of Nde1–Lis1 single and double mutations, our study showed that the most severe alteration of cell fate correlates tightly with the cell morphology and cytoarchitecture defects of radial glial neuroepithelial progenitors (Table 1). Therefore, Lis1–Nde1 is not only essential for mitotic orientation determination, but also critically required for maintaining the apical integrity and lateral contacts of radial glial neural or neuroepithelial progenitors during mitosis (Fig. 9). The differential inheritance of apical aspects may determine the fate of daughter cells, and the switch between symmetrical and asymmetrical divisions is likely to depend on a very fine balance between the amount of apical and basal membrane inheritance (37). Therefore, the alteration of apical cell morphology by Lis1–Nde1 deficiency would break this balance and result in asymmetrical division regardless of cleavage orientation. Thus, our finding on Lis1–Nde1's role in maintaining the apical morphology of bi-polar VZ progenitors reconciles the conflicting observations on the mitotic spindle regulation of the neurogenic cell fate. To achieve symmetrical division, the polarity and morphology of metaphase progenitors must be co-regulated with the mitotic spindle orientation. Either skewed mitotic spindle or altered cell morphology could result in difficulties in the precise equal partitions of cell fate determinants. Thus, the dual control of the mitotic spindle and cell morphology by Lis1–Nde1 establishes an intimate coupling between the two important elements of cell division asymmetry determinant.
Table 1.
Summary and comparison of Nde1 and Lis1 single and double mutants
| E+/− or L+/− | E−/− | E+/− L+/− | E−/− L+/− | |
|---|---|---|---|---|
| Brain size (% of WT) | 90–100% | 66% | 80% | <20% |
| Cortical layers | Near normal | Reduced II/III | Reduced II/III | Inverted; disorganized |
| VZ reduction at E12.5 (%) | Not detectable | Not detectable | Reduced 10% | Reduced 47% |
| Cell-cycle exit E12–E13 | 29% | NA | 26% | 67% |
| Apoptosis at E12.5 | Rare | Very few | Very few | Substantial |
| PP CR neuron overproduction | −/+ | + | + | +++ |
| Apical/basal mitosis by PH3 at E12.5 (%) | 76/24 | 57/43 | 53/47 | 58/42 |
| Apical/basal mitosis by 4A4 at E12.5 (%) | 94/6 | 73/27 | 74/26 | 46/54 |
| Vertical/horizontal cleavage at E12.5 (%) | 79/6 | 63/12 | 68/8 | 59/13 |
| Progenitor morphology cytoarchitecture defects | −/+ | NA | −/+ | +++ |
The majority of data were from presentations of this study and some were from (35). Phenotypes that tightly correlate to the cell fate alteration in the Nde1−/−Lis1+/− mutant were highlighted.
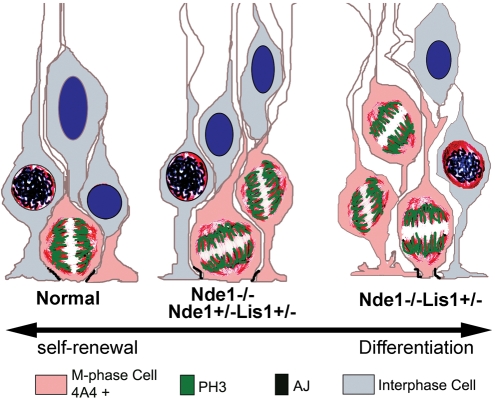
Figure 9.
A schematic presentation of cell fate control by the Lis1–Nde1 complex in radial glial progenitors, and its loss of functional defects. In normal metaphase radial glial progenitors, both the cell body and mitotic chromosomes are apically localized along the ventricular surface, and the cell is in close contact with neighboring progenitors. Such cellular architecture ensures the asymmetrical mitotic division and maintains the progenitor in proliferative state. In the Nde1−/− and Nde1+/−Lis1+/− mutants, although many cells fail to position their mitotic chromosomes apically, large degree of apical cell–cell contacts still remains, resulting in moderate neurogenesis defects. Further loss of Lis1–Nde1 function in the Nde1−/−Lis1+/− progenitors results in destabilization of apical cell membrane and loss of cell–cell contacts. These changes in the cytoarchitecture of metaphase cells act collaboratively with the mitotic spindle orientation defects and induce striking shift of progenitor fate towards neuronal differentiation.
Despite the wide tissue distribution of both Lis1 and Nde1, loss of Lis1–Nde1 function specifically affects the radial glial neuroepithelial progenitors in the developing CNS. In contrast to the significant loss of VZ progenitors in early corticogenesis, the reduction of SVZ progenitors was surprisingly mild in the Nde1−/−Lis1+/− mutant. The relatively preserved SVZ progenitor pools, along with the CNS specific phenotype of Nde1−/−Lis1+/− mutant suggested that the Lis1–Nde1 complex is specifically required in polarized progenitors like radial glial and neuroepithelial cells, but less required by non-polarized cells. This cell type specific requirement is very well in line with a unique function of the Lis1–Nde1 complex in controlling the mitotic asymmetry and neurogenic fate in CNS development.
As a protein complex that regulates the assembly and organization of both interphase and mitotic microtubules, it is not surprising that Nde1–Lis1 double deficiency caused significant alteration of radial glial morphology besides mitotic defects. Although the adherens junction and many apical markers appeared to be preserved, the cell surface membrane of the Nde1−/−Lis1+/− mutant neural progenitor cells appeared unstable and showed reduced lateral contacts to neighboring cells. The severe morphological defect may affect the symmetrical mitotic segregation directly, it may also alter the spatial cues that govern the mitotic spindle orientation (56,57). Moreover, the abnormal cell morphology and lateral contacts could affect the microenvironment and cytoarchitectural niches of neural progenitors in the cortical VZ similar to what was observed in the Numb and Numbl double mutants (58,59). While the disorganization of the pseudostratified VZ progenitors in the Nde1−/−Lis1+/− mutant also resembles the Numb–Numbl double mutants, loss of Lis1–Nde1 did not appear to induce instability or mis-targeting of the Cadherins (Supplementary Material, Fig. S6). Instead, we have found that Nde1 interacts directly with molecules that potentially regulate the actin cytoskeleton and cell-surface stability (Pawlisz and Feng, unpublished observation). Further investigation into these Lis1–Nde1's interacting proteins in regulating the morphology and cytoarchitecture of radial glial progenitors during corticogenesis will allow a better understanding of the mechanism underlying Lis1–Nde1's role in progenitor fate control.
Shaping the cerebral cortex by temporal control of cortical neurogenesis
The formation of the convoluted cerebrum surface with gyri (ridges) and sulci (grooves) is a result of the disproportional increase of neurons in the cerebral cortex relative to the rest of the brain. Moreover, the cortex expands much more laterally than vertically, resulting in a vast increase in the surface area with a relatively smaller increase in thickness during mammalian evolution. The developmental and cellular mechanisms that control the cortical surface expansion are largely unknown. In human, the lissencephalic cortex caused by LIS1 haploinsufficiency is characterized by great reduction in cortical gyri and sulci and surface area, but the thickness of the malformed cortex is usually increased significantly by abnormal neuronal migration and layer disorganization (17). Similarly, Nde1−/−Lis1+/− mutant mice displayed a neocortex that is more significantly reduced in surface area than in thickness (80 versus 20%). Our study suggested that this striking change in cortical surface area is associated with an essential function of Lis1–Nde1 in maintaining the self-renewal of neural progenitors during the early phases of cortical development. A relatively more severe thinning of the cortex, primarily caused by reducing neurons in layers II/III, was observed in the previously reported Nde1−/− mutant. In contrast to significant progenitor loss at the onset of corticogenesis in the Nde1−/−Lis1+/− mutant, reduction of VZ progenitors in the Nde1−/− cortex was not detectable until mid-corticogenesis of E14.5 (35). These experimental observations suggest that the cortical surface area is predominantly determined by the number of progenitors in the early stage of development, whereas cortical thickness relies more on progenitor self-renewal during mid-corticogenesis. Thus, our data are very well inline with the radial unit hypothesis of cortical development (40), and indicate that the Lis1–Nde1 complex regulates the size and shape of the cerebral cortex by regulating the temporal pattern of cortical neurogenesis. Moreover, Lis1 and Nde1 regulate cortical neurogenesis in a dosage-dependent manner. This dosage sensitive collaboration of two genes gives cortical radial glial neuroepithelial progenitors the potential to fine-tune their mitotic mode and cytoarchitecture. Such a regulatory mechanism appears to be important for maintaining the progenitor cells in a proliferative state during earlier phases of cortical development, which may underlie the expansion of the early progenitor pool and the evolutionary power of increasing cortical neuronal production.
Inseparable regulation of cortical neurogenesis and neuronal migration
The Nde1−/−Lis1+/− mutant and the reeler mice both show failed PP splitting and cortical layer inversion. The two mutants nevertheless present opposite defects in the level of Reelin. Contrary to the Reelin absence, over-producing Reelin and Reelin secreting C–R cells by Nde1−/−Lis1+/− mutant was associated with a ‘reeler-like’ phenotype. This suggested that maintaining proper level and distribution of Reelin is essential for cortical lamination. Because abnormal neuronal lamination is one of the most striking defect of the lissencephalic cortices, Lis1 has been believed to be primarily required by the migrating neurons (60,61), and its roles in cell fate control of the proper formation of PP and CR cells have not been appreciated. Although a cell autonomous migration defect of Nde1−/−Lis1+/− neurons remains possible, over produced PP neurons may pose a physical barrier to the incoming cortical plate neurons during PP splitting as well as cortical plate formation. It is also conceivable that the dramatically overproduced, ectopically distributed, and functionally active Reelin alters the normal migration of cortical plate neurons, and is at least partially responsible for the disorganized layer structures seen in the Nde1−/−Lis1+/− mutant. We have observed that reducing the overproduced Reelin in the Nde1−/−Lis1+/− mutant by heterozygous mutation of Reln could partially rescue the migration arrest of late-born cortical neurons (Pawlisz and Feng, unpublished observation). Further studying the effect of elevated and ectopically localized Reelin protein on migration neurons may help to better understand the mechanism of both Lis1 and Reelin in cortical neuronal migration.
In the developing cerebral cortex, radial glial cells serve both as neural progenitors and scaffolds for migrating neurons. Besides the apical destabilization during metaphase, the basal processes of radial glial cells were also severely damaged by the Nde1−/−Lis1+/− mutation. Thus, the radial glial neuroepithelial morphology defects not only alter the progenitor fate, but also impair the radial glial scaffold and contribute directly to neuronal migration defects in the Nde1−/−Lis1+/− mutant.
Neurogenesis and neuronal migration in cortical development are highly orchestrated processes. How these two important steps are coordinated both spatially and temporally to reach an accurate control of the size and structural coordination in mammalian development is yet to be fully understood. Our study demonstrated that cortical neurogenesis and neuronal migration may be regulated by a single protein complex of Lis1–Nde1. The cell division and cytoarchitectural defect of radial glial progenitors induced by Lis1–Nde1 loss of function in early corticogenesis may impair neuronal migration in both direct and indirect fashions.
MATERIALS AND METHODS
Mouse genetics
Lis1+/− and Nde1+/−mice were maintained in mixed 129Sv and NIH Black Swiss background as described (32,35). They were housed and bred according to the guidelines approved by ACUC committee at the Northwestern University. Nde1−/−Lis1+/− and Nde1+/−Lis1+/− mice were obtained by standard genetic crosses. For timed matings, the day of the vaginal plug was considered E0.5.
Histology and immunohistology
Histology and immunohistology were performed as described (35), with 5 µm paraffin sections or 12 µm frozen sections. Antibodies used are: 4A4 ( a gift from Drs M. Inagaki and I. Izawa, Aichi Cancer Center Res. Institute, Japan); BrdU, Foxp1 (Abcm); CR50 (a gift from Dr K. Nakajima, Keio University School of Medicine, Japan); β-catenin (Transduction Lab); Phospho Histone H3 (Upstate); Ki67 (Nova Castro); Pericentrin (Covance); Pax6, Pax3 (Developmental Study Hybridoma Bank); CSPG (Sigma); G10, Calretinin, Tbr1, Tbr2 and GLAST (Chemicon); Cux1 (Santa Cruz); Prominin-1 (eBioscince), and Dab1 (Cell Signalling).
Apoptosis
Apoptosis in mouse embryonic cortex was detected with the Fluorescence In Situ Cell Death Detection Kit (Roche) according to the manufacture's instruction.
Ultra-structural analysis
Fresh E12.5 embryos were fixed in 2% glutaraldehyde, and processed for standard transmission electron microscopy analysis. Specimens were examined with a JEOL 1220 transmission electron microscope equipped with Kodak digital camera.
Cortical lysates and immunoblotting
Cerebral cortices were dissected from E12.5 to E15.5 embryos and flash frozen in liquid Nitrogen. Upon obtaining genotype information, cortical samples were homogenized in 95°C SDS–PAGE sample buffer. Approximately 30 µg total protein from each sample was used for immunoblotting analyses.
In utero electroporation
A CAG-GFP plasmid (62) was used to label a subpopulation of radial glial progenitors through in utero electroporation as described (63). Electroporation was performed on embryos at E12.5 and E13.5, respectively. GFP-labeled embryos were fixed in 24 h and processed for fluorescence imaging analyses.
Quantitative analysis
Quantitative analyses were performed using Image J as described (35). Data were presented as Mean+SD. The statistical significance of differences between different samples was assessed by one-way ANOVA with pairwise comparisons or by student t-test. Western blot signals were quantified using Quantity One software (BioRad).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG Online.
FUNDING
C.A.W. is supported by NIH (2R01NS032457), and C.A.W. is an Investigator of the Howard Hughes Medical Institute. A.C. is supported by NIH (5R01NS047191) and Sontag Distinguished Scientist Award. Y.F. is supported by NIH (K01MH65338), and a career award from the Schweppes Foundation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Drs Eric C. Olson (SUNY Syracuse) and Ed Monuki (UC Irvine) for comments and critical reading of the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Noctor S.C., Flint A.C., Weissman T.A., Dammerman R.S., Kriegstein A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 2.Hanashima C., Li S.C., Shen L., Lai E., Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 3.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 4.Anthony T.E., Klein C., Fishell G., Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 5.Malatesta P., Hack M.A., Hartfuss E., Kettenmann H., Klinkert W., Kirchhoff F., Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 6.Caviness V.S., Jr, Takahashi T., Nowakowski R.S. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- 7.Mission J.P., Takahashi T., Caviness V.S., Jr Ontogeny of radial and other astroglial cells in murine cerebral cortex. Glia. 1991;4:138–148. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T., Nowakowski R.S., Caviness V.S., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J. Neurosci. 1995;15:6058–6068. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T., Nowakowski R.S., Caviness V.S., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T., Nowakowski R.S., Caviness V.S., Jr Interkinetic and migratory behavior of a cohort of neocortical neurons arising in the early embryonic murine cerebral wall. J. Neurosci. 1996;16:5762–5776. doi: 10.1523/JNEUROSCI.16-18-05762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakic P. Neuronal migration and contact guidance in the primate telencephalon. Postgrad Med J. 1978;54(Suppl. 1):25–40. [PubMed] [Google Scholar]
- 12.Goffinet A.M. Events governing organization of postmigratory neurons: studies on brain development in normal and reeler mice. Brain Res. 1984;319:261–296. doi: 10.1016/0165-0173(84)90013-4. [DOI] [PubMed] [Google Scholar]
- 13.Luskin M.B., Shatz C.J. Neurogenesis of the cat's primary visual cortex. J. Comp. Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- 14.Marin-Padilla M. Cajal–Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 15.D'Arcangelo G., Miao G.G., Chen S.C., Soares H.D., Morgan J.I., Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 16.Rice D.S., Curran T. Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger K., Rett A. Agyria-pachygyria (lissencephaly syndrome) Neuropadiatrie. 1976;7:66–91. doi: 10.1055/s-0028-1091611. [DOI] [PubMed] [Google Scholar]
- 18.Stewart R.M., Richman D.P., Caviness V.S., Jr Lissencephaly and Pachygyria: an architectonic and topographical analysis. Acta Neuropathol. (Berl.) 1975;31:1–12. doi: 10.1007/BF00696881. [DOI] [PubMed] [Google Scholar]
- 19.Dobyns W.B., Reiner O., Carrozzo R., Ledbetter D.H. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 20.Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W.B., Caskey C.T., Ledbetter D.H. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 21.Barth P.G. Disorders of neuronal migration. Can. J. Neurol. Sci. 1987;14:1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- 22.Crome L. Pachygyria. J. Pathol. Bacteriol. 1956;71:335–352. doi: 10.1002/path.1700710208. [DOI] [PubMed] [Google Scholar]
- 23.Hattori M., Adachi H., Tsujimoto M., Arai H., Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase [corrected] Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- 24.Shu T., Ayala R., Nguyen M.D., Xie Z., Gleeson J.G., Tsai L.H. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T., Serneo F.F., Higgins C., Gambello M.J., Wynshaw-Boris A., Gleeson J.G. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faulkner N.E., Dujardin D.L., Tai C.Y., Vaughan K.T., O'Connell C.B., Wang Y., Vallee R.B. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- 27.Sheen V.L., Ferland R.J., Harney M., Hill R.S., Neal J., Banham A.H., Brown P., Chenn A., Corbo J., Hecht J., et al. Impaired proliferation and migration in human Miller-Dieker neural precursors. Ann. Neurol. 2006;60:137–144. doi: 10.1002/ana.20843. [DOI] [PubMed] [Google Scholar]
- 28.Siller K.H., Serr M., Steward R., Hays T.S., Doe C.Q. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol. Cell. 2005;16:5127–5140. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yingling J., Youn Y.H., Darling D., Toyo-Oka K., Pramparo T., Hirotsune S., Wynshaw-Boris A. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith D.S., Niethammer M., Ayala R., Zhou Y., Gambello M.J., Wynshaw-Boris A., Tsai L.H. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- 31.Tsai J.W., Chen Y., Kriegstein A.R., Vallee R.B. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirotsune S., Fleck M.W., Gambello M.J., Bix G.J., Chen A., Clark G.D., Ledbetter D.H., McBain C.J., Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat. Genet. 1998;19:333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- 33.Gambello M.J., Darling D.L., Yingling J., Tanaka T., Gleeson J.G., Wynshaw-Boris A. Multiple dose-dependent effects of Lis1 on cerebral cortical development. J. Neurosci. 2003;23:1719–1729. doi: 10.1523/JNEUROSCI.23-05-01719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y., Olson E.C., Stukenberg P.T., Flanagan L.A., Kirschner M.W., Walsh C.A. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y., Walsh C.A. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Haydar T.F., Ang E., Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc. Natl Acad. Sci. USA. 2003;100:2890–2895. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosodo Y., Roper K., Haubensak W., Marzesco A.M., Corbeil D., Huttner W.B. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chenn A., McConnell S.K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 39.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 40.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 41.Rakic S., Davis C., Molnar Z., Nikolic M., Parnavelas J.G. Role of p35/Cdk5 in preplate splitting in the developing cerebral cortex. Cereb. Cortex. 2006;16(Suppl. 1):i35–i45. doi: 10.1093/cercor/bhj172. [DOI] [PubMed] [Google Scholar]
- 42.Caviness V.S., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 43.Arnaud L., Ballif B.A., Cooper J.A. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell Biol. 2003;23:9293–9302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rakic S., Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb. Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- 45.Bielle F., Griveau A., Narboux-Neme N., Vigneau S., Sigrist M., Arber S., Wassef M., Pierani A. Multiple origins of Cajal–Retzius cells at the borders of the developing pallium. Nat. Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 46.Haubensak W., Attardo A., Denk W., Huttner W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl Acad. Sci. USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noctor S.C., Martinez-Cerdeno V., Ivic L., Kriegstein A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 48.Roegiers F., Jan Y.N. Asymmetric cell division. Curr. Opin. Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Zhong W., Jiang M.M., Schonemann M.D., Meneses J.J., Pedersen R.A., Jan L.Y., Jan Y.N. Mouse numb is an essential gene involved in cortical neurogenesis. Proc. Natl Acad. Sci. USA. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahringer J. Control of cell polarity and mitotic spindle positioning in animal cells. Curr. Opin. Cell Biol. 2003;15:73–81. doi: 10.1016/s0955-0674(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki S., Shionoya A., Ishida M., Gambello M.J., Yingling J., Wynshaw-Boris A., Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 52.Sapir T., Elbaum M., Reiner O. Reduction of microtubule catastrophe events by LIS1, platelet- activating factor acetylhydrolase subunit. EMBO J. 1997;16:6977–6984. doi: 10.1093/emboj/16.23.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derewenda U., Tarricone C., Choi W.C., Cooper D.R., Lukasik S., Perrina F., Tripathy A., Kim M.H., Cafiso D.S., Musacchio A., et al. The structure of the coiled-coil domain of Ndel1 and the basis of its interaction with Lis1, the causal protein of Miller-Dieker lissencephaly. Structure. 2007;15:1467–1481. doi: 10.1016/j.str.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Stehman S.A., Chen Y., McKenney R.J., Vallee R.B. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J. Cell Biol. 2007;178:583–594. doi: 10.1083/jcb.200610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weissman T., Noctor S.C., Clinton B.K., Honig L.S., Kriegstein A.R. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb. Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- 56.Thery M., Jimenez-Dalmaroni A., Racine V., Bornens M., Julicher F. Experimental and theoretical study of mitotic spindle orientation. Nature. 2007;447:493–496. doi: 10.1038/nature05786. [DOI] [PubMed] [Google Scholar]
- 57.Thery M., Racine V., Pepin A., Piel M., Chen Y., Sibarita J.B., Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- 58.Rasin M.R., Gazula V.R., Breunig J.J., Kwan K.Y., Johnson M.B., Liu-Chen S., Li H.S., Jan L.Y., Jan Y.N., Rakic P., et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat. Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 59.Petersen P.H., Zou K., Krauss S., Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat. Neurosci. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- 60.Kato M., Dobyns W.B. Lissencephaly and the molecular basis of neuronal migration. Hum. Mol. Genet. 2003;12(Spec No. 1):R89–R96. doi: 10.1093/hmg/ddg086. [DOI] [PubMed] [Google Scholar]
- 61.Wynshaw-Boris A. Lissencephaly and LIS1: insights into the molecular mechanisms of neuronal migration and development. Clin. Genet. 2007;72:296–304. doi: 10.1111/j.1399-0004.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda T., Cepko C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodhead G.J., Mutch C.A., Olson E.C., Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J. Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan W., Assadi A.H., Wynshaw-Boris A., Eichele G., Matzuk M.M., Clark G.D. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci USA. 2003;100:7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.