Abstract
Arterial vein grafts (AVGs) often fail due to intimal hyperplasia, thrombosis, or accelerated atherosclerosis. Various approaches have been proposed to address AVG failure, including delivery of temporary mechanical support, many of which could be facilitated by peri-vascular placement of a biodegradable polymer wrap. The purpose of this work was to demonstrate that a polymer wrap can be applied to vein segments without compromising viability/function, and to demonstrate one potential application; i.e., gradually imposing the mid-wall circumferential wall stress (CWS) in wrapped veins exposed to arterial levels of pressure.
Poly(ester urethane)urea, collagen, and elastin were combined in solution, and then electrospun onto freshly-excised porcine internal jugular vein segments. Tissue viability was assessed via Live/Dead™ staining for necrosis, and vasomotor-challenge with epinephrine and sodium nitroprusside for functionality. Wrapped vein segments were also perfused for 24-hrs within an ex vivo vascular perfusion system under arterial conditions (pressure=120/80 mmHg; flow=100 mL/min), and CWS was calculated every hour.
Our results showed that the electrospinning process had no deleterious effects on tissue viability, and that the mid-wall CWS vs. time profile could be dictated through the composition and degradation of the electrospun wrap. This may have important clinical applications by enabling the engineering of an improved AVG.
Keywords: Electrospinning, Vein Graft, Intimal Hyperplasia, Vein Wrap
1. INTRODUCTION
Coronary artery bypass grafting is the most effective and most widely used treatment for coronary artery disease, with nearly 500,000 procedures being performed annually in the US [1]. In addition there are approximately 80,000 lower extremity bypass surgeries performed each year [2]. The autologous saphenous vein graft remains the graft of choice for 95% of surgeons performing these bypass procedures [3]. Despite their wide use, failure of arterial vein grafts (AVGs) remains a major problem [4]: 12% to 27% of AVGs become occluded in the first year with a subsequent annual occlusive rate of 2% to 4% [2]. Patients with failed grafts will die or require re-operation.
Many attempts have been made to inhibit or counter-act the AVG failure modes of early thrombosis, intimal hyperplasia (IH) and accelerated atherosclerosis, including local delivery of genes [5] and drugs [6], as well as minimization of the compliance mismatch at the anastomoses via external mechanical support [7–12]. IH has received the most attention as it accounts for 20% to 40% of all AVG failures within the first 5 years [13].
There is substantial evidence implicating the abrupt exposure of AVGs to the dynamic mechanical environment of the arterial circulation in the initiation of IH [7,14,15]. In particular, the law of Laplace states that the circumferential wall stress (CWS) in a thin-walled blood vessel such as a vein is directly proportional to its diameter and acting intraluminal pressure. Indeed, the CWS can be increased 140 fold in a distended vein transposed to the arterial circulation compared to that in a vein under normal circumstances [14], and this increase in CWS has been shown to promote IH formation [7,16]. On the other hand, it has been shown that increased levels of shear stress tend to modulate IH [17]. These two biomechanical factors, seemingly causing opposing hyperplastic responses by AVGs, were carefully explored by Dobrin et al., who showed that the increased circumferential stretch plays a more significant role in promoting intimal thickening than the increased shear stress does in preventing it [16]. The thickening associated with IH is thought to be an attempt to return the CWS to venous levels. However, many times this response is uncontrolled and can over-compensate, leading to stenosis or occlusion instead of the desired thickening or “arterialization” of the vein segment.
Preventing acute distension, and hence an abrupt increase in CWS (based on the law of Laplace) of AVGs by adding an external support or sheath has seemingly improved various pathologic responses [7–12]. However, these previous approaches have not resulted in a clinically viable means for improving AVG patency, possibly because they utilized adventitially-placed wraps/sheaths that were biodurable, and/or loose-fitting.
Attempts to inhibit or counter-act AVG failure mechanisms may be facilitated by the placement of a conformal biodegradable polymer wrap on the adventitial surface by gradually imposing arterial levels of CWS to the AVG. For such a wrap the following characteristics would be desirable:
The biodegradation rate of the wrap should be tunable
The wrap should be tight-fitting to control distension under arterial pressure
The wrap must not affect tissue viability or functionality
One possible means to achieve the conformal placement of a biodegradable polymer wrap onto an AVG is through the deposition of a microfibrils by electrospinning [18] immediately following harvest and just prior to implantation. To our knowledge there have been no previous reports of electrospinning a polymer onto living tubular tissues. The purpose of this work was to demonstrate the feasibility of conformally depositing a tunable biodegradable polymer composite as an adventitial wrap to living vascular segments by electrospinning and to assess its effects on tissue viability and functionality.
2. MATERIALS AND METHODS
2.1. Tissue harvest and transport
All animal procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. The porcine internal jugular vein (PIJV) was chosen as a model because of its similarity in inner diameter and wall thickness to the human greater saphenous vein, and because this tissue has previously been used to investigate the pathologic response of veins exposed to arterial hemodynamic conditions [8,12,19]. The surgical harvest procedure was performed in the manner of a saphenectomy for bypass. Briefly, the anesthetized animal was placed in supine position, cervical incisions were made bilaterally, and dissection was done in layers to the vascular fascia of the neck. Each PIJV was identified and dissected proximal to the jugular confluence and distal to the jugular foramen. All tributaries were identified and carefully ligated to avoid leakage. After the desired length (6–8 cm) was exposed, the segment was cannulated on each end with duck billed vessel cannulae. Just prior to explant, a custom-designed vascular clamp [20] was attached onto the ends of the cannulae to maintain the in vivo length of the vessel following removal. The vessel was then cut on either side between the clamped cannulae and the ligations. Immediately after removal, the vessels were placed in a sterile transport box containing lactated ringers solution supplemented with heparin (500 units/L), papaverine (60 mg/L), and Cefoxitin (1.0 g/L). The time between tissue harvest and mounting into the perfusion system described below was always less than one hour. The animal was euthanized upon completion of surgery according to university guidelines.
2.2. Perivascular placement of electrospun polymer wrap
The biodegradable polymer composite used to form the adventitial wrap was based on the poly(ester urethane)urea (PEUU) material developed by Guan et al. [21] and further characterized in electrospun format by Stankus et al. [22,23]. This polymer undergoes hydrolytic degradation in vitro into non-cytotoxic degradation products and has been shown to degrade to near completion in vivo at approximately 3 months [24]. To control the degradation rate of the wrap, a composite of PEUU, collagen, and elastin proteins was utilized, with protein addition used to hasten mass loss.
PEUU was synthesized from poly(ε-caprolactone )diol and 1,4-diisocyanatobutane with putrescine chain extension. PEUU, collagen, and elastin were combined in solution in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), and then electrospun onto a PIJV segment using a procedure explained in detail elsewhere [23]. Briefly, electrospinning conditions included a mixture solution volumetric flowrate of 0.28 µL/s, a distance between nozzle and target of 17 cm, and electrical charges of +12 kV to the nozzle and −3 kV to the target. The target used for fabrication of spun AVGs for implantation was a Type 316 stainless steel mandrel of 3 mm diameter that was carefully inserted into the AVG lumen to avoid endothelial injury. The mandrel and coaxial vein were rotated together at 250 rpm, and translated axially on a linear stage at a speed of approximately 8 cm/s over 10 cm to produce a more uniform coating thickness.
There were three parameters used to tune the mechanical properties and degradation rate of the polymer: 1) the final polymer concentration in a mixture solution; 2) the PEUU:collagen:elastin ratio in the mixture solution; and 3) the wrap thickness, which was proportional to electrospinning time. A summary of all tested combinations of these parameters is shown in Table 1.
Table 1.
Summary of polymer tuning parameter combinations.
| Combination | PEUU:collagen:elastin (%) | Electrospinning Time (min) | Final Concentration (%) |
|---|---|---|---|
| A | 14.3:42.3:42.3 | 20 | 6 |
| B | 25:75:0 | 15 | 6 |
| C | 50:50:0 | 15 | 6 |
| D | 50:50:0 | 20 | 12 |
2.3. Scanning electron microscopy
The electrospun wrapped PIJVs were examined under scanning electron microscopy (SEM). In short, tissue segments designated for SEM were fixed in ultrapure 2.5 % gluteraldehyde, dehydrated through a graded series of ethanol solutions (30–100%), critical point dried (Emscope, CPD 750, Ashford, Kent, UK), then overcoated with vaporized carbon (Cressington Freeze Fracture Device, Cressington, Cranberry, PA, USA). The tissue was visualized using a JEOL JEM-6335F field emission gun SEM (JEOL, Peabody, MA, USA).
2.4. Tissue viability
To assess the effects of the electrospinning process on tissue viability we examined PIJV segments with (“spun”) and without (“sham”) the polymer wrap in place, as well as untreated freshly excised (“control”) tissue. For the sham PIJV segments without the electrospun polymer wrap, we mimicked the electrospinning process up to the point of actually placing the polymer wrap (i.e., including the insertion of the mandrel and rotating/translating the vein within the electrical field). Tissue functionality was assessed using an ex vivo vasomotor challenge as previously described [20]. In short, vessel segments were cannulated, placed under a constant intraluminal pressure of 20 mmHg, and exposed to incremental doses of epinephrine (EPI). Throughout the experiment, outer vessel diameter (D) was continuously measured with a laser micrometer [20]. The baseline diameter (Dbaseline) was measured before injection of the first dose of EPI. EPI was subsequently injected to final concentrations of 2×10−5, 2×10−4, and 2×10−3 mg/mL at 1, 4.5, and 10 minutes, respectively. Following observation of maximal vasoconstriction with each dose, each subsequent dose was administered. After administration of the maximal dose of EPI, and observation of maximal level of constriction (Dconstricted), a 2 mL bolus of 25 mg/mL sodium nitroprusside (SNP) was injected to give a final concentration of 0.125 mg/mL. The diameter at full dilation (Ddilated) was then recorded. The level of constriction in response to EPI was calculated as:
| (1) |
Similarly, the level of dilation in response to SNP was calculated as:
| (2) |
Tissue viability was further examined using Live/Dead™ staining (Molecular Probes, Carlsbad, CA, USA) of cryosections, according to manufacturer’s instructions to assess the level of necrosis. Nuclei were counterstained with Hoechst 33258. Each segment (control, sham control and spun) intended for Live/Dead™ staining was cut in half and placed in static culture within a Petri-dish under standard incubator conditions. One-third of each segment was assessed immediately after electrospinning, one-third after 18 hours of culture, and one-third after 92 hours. 5 mm rings were cut from each sample and embedded in cryomatrix (TBS, Durham, NC) then frozen. Five 8 µm sections were cut from each ring and imaged under 20x magnification using an epifluorescent microscope (Nikon, Model E800, Melville, NY, USA). Two images were taken per section so that a total of 10 fields of view were quantified per PIJV segment. Scion Image (Version Beta 4.02, NIH, Bethesda, MD) was used to count the total number of cells in a field of view. To determine the percentage of live cells in a field of view, dead cells were counted manually, divided by the total number of cells, and multiplied by 100%. The percentage of dead cells was subtracted from 100% to calculate the percentage of live cells.
2.5. Demonstrative application: Gradual imposition of CWS to AVGs
Since it is believed that an abrupt exposure of AVGs to arterial levels of CWS may contribute to their failure modalities [7,14,15], we believe that one potential application of the electrospun biodegradable polymer wrap would be to gradually expose AVGs to arterial levels of CWS. Previous attempts to limit CWS using an external sheath have not been fully successful because they were either biodurable and/or loose fitting [7–12]. To demonstrate how the wrap described here may modulate CWS, and may be tuned to achieve desired results, we examined the CWS-over-time profile for each of the wrap combinations given in Table 1 and compared these to unwrapped vein segments exposed to venous or arterial conditions. This was achieved using the data collected from ex vivo perfusion experiments and a mathematical model for CWS.
Vein segments were mounted in our well established, validated ex vivo vascular perfusion/organ culture system [19,20]. Briefly, the closed loop perfusion design allows the circulation of sterile perfusate (330 mL tissue culture Media 199 supplemented with 1% fetal bovine serum and 1.0 g/L cefoxitin) through the vascular segment as well as circulation of an adventitial bath (1 L DMEM with 1% fetal bovine serum and 1.0 g/L cefoxitin) within a sealed chamber. Both the perfusate and bathing media were maintained at 37 °C and physiologic levels of dissolved gasses. Experiments utilized one of two simulated hemodynamic conditions [19] – either native venous (VEN) or arterial (ART) conditions. To simulate VEN hemodynamics the perfusion loop was set to provide nonpulsatile flow of 20 mL/min and pressure of 20 mmHg. To simulate ART hemodynamics, the system was set to provide a pulsatile pressure waveform of 120/80 mmHg with a mean perfusate flow of 100 mL/min. Separate experiments were performed to examine unwrapped veins under VEN or ART conditions, and wrapped veins under ART conditions (wART). Each perfusion experiment lasted for 24 hours with intraluminal pressure, outer diameter and flowrate being measured every hour. Vein segments were then analyzed histologically as described below.
For biomechanical modeling purposes, consider the schematic in Figure 1 showing an idealized cross section of the vein/wrap complex. The outer layer of the bi-layer compound tube is taken as the electrospun polymer wrap and the concentric inner layer is the vein segment. The mathematical model developed by Vorp et al. for a three-layered bioresorbable graft [25] was adapted for the two-layered geometry represented by Figure 1. Following a similar derivation under similar assumptions as previously described [25], the circumferential wall stress, CWS, at any radius, r, within the inner (vein) layer shown in Figure 1, we obtain:
| (3) |
where a and b are the inner and outer radii, respectively, of the vein layer. Pi is the internal pressure, and P2 is the interfacial pressure acting between the two layers of the concentric cylinder resulting from their difference in mechanical properties. If we assume that for the case shown in Figure 1 Po = 0 (i.e., atmospheric pressure), and use a similar derivation as done previously [25], we find for P2:
| (4) |
Here, ν is the Poisson’s ratio (assumed equal for the wrap and the vein), EV is the Young’s modulus of elasticity for the vein and EW that for the wrap. Pi and the outer diameter (c) were measured in our ex vivo perfusion experiments. Again using derivations and assumptions as described in our previous report [25], we estimated the inner (a) and interfacial (b) radii for each set of measured Pi and c, to yield:
| (5) |
and
| (6) |
where L is the length of the vein segment, and the subscripts p and u refer a quantity to its pressurized state within the perfusion system and its unpressurized state on the benchtop, respectively. Substituting equation (4), equation (5) and equation (6) into equation (3), and evaluating at the mean arterial pressure and at , we can calculate the mid-wall CWS in the polymer wrapped vein from equation 3. We used EW = 7.5 MPa [23], EV = 600 KPa [26], and ν = 0.5 (i.e., both materials assumed to be incompressible) in our calculations.
Figure 1.
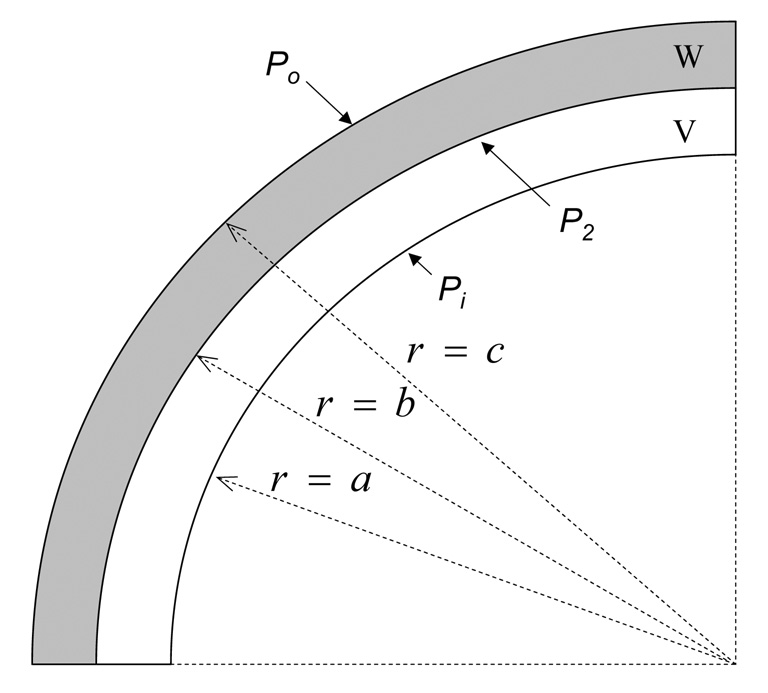
Schematic showing a cross-sectional view of the vein /wrap complex.
2.6 β-stiffness
Inner diameter (OD) and intraluminal pulsatile pressure (P) were measured each hour and used to calculate the β-stiffness (β) of both spun and sham control PIJVs. Using a sampling frequency of 150 Hz, the hourly measurements were made for 5 seconds so that approximately 5 complete pulse cycles of data were collected. The acquired signals were then filtered and plotted. Using the maximum (ODs and Ps) and minimum (ODd and Pd) values for each cycle, β was calculated from [27]:
| (7) |
The 5 values were averaged and a single value β was calculated every hour over the 24 hour perfusion.
2.7. Histology
Following removal of the veins from the ex vivo perfusion system, they were fixed in 2% paraformaldehyde for 4 hours at 4°C followed by 30% sucrose at 4°C overnight. 5 mm tissue rings were cut, washed with PBS, embedded in paraffin, and cut into 5 µm sections. The tissue sections were either stained with hematoxylin and eosin (H&E), or Masson’s trichrome (MTC) stains. Stained tissue sections were then visualized using an Olympus Provis light microscope (Olympus, Center Valley, PA, USA) and compared qualitatively.
2.8. Statistics
For the vasomotor challenge data, a paired student’s t-test for means was performed, and P<0.05 was considered statistically significant. Unless indicated otherwise, data are given as mean ± standard error of the mean.
3. RESULTS
The electrospun adventitial wrap exhibited high porosity and tight adherence to the adventitial surface of the veins (Figures 2A–C), which suggests that the wrap would provide structural support to an AVG without inhibiting adventitial nutrient and gas diffusion into the tissue. Another important observation was that the electrospinning process did not appear to damage the endothelial layer, which remained continuous (Figure 2D).
Figure 2.
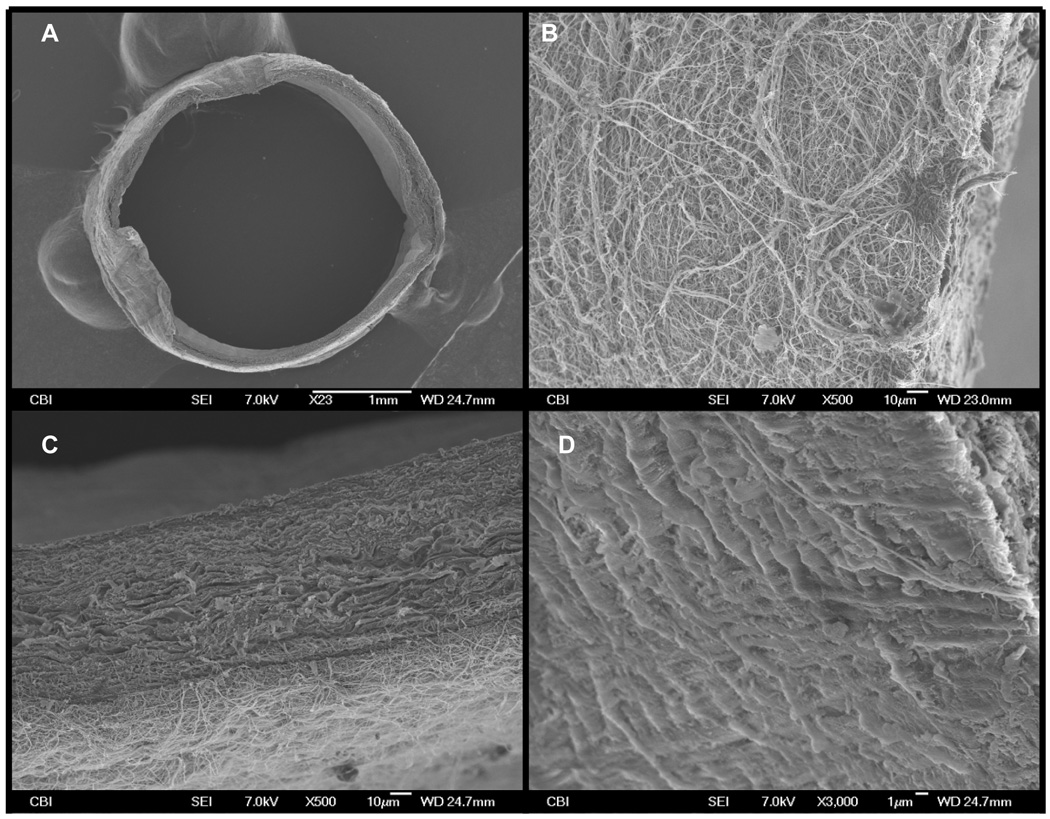
(A) shows a low magnification SEM image of the PIJV segment with the electrospun polymer deposited onto its adventitial surface. (B) is an SEM image (taken at 500x magnification) of the adventitial surface of the PIJV after the polymer wrap was applied. Note the high porosity of the polymer wrap. (C) is an SEM image (taken at 500x magnification) showing the attachment of the polymer wrap to the vein. (D) is an SEM image (taken at 500x magnification of the luminal surface of the vein and shows a continuous endothelium layer which appears to have remained intact.
The results of a typical vasomotor challenge experiment are shown in Figure 3. The sham PIJV segment responded in a predictable dose-dependent manner to stimulation with EPI, while the spun PIJV exhibited a single contraction commencing with the lowest dose of EPI. Vasodilation in response to SNP was similar for both the control and spun PIJVs, each resulting in a larger outer diameter than that at baseline, suggesting a certain level of basal tone in both the sham and spun PIJVs. Overall, there was no significant difference in the level of contraction (Figure 4A) or dilation (Figure 4B) between sham and spun PIJV segments. Furthermore, there was no significant difference in Live/Dead™ staining between each experimental group for each timepoint (data not shown).
Figure 3.
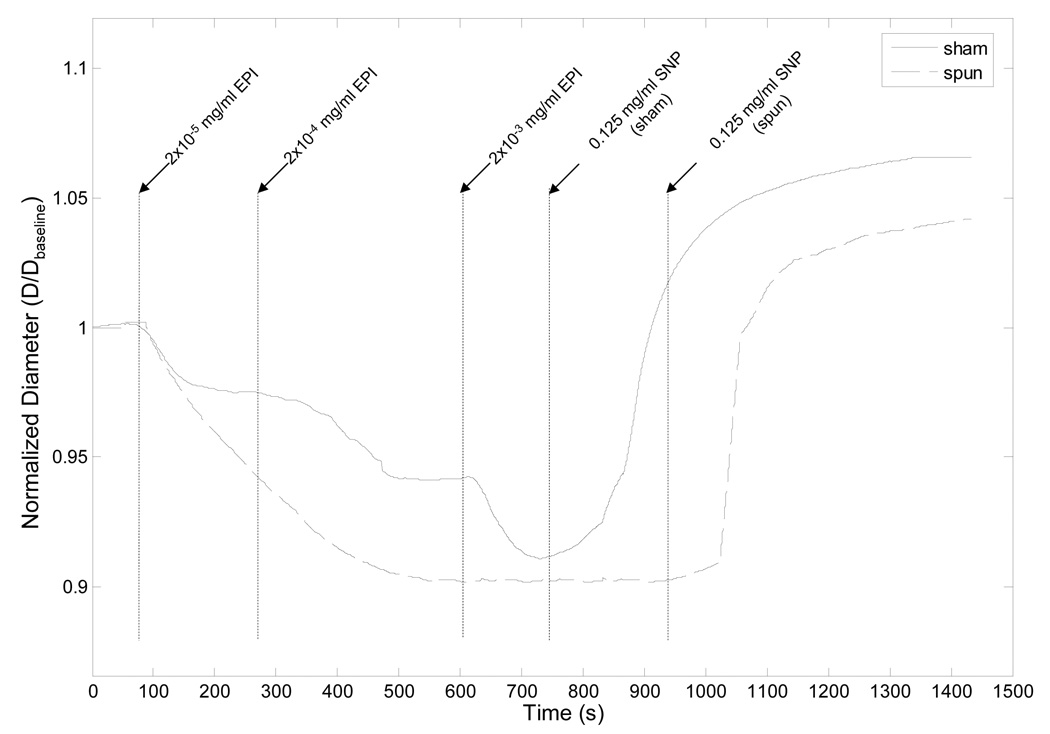
Representative vasomotor challenge results obtained using epinephrine (EPI) and sodium nitroprusside (SNP) to stimulate both a spun and a sham control PIJV segment. Since SNP was not administered until a full relaxation of the tissue was observed following stimulation with EPI, SNP was administered at different times for the sham and spun PIJVs. Outer diameter measurements of each PIJV segment over the duration of the experiments were normalized to the baseline outer diameter which was measured prior to administration of the first dose of EPI.
Figure 4.
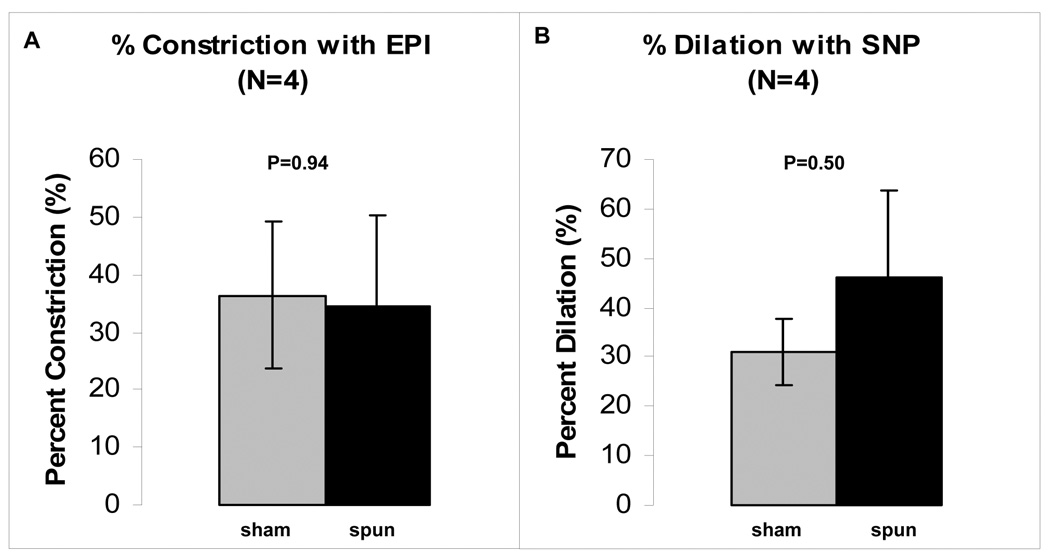
Results from vasomotor challenge experiments (N=4). There appears to be no significant difference in the level of contraction or dilation between the sham control and spun PIJVs. The data are presented as mean ± standard error of the mean.
Histologic images were consistent with the SEM images in that they also showed the polymer wrap to be well attached to the adventitial surface of the vein and that the electrospun polymer composite achieved an approximately uniform thickness of 200 µm (Figure 5A and 5C). Further, the fibrillar coating was nearly completely lost following the 24 hour perfusion period (Figures 5B and 5D).
Figure 5.
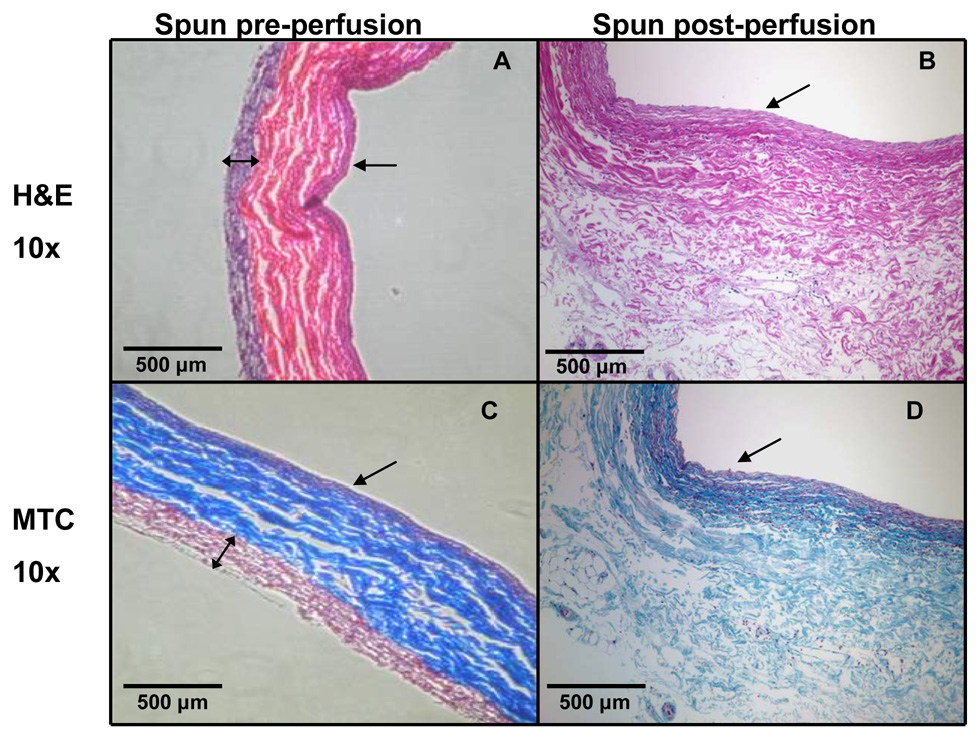
H&E (A,B) and Masson’s trichrome images (C,D) for both before perfusion and after wrapping procedure (A,C) and after 24 hours of ex vivo perfusion (B,D). Note the uniform thickness of the polymer wrap prior to perfusion, and the absence of the polymer wrap in the post-perfusion images. The single-headed arrow indicates the vessel lumen. The double-headed arrow in (A) and (C) indicates the thickness of the polymer wrap, which was not detectable in (B) or (D).
The structural support offered to veins by the wrap is evident when the outer diameter of wrapped veins under ART conditions is compared to that for unwrapped veins (Figure 6A). Figure 6B shows that spun PIJVs appear to be less stiff than sham controls when exposed to ART conditions.
Figure 6.
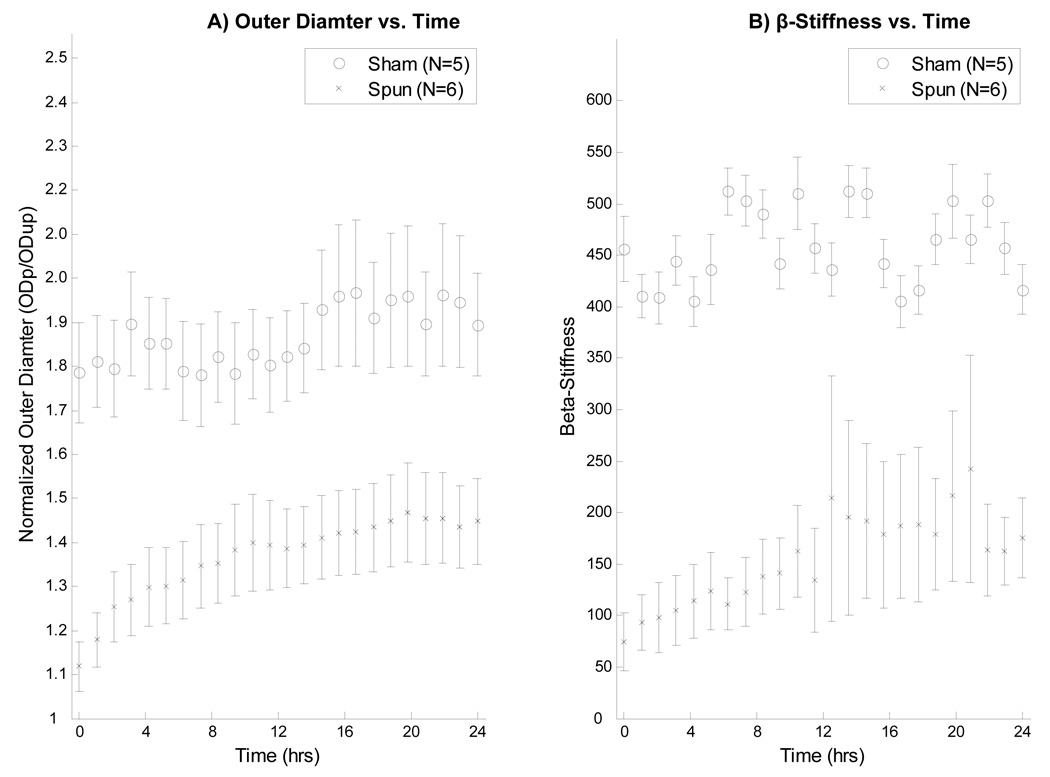
(A): Normalized outer diameter over time for both sham and spun PIJVs under ART conditions. Note that the normalized diameter of the spun veins is markedly reduced compared to sham controls. Pressurized outer diameter (ODp) was normalized to unpressurized outer diameter (ODup) for each vein. (B): β-stiffness values for both sham and spun PIJVs under ART conditions over 24 hours.
The CWS-over time profile for the polymer solution combinations of Table 1 were quite variable (Figure 7). In one case (combination B), the wrap degraded too quickly and resulted in a rapid increase in CWS under ART conditions. Other combinations (C and D) did not degrade quickly enough and resulted in no appreciable increase in CWS over a 24-hour period. Combination A degraded at a rate which resulted in a nearly linear variation over the 24-hour period between VEN and ART levels of CWS. This combination was repeated (N=7) and the effect was found to be repeatable.
Figure 7.
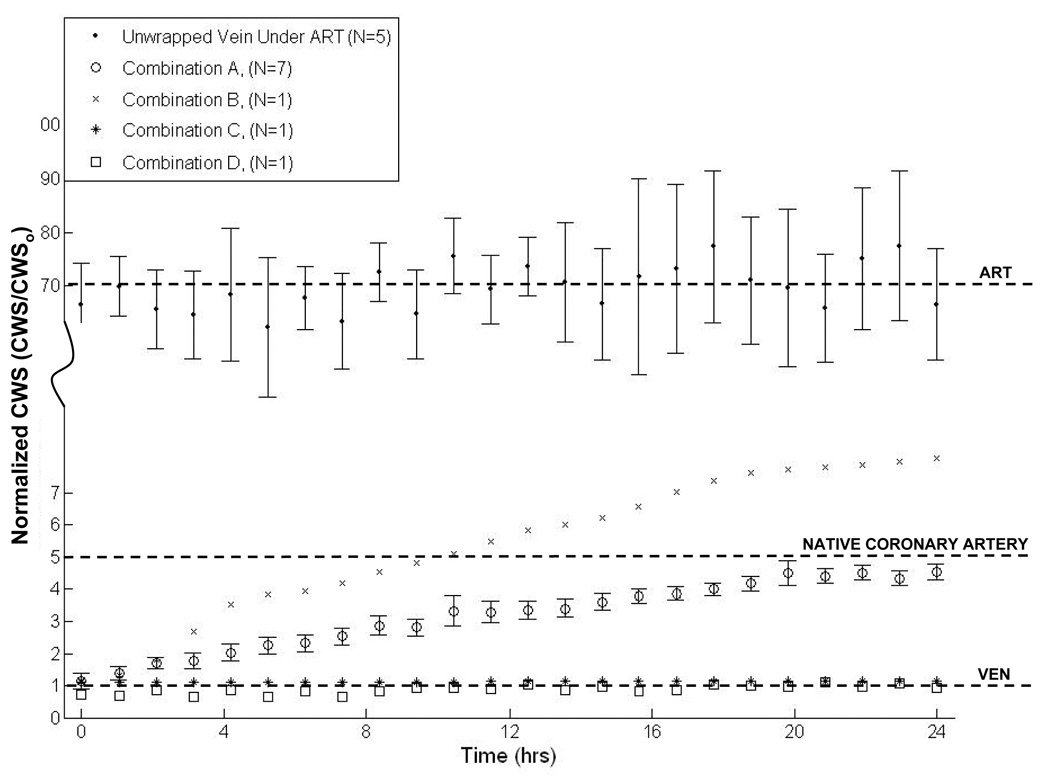
Normalized CWS vs. time results from 24 hour ex vivo perfusions of electrospun polymer wrapped PIJV segments for each combination of polymers as shown in Table 1. Normalization was to CWSo, the mean CWS level measured in an unwrapped vein under venous conditions (~25 KPa), which is represented by the lower dashed horizontal line. The middle dashed horizontal line indicates the mean CWS in a coronary artery (~120 KPa) [32]. The upper dashed line represents the mean CWS measured in an unwrapped vein (sham control) under ART conditions (~1.75 MPa). In the legend, ET stands for electrospinning time.
4. DISCUSSION
The work presented in this report shows, that a biodegradable electrospun polymer wrap can be safely (Figure 2–Figure 4) and uniformly (Figure 5) electrospun onto vein segments, and that the wrap can be tuned to completely degrade (Figure 5) such that CWS is applied to an AVG at a desired rate (Figure 7).
The use of an external sheath around vein grafts was first described by Parsonnet et al. [11]. They showed that the sheath prevented dilatation and that it was well accepted by the host tissue [11]. Karayannacos et al. showed reduced thrombosis and sub-endothelial proliferation in AVGs with both loose- and tight-fitting Dacron mesh sheaths compared with unsupported control grafts [9]. Mehta et al. demonstrated that placement of an external, macroporous, nonrestrictive, polyester stent reduces neointima formation in porcine vein grafts [28]. More recently, polytetrofluoroethylene sheaths were used to permanently restrict AVGs from expansion under arterial pressure and this led to reduced IH formation in a pig model [10]. Clinical translation of permanent mechanical support to AVGs has not yet been reported, most likely due to the unfavorable inflammatory response to biodurable synthetic materials in vascular applications [29]. This limitation motivated Vijayan et al. and Jeremy et al. to use a polyglactin based biodegradable sheath to reduce IH in AVGs [8,12]. The noted beneficial effects included enhanced neo-vasa-vasorum development over unwrapped controls [12]. However, these biodegradable sheaths were loose-fitting and allowed the AVGs to expand to their maximum diameters under arterial pressure, and thus did not offer mechanical support against the increased level of CWS. Huynh et al. used a temporary, non-restrictive, external collagen tube support to reduce IH formation in rabbit vein grafts, but no mention of the degradation kinetics was reported [7]. It is known, however, that cross-linked collagen degrades very rapidly in an aqueous solution [30] and hence the structural support offered to AVGs by sheaths made of collagen alone may not be effective over the long-term. An external AVG sheath developed by Liao et al. was designed to degrade at a desired rate in order to transfer CWS to an AVG gradually over time [31]. Poly lactic-co glycolic acid sheets were prefabricated into tubes by wrapping around a Teflon rod, and therefore are not customizable to each AVG [31]. Therefore, this approach also allows expansion of an AVG under arterial pressure before delivering any mechanical support. The degradation kinetics and resulting CWS vs. time profile in the sheaths, not in the mid-AVG-wall as described here, were reported [31]. Our approach addresses the two major limitations associated with this previous work, specifically with respect to biodurable and/or non-restrictive external sheaths. Another advantage of a tight-fitting AVG wrap would be that a restriction in diameter would have a dually beneficial effect. That is, there would be a reduction in CWS as well as an increase in shear stress which both have been shown to attenuate IH development in AVGs [7,17].
Delivery of mechanical support to AVGs is but one possibility for an adventitial wrap. Other applications could be as a vehicle for the local delivery of biochemicals, drugs, genes, or cells. Kohler et al. used a biodegradable mesh to deliver paclitaxel to effectively reduce IH at the graft-vein anastomosis in a sheep model of dialysis access [6]. Such activities could theoretically be incorporated using the electrospun polymer wrap technique, with the potential to control the delivery rate to some extent by tuning the degradation rate of the electrospun polymer wrap.
Some limitations of this report should be noted. Although the Live/Dead™ assay is widely used to evaluate necrosis in living cells and tissues, it arguably was not ideally suited for our application. This was due to the limited distance the reagents were able to diffuse through the thickness of vascular tissue. However, the vasomotor challenge data indicated that the spun PIJV was able to contract with the same intensity as the sham control which demonstrated the functional viability of the SMCs comprising tissue. Finally, we would have ideally compared the vasomotor responses of the sham and spun PIJVs to a baseline control response. However, obtaining a third segment of PIJV for immediate testing was not feasible since we could only harvest two PIJV segments per animal.
5. CONCLUSIONS AND FUTURE OUTLOOK
We showed here that a tunable, biodegradable polymer wrap can be applied to vein segments via electrospinning without compromising viability or function, and demonstrated one potential application; i.e., gradually imposing the mid-wall CWS in wrapped veins exposed to arterial levels of pressure. The gradual imposition of arterial levels of CWS, rather than abrupt exposure, may be an important new means to reduce the hyperplastic response of AVGs, promoting instead safe arterialization.
ACKNOWLEDGEMENTS
The authors would like to thank Jennifer Debarr for her help with histology, and the Center for Biological Imaging for their help with the scanning electron microscopy. The authors would also like to thank J. Scott VanEpps, PhD, Alejandro Nieponice, MD, Edith Tzeng, MD and Rabih Chaer, MD, for their help with animal surgeries. This study was funded by the NIH (grant # RO1 HL65745 to D. A. Vorp), the Competitive Medical Research Fund of the University of Pittsburgh Medical Center, and the Department of Health, Commonwealth of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.AHA. Heart disease and stroke statistics: 2006 update. Dallas, TX: American Heart Association; 2006. [Google Scholar]
- 2.Feinglass J, Kaushik S, Handel D, Kosifas A, Martin G, Pearce WH. Peripheral bypass surgery and amputation. Arch. Surg. 2000;135:75. doi: 10.1001/archsurg.135.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Shears LL, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, et al. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998;187(3):295–306. doi: 10.1016/s1072-7515(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 4.Statistics, NCfH. Quarterly fact sheet: Monitoring health care in america. Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 5.Petrofski JA, Hata JA, Gehrig TR, Hanish SI, Williams ML, Thompson RB, et al. Gene delivery to aortocoronary saphenous vein grafts in a large animal model of intimal hyperplasia. J Thorac Cardiovasc Surg. 2004;127(1):27–33. doi: 10.1016/j.jtcvs.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable vascular wrap paclitaxel-eluting mesh. J Vasc Surg. 2007;45(5):1029–1037. doi: 10.1016/j.jvs.2007.01.057. discussion 1037–8. [DOI] [PubMed] [Google Scholar]
- 7.Huynh TT, Davies MG, Trovato MJ, Svendsen E, Hagen PO. Alterations in wall tension and shear stress modulate tyrosine kinase signaling and wall remodeling in experimental vein grafts. J Vasc Surg. 1999;29(2):334–344. doi: 10.1016/s0741-5214(99)70386-1. [DOI] [PubMed] [Google Scholar]
- 8.Jeremy JY, Bulbulia R, Johnson JL, Gadsdon P, Vijayan V, Shukla N, et al. A bioabsorbable (polyglactin), nonrestrictive, external sheath inhibits porcine saphenous vein graft thickening. J Thorac Cardiovasc Surg. 2004;127(6):1766–1772. doi: 10.1016/j.jtcvs.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Karayannacos PE, Hostetler JR, Bond MG, Kakos GS, Williams RA, Kilman JW, et al. Late failure in vein grafts: Mediating factors in subendothelial fibromuscular hyperplasia. Ann Surg. 1978;187(2):183–188. doi: 10.1097/00000658-197802000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SQ, Moore MM, Glucksberg MR, Mockros LF, Grotberg JB, Mok AP. Partial prevention of monocyte and granulocyte activation in experimental vein grafts by using a biomechanical engineering approach. J Biomech. 1999;32(11):1165–1175. doi: 10.1016/s0021-9290(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 11.Parsonnet V, Lari AA, Shah IH. New stent for support of veins in arterial grafts. Arch Surg. 1963;87:696–702. doi: 10.1001/archsurg.1963.01310160158031. [DOI] [PubMed] [Google Scholar]
- 12.Vijayan V, Shukla N, Johnson JL, Gadsdon P, Angelini GD, Smith FC, et al. Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. J Vasc Surg. 2004;40(5):1011–1019. doi: 10.1016/j.jvs.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 13.Davies MG, Hagen PO. Pathophysiology of vein graft failure: A review. Eur J Vasc Endovasc Surg. 1995;9(1):7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu SQ, Fung YC. Changes in the organization of the smooth muscle cells in rat vein grafts. Ann Biomed Eng. 1998;26(1):86–95. doi: 10.1114/1.52. [DOI] [PubMed] [Google Scholar]
- 15.Sumpio B. Hemodynamic forces and vascular cell biology. Austin: R.G. Landes Company; 1993. [Google Scholar]
- 16.Dobrin PB, Littooy FN, Endean ED. Mechanical factors predisposing to intimal hyperplasia and medial thickening in autogenous vein grafts. Surgery. 1989;105(3):393–400. [PubMed] [Google Scholar]
- 17.Gusic RJ, Myung R, Petko M, Gaynor JW, Gooch KJ. Shear stress and pressure modulate saphenous vein remodeling ex vivo. J Biomech. 2005;38(9):1760–1769. doi: 10.1016/j.jbiomech.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Taylor GI. Disintegration of water drops in an electric filed. Proc R Soc Lond. 1964;280:383–397. [Google Scholar]
- 19.Severyn DA, Muluk SC, Vorp DA. The influence of hemodynamics and wall biomechanics on the thrombogenicity of vein segments perfused in vitro. J Surg Res. 2004;121(1):31–37. doi: 10.1016/j.jss.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Ligush J, Labadie RF, Berceli SA, Ochoa JB, Borovetz HS. Evaluation of endothelium-derived nitric oxide mediated vasodilation utilizing ex vivo perfusion of an intact vessel. J Surg Res. 1992;52(5):416–421. doi: 10.1016/0022-4804(92)90305-j. [DOI] [PubMed] [Google Scholar]
- 21.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61(3):493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 22.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stankus JJ, Guan J, Wagner WR. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res A. 2004;70(4):603–614. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto KL, Guan J, Oshima H, Sakai T, Wagner WR. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg. 2007;83(2):648–654. doi: 10.1016/j.athoracsur.2006.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorp DA, Raghavan ML, Borovetz HS, Greisler HP, Webster MW. Modeling the transmural stress distribution during healing of bioresorbable vascular prostheses. Ann Biomed Eng. 1995;23(2):178–188. doi: 10.1007/BF02368324. [DOI] [PubMed] [Google Scholar]
- 26.Wesly RL, Vaishnav RN, Fuchs JC, Patel DJ, Greenfield JC. Static linear and nonlinear elastic properties of normal and arterialized venous tissue in dog and man. Circ Res (Online) 1975;37(4):509–520. doi: 10.1161/01.res.37.4.509. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi K. Experimental approaches on measuring the mechanical properties and constitutive laws of arterial walls. J Biomech Eng. 1993;115(4B):481–488. doi: 10.1115/1.2895528. [DOI] [PubMed] [Google Scholar]
- 28.Mehta D, George SJ, Jeremy JY, Izzat MB, Southgate KM, Bryan AJ, et al. External stenting reduces long-term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arteriovenous bypass grafting. Nat Med. 1998;4(2):235–239. doi: 10.1038/nm0298-235. [DOI] [PubMed] [Google Scholar]
- 29.Bunt TJ. Synthetic vascular graft infections. I. Graft infections. Surgery. 1983;93(6):733–746. [PubMed] [Google Scholar]
- 30.Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27(8):1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Liao SW, Lu X, Putnam AJ, Kassab GS. A novel time-varying poly lactic-co glycolic acid external sheath for vein grafts designed under physiological loading. Tissue Eng. 2007;13(12):2855–2862. doi: 10.1089/ten.2007.0009. [DOI] [PubMed] [Google Scholar]
- 32.Holzapfel GA, Sommer G, Gasser CT, Regitnig P. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am J Physiol Heart Circ Physiol. 2005;289(5):H2048–H2058. doi: 10.1152/ajpheart.00934.2004. [DOI] [PubMed] [Google Scholar]


