Abstract
The c-Jun N-terminal kinase (JNK) is a stress-activated member of MAP kinase family. JNK activation has been strongly implicated in inflammatory responses, neurodegeneration, and apoptosis. Recent evidence shows that JNK pathway is also transiently activated in primary sensory neurons after tissue or nerve injury, which is required for the development of hyperalgesia and allodynia. In particular, JNK is persistently activated in astrocytes of the spinal cord after nerve injury, and this activation can maintain central sensitization and mechanical allodynia. In this mini-review, we will provide evidence for the involvement of JNK pathway in regulating persistent pain sensitization. We will also discuss possible upstream signaling mechanisms that cause JNK activation and downstream signaling mechanisms by which JNK modulates pain sensitivity. Thus, targeting JNK pathway might be a useful strategy to treat both neurodegeneration and chronic pain.
Chronic pain, resulting from tissue injury (inflammatory pain) or nerve damage (neuropathic pain) or tumor growth (cancer pain), is a real clinical challenge[10, 22, 30]. Although it is generally believed that chronic pain is caused by neural plasticity that occurs both in the peripheral nervous system (peripheral sensitization) and central nervous system (central sensitization), the mechanisms underlying the induction and maintenance of chronic pain are incompletely understood [15, 46]. Inflammation is a driving force for the pathogenesis of chronic pain by producing multiple inflammatory mediators such as prostaglandin (PGE2), nerve growth factor (NGF), and proinflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α) and interleukin-1beta (IL-1β)] in inflamed or damaged tissues [16, 37]. These inflammatory mediators are not only produced by peripheral tissues, but also by glial cells (e.g. microglia and astrocytes) in the central nervous system. Recently, accumulating evidence supports an important role of spinal microglia and astrocytes in promoting chronic pain [8, 14, 41, 43].
In particular, mitogen-activated protein kinases (MAPKs) are activated in spinal glia after nerve injury and play an important role in chronic pain sensitization by signaling to the inflammatory mediators. On the one hand, MAPK pathways are activated by different inflammatory mediators. On the other hand, activation of MAPK pathways also increases the synthesis of multiple inflammatory mediators. This positive feedback loop leads to more production of inflammatory mediators [16]. There are three major members in the MAPK family, extracellular signal regulated kinase (ERK), p38, and c-Jun N-terminal kinases (JNK) [19]. Recent studies have demonstrated that ERK and p38 MAPKs play important roles in regulating neuronal plasticity and persistent pain sensitization via both neuronal and glial mechanisms [16, 18, 32, 36].
Compared to ERK and p38, much less is known about the role of JNK in pain regulation. JNK is activated by upstream kinase MKK4/7. As a stress-activated protein kinase (SAPK), JNK is normally activated by cellular stress such as heat shock, direct DNA damage, and reactive oxygen species, proinflammatory cytokines, rise of intracellular Ca2+, and under several neurodegenerative conditions [4]. JNK plays an important role in the induction of apoptosis in neurons. Thus, inhibition of JNK pathway can protect against cerebral ischemia and other neurodegenerative conditions [4]. Peripheral neuropathy is a common neurological symptom in AIDS patients. Treatment of neonatal dorsal root ganglion (DRG) neurons with HIV envelope glycoprotein gp120 produces apoptosis, which can be blocked by inhibition of the JNK pathway [2]. The JNK family consists of three genes, jnk1, jnk2 and jnk3. JNK3 is found primarily in brain and has different functions than JNK1 or JNK2 [25]. In a sciatic axotomy model of neuronal injury, death of neonatal DRG neurons is reduced by JNK3 deficiency [23]. Accumulating evidence suggests that JNK also plays a role in the development and maintenance of chronic pain.
Most studies on JNK pathway in DRG neurons are related to apoptosis [2, 23, 42] Interestingly, activation of JNK in DRG neurons only causes death of neonatal neurons but not adult neurons, indicating that adult neurons have a downstream block to apoptosis [42]. After L5-spinal nerve ligation (SNL) in adult rats, there is a rapid (< 1 day) but transient (<10 days) JNK activation in the L5 DRG [49]. This JNK activation in DRG neurons does not results in apoptosis, because death of DRG neurons is not noticeable in the first several weeks after nerve injury [40]. After nerve injury, JNK activation primarily occurs in small diameter C-fiber neurons [32, 49]. This transient JNK activation is involved in the early development of mechanical allodynia after nerve injury, because DRG infusion of the peptide JNK inhibitor D-JNKI-1 prevents the development of SNL-induced mechanical allodynia for a week but does not reverse mechanical allodynia [49].
Although JNK activation in DRG neurons is very prominent in nerve injury conditions, acute inflammation may also cause moderate and transient activation of JNK [9]. Intraplantar injection of capsaicin induces a rapid (within 15 min) heat hyperalgesia that recovers after 3 hours. Intraplantar pretreatment of the JNK inhibitor SP600125 largely prevents the development of the heat hyperalgesia (Fig 1). This acute hyperalgesic effect of JNK is likely to be mediated by JNK activation in nociceptor axons/terminals of the injured skin. Moreover, acute hyperalgesia induced by intraplantar endothelin-1, complete Freund’s adjuvant, is also suppressed by SP600125 [9, 31].
Figure 1. Prevention of capsaicin-induced heat hyperalgesia by JNK inhibition.
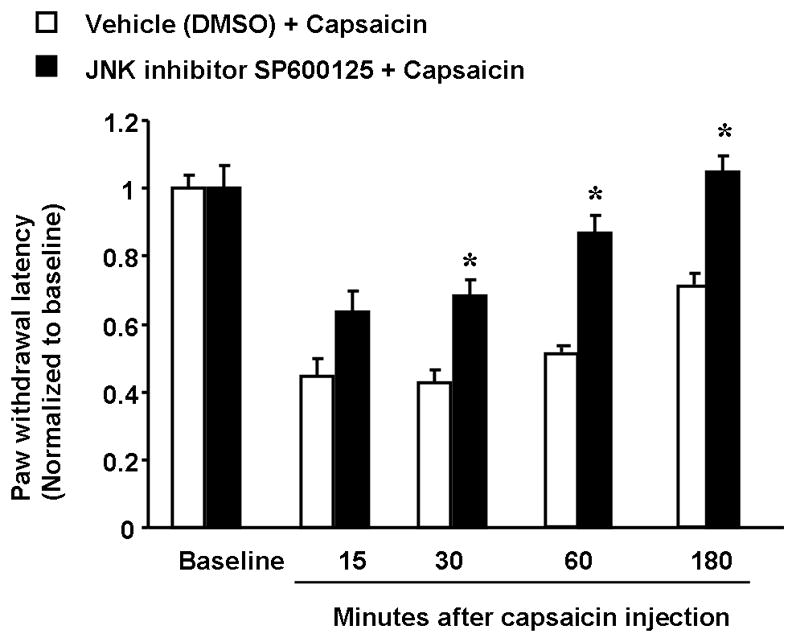
Hindpaw injection (intraplantar) of the JNK inhibitor SP600125 (5 μg in 20 μl) inhibits capsaicin-induced heat hyperalgesia. Capsaicin (15 μg in 5 μl) was injected into the same site of hindpaw 20 min after SP600125 injection. The heat hyperalgesia was measured using Hargreaves apparatus (radiant heat) and paw withdrawal latencies from 15 to 180 minutes after capsaicin stimulation were determined. *, P<0.05, compared to corresponding vehicle control (10% DMSO), n=6, t-test. The baseline paw withdrawal latency is adjusted to 12–15 seconds.
Nerve injury is known to activate both microglia and astrocytes in the spinal cord, but with different time courses. The persistent expression of astrocytic markers correlates well with the persistence of neuropathic pain symptom such as mechanical allodynia [5, 8, 14]. Intrathecal injection of L-alpha-aminoadipate, a relatively specific cytotoxin for astrocytes, was shown to reverse nerve injury-induced mechanical allodynia, supporting a role of spinal astrocytes in maintaining neuroapthic pain symptom [49].
In contrast to transient JNK activation in the DRG, spinal nerve ligation (SNL), induces persistent activation (> 3 weeks) of JNK in the spinal cord [49]. In particular, JNK1 is predominant isoform that is involved in pain modulation. Although both JNK1 and JNK2 are constitutively expressed in the spinal cord, only phosphorylated JNK1 (pJNK1, active form) is expressed in the spinal cord and increased after SNL. Double immunofluorescence has shown that JNK1 is expressed in spinal astrocytes, because it is colocalized with GFAP (astrocytic marker) but not with NeuN (neuronal marker) or OX-42 (microglial marker) [49]. In primary astrocyte cultures, pJNK1 is also the predominant isoform [14]. In the intact spinal cord, pJNK is found almost exclusively in astrocytes, though not all astrocytes expressed pJNK. JNK activation also occurs in spinal astrocytes after partial sciatic nerve injury [27]. We also found that JNK1 but not JNK2 was activated in another chronic pain condition following adjuvant-induced inflammation (unpublished data).
To determine the role of JNK in chronic pain, we used a potent and highly specific peptide inhibitor of JNK [4]. Most MAPK inhibitors are small molecules, designed to target the ATP binding sites of these kinases. However, the peptide inhibitor is derived from JNK binding domain of JNK-interacting protein-1 (JIP-1), and can block selectively the access of JNK to c-Jun and other substrates by a competitive mechanism. A TAT sequence (transporter sequence) is linked to the peptide, enabling the peptide membrane permeable. A convert to D-form amino acids further makes the peptide resistant to proteinases. This peptide inhibitor, named as D-JNKI-1 (D-form JNK inhibitor-1), is an extremely potent neuroprotectant [4]. Spinal infusion of D-JNKI-1 prevents mechanical allodynia for more than 10 days [49]. Because JNK is also activated transiently in primary sensory neurons, the preventive effect of D-JNKI-1 in the first few days might be mediated by JNK activation in the DRG. However, the late phase of allodynia is predominantly, if not exclusively, mediated by JNK activation in the spinal cord. In parallel, a single bolus injection of D-JNKI-1, given 10 days after nerve injury, can block mechanical allodynia for more than 12 hours, and this effect is more potent and prolonged that the small molecule inhibitor SP600125 [49], which has been shown to suppress neuropathic pain in animals with diabetes [6].
What are the upstream mechanisms that cause JNK activation? Complete activation JNK requires dual phosphorylation of threonine and tyrosine residues by the MAPK kinases (MAP2Ks), MKK4 and MKK7. These kinases are in turn activated by the MAPK kinase kinases (MAP3Ks), such as MLKs, ASK1, TAK1, MEKK1, and MEKK4. These MAP3Ks are activated by upstream kinases including cdc42, Rac1, PAK1 that link to a variety of cell receptors sensing stress and inflammation (Fig 2) [3, 7]. Therefore, a diversity of stimuli can activate JNK pathways. Besides stress-related stimuli, the JNK pathway is activated by proinflammatory cytokines such as IL-1β and TNF-α, and growth factors such as basic fibroblast growth factor (bFGF) and transforming growth factor (TGF). Furthermore, the JNK pathway is activated in the innate response following the activation of Toll-like receptor (e.g. TLR-4 and TLR-9) by invading pathogens [44].
Figure 2. Signal transduction of the JNK pathway.
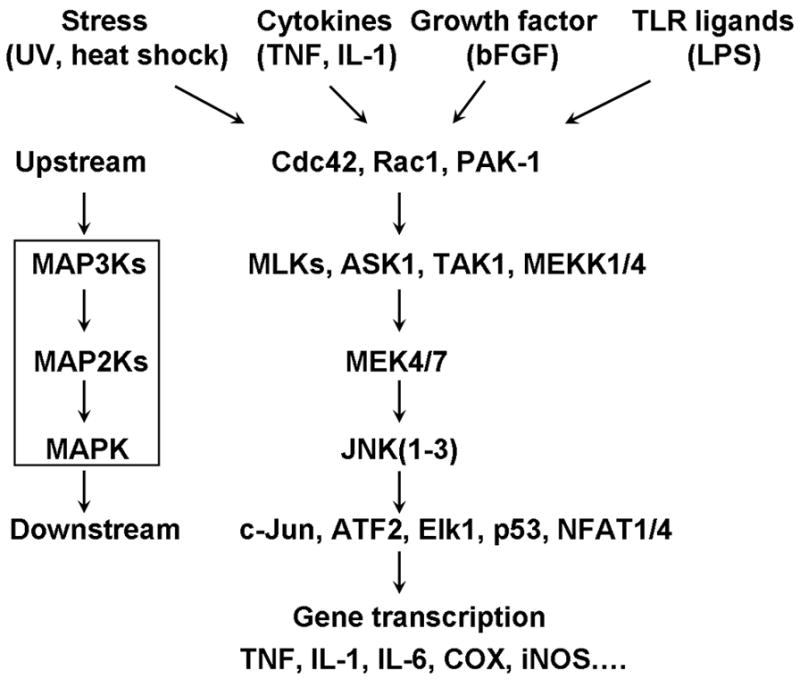
JNK family consists of 3 genes: jnk1, jnk2, and jnk3. While JNK1 is the dominant form in the spinal cord (especially in astrocytes), other forms may exist in the DRG. The JNK pathway can be activated by the exposure of cells to a diversity of extracellular stimuli, such as stress (UV irradiation, heat shock), inflammatory cytokines (TNF-α, IL-1β), growth factors (bFGF, TGF), as well as by the activation of Toll-like receptors. The activated receptors are communicated to upstream kinases of the JNK/MAPK cascade, such as Cdc42, Rac1, PAK-1, p21Rho-GTPase. This MAPK cascade includes various members of the MAP3Ks (such as MLKs, ASK1, TAK1, MEKK1, and MEKK4) and the MAP2Ks MKK4 and MKK7 and leads to the activation of JNK. The activated JNKs translocate to the nucleus and phosphorylate a range of substrates, such as the transcription factors c-Jun, Elk-1, p53, ATF-2, c-Myc, and the NFAT family, leading to gene transcription. The protein products of these genes (e.g., TNF-α, IL-1β, COX1/2) will enhance pain sensitivity. In addition to transcriptional regulation, non-transcriptional regulation can also mediate JNK’s action on pain.
As an activator of astrocytes, bFGF is also a strong activator of JNK in cultured astrocytes [14]. It is produced by astrocytes and strongly induces their mitosis, growth, differentiation and gliosis [11]. After SNL, bFGF immunoreactivity increases in reactive astrocytes in the ipsilateral dorsal horn after nerve injury [29]. Intrathecal infusion of bFGF produces persistent mechanical allodynia [14], whereas bFGF neutralizing antibody reverses nerve injury-evoked tactile allodynia [28]. In cultured astrocytes, bFGF induces robust and persistent activation of JNK [14]. Apart from astrocytes, bFGF is also produced in DRG neurons after nerve injury [17]. Further, nerve injury increases phosphorylation of FGF receptors (FGFR1-4) in DRG neurons [20]. Thus bFGF from DRG neurons may also cause JNK activation in DRG neurons in an autocrine or paracrine manner, as well as JNK activation in spinal astrocytes via bFGF release from central terminals.
Proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 are primary activators of JNK pathway. After tissue or nerve damage, they are upregulated not only in the damaged tissues (e.g., inflamed paws and the peripheral nerves) but also in the DRG and spinal cord, and play important roles in the pathogenesis of pain [8, 35, 37, 38, 43, 45]. In cultured DRG neurons, JNK is activated by both TNF-α [34] and IL-1β [13]. In the spinal cord, these proinflammatory cytokines are mainly produced by glial cells, although they are also made by neurons [8, 33, 43, 47]. In cultured astrocytes, TNF-α has been shown to induce a transient JNK activation with a peak at 15 min and decline after 30 min [48]. It has been well documented that TNFα activates TNF-α associated factor 2 (TRAF2) in cytoplasm by binding to TNF-α receptor types 1 (TNFR1, p55) and 2 (TNFR2, p75) on the cell membrane, leading to activation of JNK pathway [1]. IL-1 was shown to elicit a prominent JNK activation in astrocytes but not in oligodendrocytes [48]. A cytokines mix containing TNF-α, IL-1β and IFN-γ induce a long-lasting activation of JNK in astrocytes [12]. In particular, Liu et al. have shown that TNF-α is able to induce long-term potentiation (LTP) in the spinal cord in rats with neuropathic pain, and this LTP is completely blocked by spinal application of JNK inhibitor, suggesting an important role of TNF-α/JNK pathway in spinal cord neurons sensitization in neuropathic pain states [26]. While JNK can be activated by proinflammatory mediators, it can also be inhibited in astroctyes by anti-inflammatory/anti-hyperalgesic reagent such as lipoxin A4 [39].
What are the downstream mechanisms that mediate the action of JNK activation? As a protein kinase, JNK can phosphorylate a range of substrates. So far, over 50 substrates have been identified for JNKs even in the absence of any large-scale or systemic screening strategy [3]. The activated JNK can be translocated to the nucleus to phosphorylate many nuclear substrates. Apart from some hormone receptors, most nuclear substrates are transcription factors, such as the Jun family (c-Jun, JunB, JunD), Elk-1, p53, ATF-2, JDP2, c-Myc, the NFAT family, the forkhead family, the STAT family, and the Pax family [3]. Phosphorylation of these substrates can modulate their activity in both positive and negative fashion. But for most of transcription factors including c-Jun, ATF2, Elk-1, and P53, phosphorylation increases their transcriptional activity, and subsequently induces gene expression (Fig 2) [3]. In DRG neurons, JNK can regulate the synthesis of CGRP [13] and matrix metalloproteinase-9, a proinflammatory enzyme (Zhuang and Ji, unpublished observation) after stimulation of IL-1β and TNF-α, respectively.
c-Jun is the best known substrate of JNK. After nerve injury, c-Jun phosphorylation is increased in injured DRG neurons [49]. Interestingly, SNL also induces phosphorylation of c-Jun in spinal astrocytes, which is suppressed by JNK inhibition [49]. Therefore, JNK activation after SNL is likely to regulate gene transcription in spinal astrocytes via activation of the transcription factor c-Jun or other transcription factors. As mentioned above, nerve injury induces spinal synthesis of inflammatory mediators such as IL-1β, TNF-α, IL-6, nitric oxide (NO) that is produced by inducible NO synthase (iNOS), and prostaglandin E2 (PGE2) that is produced by either COX-1 or COX-2; all these mediators and enzymes positively contribute to pain sensitization [8, 16, 43]. In cultured astrocytes, inhibition JNK activation by CEP-1347, an inhibitor of JNK upstream kinase MLK (mixed lineage kinases), blocks the expression of COX-2 and iNOS, as well as the release of NO, PGE2 and IL-6 following challenge with a mixture of cytokines [12]. JNK activation in astrocytes also leads to the production of reactive oxygen species (ROX) that is known to have a role in naturopathic pain [21, 24].
In addition to well-establisher transcriptional regulation, JNK also regulates pain sensitivity via rapid posttranslational regulation. For example, we have shown that intraplantar JNK inhibition can prevent heat hyperalgesia within half hour (Fig 1), indicating a possible posttranslational regulation. Given that heat hyperalgesia is mediated by the heat transuding molecule TRPV1 (transient receptor potential subtype V1), JNK may regulate the activity of TRPV1. Also, JNK is likely to mediate TNF-α evoked spinal LTP via posttranslational mechanism, due to its rapid action [26].
In summary, as a stress-activated MAPK, JNK appears to contribute importantly to inflammatory mechanisms of pain that can be dissociated from well-known apoptosis-promoting role of this pathway. JNK activation contributes to pain sensitization via both neuronal and glial mechanisms that may be mediated by different isoforms of JNK. Notably, JNK1 activation in spinal cord astrocytes contributes to the maintenance of chronic pain. Although increasing evidence supports an important role of JNK in pain regulation, the upstream and downstream mechanisms of JNK activation are still elusive. In particular, the key molecular targets that mediate the action of JNK in pain modulation remain to be identified. Given to potent effect of JNK inhibition on neuroprotection and pain relief, targeting JNK pathway might be useful to treat both neurodegeneration and chronic pain in patients suffering from neurodegenerative diseases such as spinal cord injury and diabetic neuropathy.
Acknowledgments
This study was funded by NIH grants DE17794, NS 54932, and TW7180.
References
- 1.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–7. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 2.Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB-and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol. 2004;188:246–53. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–95. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonny C, Borsello T, Zine A. Targeting the JNK pathway as a therapeutic protective strategy for nervous system diseases. Rev Neurosci. 2005;16:57–67. doi: 10.1515/revneuro.2005.16.1.57. [DOI] [PubMed] [Google Scholar]
- 5.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 6.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–54. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- 7.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 8.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 9.Doya H, Ohtori S, Fujitani M, Saito T, Hata K, Ino H, Takahashi K, Moriya H, Yamashita T. c-Jun N-terminal kinase activation in dorsal root ganglion contributes to pain hypersensitivity. Biochem Biophys Res Commun. 2005;335:132–8. doi: 10.1016/j.bbrc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 11.Eclancher F, Perraud F, Faltin J, Labourdette G, Sensenbrenner M. Reactive astrogliosis after basic fibroblast growth factor (bFGF) injection in injured neonatal rat brain. Glia. 1990;3:502–9. doi: 10.1002/glia.440030609. [DOI] [PubMed] [Google Scholar]
- 12.Falsig J, Porzgen P, Lotharius J, Leist M. Specific modulation of astrocyte inflammation by inhibition of mixed lineage kinases with CEP-1347. J Immunol. 2004;173:2762–70. doi: 10.4049/jimmunol.173.4.2762. [DOI] [PubMed] [Google Scholar]
- 13.Hou L, Li W, Wang X. Mechanism of interleukin-1 beta-induced calcitonin gene-related peptide production from dorsal root ganglion neurons of neonatal rats. J Neurosci Res. 2003;73:188–97. doi: 10.1002/jnr.10651. [DOI] [PubMed] [Google Scholar]
- 14.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE 2004. 2004:reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- 17.Ji RR, Zhang Q, Zhang X, Piehl F, Reilly T, Pettersson RF, Hokfelt T. Prominent expression of bFGF in dorsal root ganglia after axotomy. Eur J Neurosci. 1995;7:2458–68. doi: 10.1111/j.1460-9568.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 18.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 20.Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of extracellular signal-regulated protein kinases 5 in primary afferent neurons contributes to heat and cold hyperalgesia after inflammation. J Neurochem. 2007;102:1614–24. doi: 10.1111/j.1471-4159.2007.04698.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki T, Kitao T, Nakagawa K, Fujisaki H, Takegawa Y, Koda K, Ago Y, Baba A, Matsuda T. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–33. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- 22.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 23.Keramaris E, Vanderluit JL, Bahadori M, Mousavi K, Davis RJ, Flavell R, Slack RS, Park DS. c-Jun N-terminal kinase 3 deficiency protects neurons from axotomy-induced death in vivo through mechanisms independent of c-Jun phosphorylation. J Biol Chem. 2005;280:1132–41. doi: 10.1074/jbc.M410127200. [DOI] [PubMed] [Google Scholar]
- 24.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–24. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–9. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, Li YY, Liu XG. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–15. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99:175–84. doi: 10.1016/s0304-3959(02)00097-0. [DOI] [PubMed] [Google Scholar]
- 28.Madiai F, Goettl VM, Hussain SR, Clairmont AR, Stephens RL, Jr, Hackshaw KV. Anti-fibroblast growth factor-2 antibodies attenuate mechanical allodynia in a rat model of neuropathic pain. J Mol Neurosci. 2005;27:315–24. doi: 10.1385/JMN:27:3:315. [DOI] [PubMed] [Google Scholar]
- 29.Madiai F, Hussain SR, Goettl VM, Burry RW, Stephens RL, Jr, Hackshaw KV. Upregulation of FGF-2 in reactive spinal cord astrocytes following unilateral lumbar spinal nerve ligation. Exp Brain Res. 2003;148:366–76. doi: 10.1007/s00221-002-1286-3. [DOI] [PubMed] [Google Scholar]
- 30.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 31.Motta EM, Calixto JB, Rae GA. Mechanical hyperalgesia induced by endothelin-1 in rats is mediated via phospholipase C, protein kinase C, and MAP kinases. Exp Biol Med (Maywood) 2006;231:1141–5. [PubMed] [Google Scholar]
- 32.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–22. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–8. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42:93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- 35.Schafers M, Sommer C. Anticytokine therapy in neuropathic pain management. Expert Rev Neurother. 2007;7:1613–27. doi: 10.1586/14737175.7.11.1613. [DOI] [PubMed] [Google Scholar]
- 36.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–21. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–7. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–62. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 39.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–52. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol. 2000;422:172–80. doi: 10.1002/(sici)1096-9861(20000626)422:2<172::aid-cne2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Walsh GS, Orike N, Kaplan DR, Miller FD. The invulnerability of adult neurons: a critical role for p73. J Neurosci. 2004;24:9638–47. doi: 10.1523/JNEUROSCI.1299-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–85. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 44.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–39. [PubMed] [Google Scholar]
- 46.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 47.Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–21. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Miller BS, Rosenzweig SA, Bhat NR. Activation of C-jun N-terminal kinase/stress-activated protein kinase in primary glial cultures. J Neurosci Res. 1996;46:114–21. doi: 10.1002/(SICI)1097-4547(19961001)46:1<114::AID-JNR14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–60. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


