Abstract
Genome replication of mammalian orthoreovirus (MRV) occurs in cytoplasmic inclusion bodies called viral factories. Nonstructural protein μNS, encoded by genome segment M3, is a major constituent of these structures. When expressed without other viral proteins, μNS forms cytoplasmic inclusions morphologically similar to factories, suggesting a role for μNS as the factory framework or matrix. In addition, most other MRV proteins, including all five core proteins (λ1, λ2, λ3, μ2, and σ2) and nonstructural protein σNS, can associate with μNS in these structures. In the current study, small interfering RNA targeting M3 was transfected in association with MRV infection and shown to cause a substantial reduction in μNS expression as well as, among other effects, a reduction in infectious yields by as much as 4 log10 values. By also transfecting in vitro-transcribed M3 plus-strand RNA containing silent mutations that render it resistant to the small interfering RNA, we were able to complement μNS expression and to rescue infectious yields by ~100-fold. We next used μNS mutants specifically defective at forming factory-matrix structures to show that this function of μNS is important for MRV growth; point mutations in a C-proximal, putative zinc-hook motif as well as small deletions at the extreme C terminus of μNS prevented rescue of viral growth while causing μNS to be diffusely distributed in cells. We furthermore confirmed that an N-terminally truncated form of μNS, designed to represent μNSC and still able to form factory-matrix structures, is unable to rescue MRV growth, localizing one or more other important functions to an N-terminal region of μNS known to be involved in both μ2 and σNS association. Thus, factory-matrix formation is an important, though not a sufficient function of μNS during MRV infection; μNS is multifunctional in the course of viral growth.
Keywords: inclusion body, Reoviridae, silencing, siRNA, viral factory, viroplasm
Introduction
Mammalian orthoreovirus (MRV) is the type species of family Reoviridae (reviewed in Schiff et al., 2007). MRV contains ten genome segments of double-stranded RNA (dsRNA)—three large (L), three medium (M), and four small (S)—enclosed within a bilayered protein capsid that lacks a lipid envelope. Cell entry results in delivery of the inner-capsid (“core”) particle into the target cytoplasm. Once in the cytoplasm, the core particle begins to transcribe the viral genome to produce full-length, capped plus-strand RNAs ([+]RNAs) (Banerjee and Shatkin, 1970; Levin et al., 1970; Skehel and Joklik, 1969), which can function as mRNAs for the translation of viral proteins.
MRV replicates its genome in distinctive cytoplasmic inclusion bodies, often called viral factories (Gomatos, 1967; Fields et al., 1971; Parker et al., 2002). These structures form early in infection as small round inclusions, which then grow larger and move toward the nucleus as infection proceeds. Later in infection with most, but not all MRV strains, they are anchored to and spread along stabilized microtubules (Dales, 1963; Parker et al., 2002; Spendlove et al., 1963). Previous studies have shown that the factories contain fully and partially assembled viral particles, viral proteins, dsRNA, microtubules, and “kinky” filaments proposed to be intermediate filaments (Dales, 1963; Dales et al., 1965; Fields et al., 1971; Spendlove et al., 1963; Sharpe et al., 1982; Tournier and Plissier, 1960). Factories appear not to contain ribosomes or membrane-bound compartments, nor are they membrane-bound themselves (Gomatos et al., 1967; Rhim et al., 1962; Sharpe et al., 1982; Tournier and Plissier, 1960). The localized sequestration and concentration of viral RNAs, viral proteins, and cellular proteins is what defines the viral factory, although the exact composition and functions of the factory are only partially characterized.
The MRV nonstructural protein μNS comprises 721 amino-acid residues (80 kDa) and is encoded by genome segment M3 (McCrae and Joklik, 1978; Mustoe et al., 1978). Expression of μNS in transfected cells is sufficient to form cytoplasmic inclusions morphologically similar to viral factories at low resolution (Becker et al., 2003; Broering et al., 2002). We have therefore hypothesized that μNS forms the factory framework or “matrix” (for early use of this term, see Rhim et al., 1962; Tournier and Plissier, 1960). Most other viral proteins, including all five core proteins (λ1, λ2, λ3, μ2, and σ2) and nonstructural protein σNS, have been shown to associate specifically and independently with μNS in the factory-matrix structures (often called factory-like structures, or FLS, in previous literature) (Becker et al., 2003; Broering et al., 2002, 2004; Miller et al., 2003, 2007). We have thus also hypothesized that μNS plays further roles in recruiting such components to the factories. These proteins may be individually or cooperatively involved in RNA assortment, minus-strand synthesis, and core assembly.
After outer-capsid assembly, the transcriptional activity of MRV core particles is repressed (Astell et al., 1972; Drayna and Fields, 1982; Joklik, 1972; Yamakawa et al., 1982). When complexed with μNS in vitro, outer-capsid proteins are prevented from recoating the cores, which remain active for transcription and capping despite the presence of μNS (Broering et al., 2000). This suggests that μNS may also delay outer-capsid assembly during infection so that larger amounts of [+]RNAs can be produced by cores. Entering core particles can be localized to factory-matrix structures (Broering et al., 2004), and during viral infection the synthesis of viral RNA can be detected in the factories (C.L. Miller and M.L.N., manuscript in preparation).
A second form of μNS, called μNSC, lacks about 5 kDa from its N terminus and is routinely detected in infected cells (Lee et al., 1981; Wiener et al., 1989). It appears to be an independent translation product of M3, resulting from initiation at Met41 (instead of Met1) in the μNS sequence (McCutcheon et al., 1999; Wiener et al., 1989). The role of μNSC in viral infection has not been determined, but a recent report has provided evidence that expression of μNSC is neither necessary nor sufficient to support MRV growth in cell culture (Kobayashi et al., 2006).
Rotavirus also forms non-membrane-bound cytoplasmic inclusion bodies, commonly called viroplasms, which appear similar to MRV factories (Petrie et al., 1984). Nonstructural proteins NSP2 and NSP5 are needed in combination to form viroplasm-like structures, or VLS, in transfected cells (Fabbretti et al., 1999). A number of studies with rotavirus have described the silencing of viral protein expression with small interfering RNAs (siRNAs), often accompanied by effects on viral growth (reviewed by Arias et al., 2004). Silencing of either NSP2 or NSP5 expression, for example, inhibits several events in the viral life cycle, including formation of viroplasms and genome replication (Campagna et al., 2005; Lopez et al., 2005; Silvestri et al., 2004). Complementation with NSP2 expressed in trans after silencing of the virus-produced transcripts has been additionally useful in the study of NSP2 functions during rotavirus infection, rescuing virion assembly by ~6-fold (Taraporewala et al., 2006).
More recently, a complementation system for MRV μNS has been shown to rescue viral growth by ~40-fold after M3 silencing by siRNA (Kobayashi et al., 2006). That system has used cell lines stably expressing a short hairpin RNA against M3/μNS to knock down protein expression and has shown that infectious yields of MRV can be diminished by >3 log10 values. Importantly, that study has been the first to provide direct evidence that μNS is an “essential” protein for MRV growth, but did not specifically probe whether formation of factory-matrix structures is one of its important properties.
We have hypothesized that μNS plays a central role in the formation of functional viral factories (Broering et al., 2000, 2002, 2004; Miller et al., 2003, 2007), and in this study we tested some of its important properties for MRV growth. We first knocked down μNS expression using siRNA. In the absence of high levels of μNS expression, viral growth was inhibited. We then developed a system to rescue MRV growth by complementing with in vitro-transcribed M3 [+]RNA that expressed μNS in the presence of siRNA. We were able to rescue infectious yields by ~100-fold with this method of μNS trans-expression. The complementation system then allowed us to test μNS mutants specifically defective at forming factory-matrix structures, and we were thereby able to show that this function of μNS is important for rescuing viral growth. In addition, we confirmed that expression of an N-terminally truncated form of μNS, designed to represent μNSC and still able to form factory-matrix structures, is unable to rescue MRV growth, localizing one or more other important functions of μNS to its N terminus. Thus, factory-matrix formation is an important, though not a sufficient function of μNS during MRV infection.
Results
M3-targeting siRNAs reduce μN in a strain-dependent manner
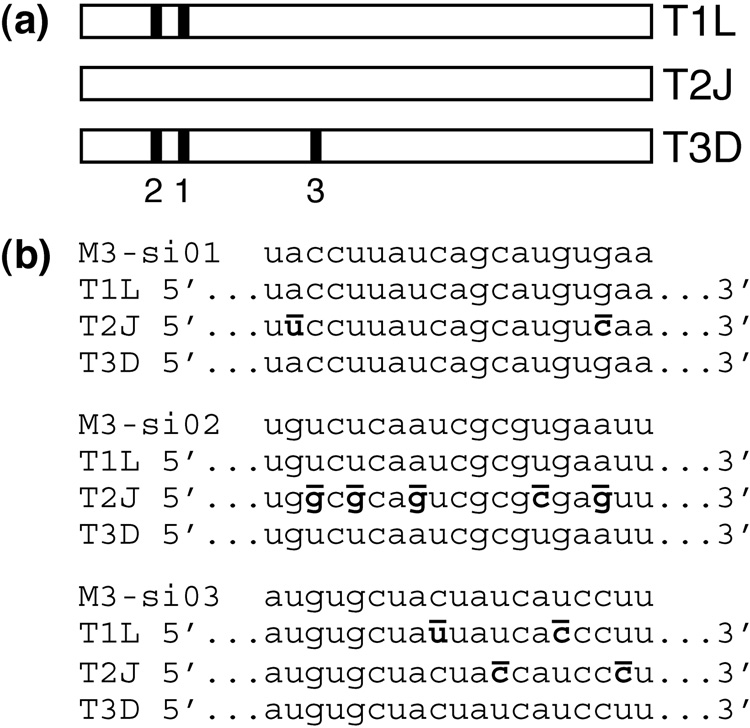
To reduce the expression of μNS during MRV infection, three different siRNAs (M3-si01, M3-si02, and M3-si03) were designed to target the M3 [+]RNA encoding μNS with the following strain specificities (Fig. 1a, b). M3-si01 and M3-si02 were designed to target strains Type 1 Lang (T1L) and Type 3 Dearing (T3D). M3-si03, in comparison, was designed to target only T3D, since T1L has two nucleotide mismatches with the M3-si03 sequence. None of the three siRNAs were designed to target strain Type 2 Jones (T2J), since T2J has two or more nucleotide mismatches with each of the M3-si sequences.
Fig. 1.
siRNAs targeting MRV M3 transcripts. (a) Schematic of the M3 gene depicting locations of the siRNA target sequences in MRV strains T1L, T2J, and T3D. Nucleotides 194–212 of T1L and T3D are targeted by M3-si01 (1). Nucleotides 140–158 of T1L and T3D are targeted by M3-si02 (2). Nucleotides 1369–1387 of T3D are targeted by M3-si03 (3). (b) Sequences of the three M3-targeting siRNAs are shown, each followed by the corresponding region of the T1L, T2J, and T3D M3 genes. Mismatches between siRNA and gene are bolded and overlined in the gene sequence.
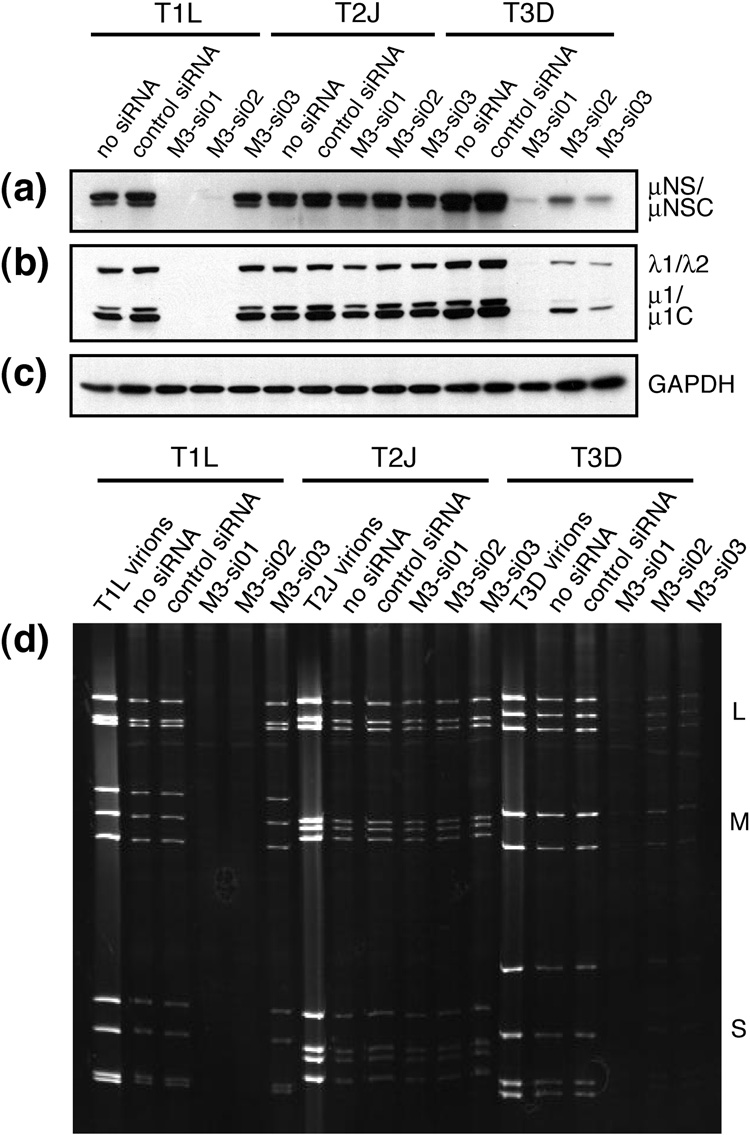
To test the ability of each siRNA to knock down μNS expression, the siRNAs were individually transfected by electroporation into CV-1 cells. At 24 h post-transfection (p.t.), the cells were infected with purified stocks of MRV T1L, T2J, or T3D at a multiplicity of infection (MOI) of 5 PFU/cell. At 24 h post-infection (p.i.), whole-cell lysates were collected, separated by SDS/polyacrylamide gel electrophoresis (SDS/PAGE), transferred to nitrocellulose, and probed with anti-μNS polyclonal antibodies (Fig. 2a). In the case of T1L infection, similarly strong expression of μNS was observed in cells that were electroporated without siRNA, cells that received an irrelevant control siRNA, and cells that received M3-si03, which did not target T1L M3. M3-si01 and M3-si02, however, reduced μNS expression to near (M3-si02) or below (M3-si01) the limit of detection, consistent with the fact that they both targeted T1L. In the case of T2J infection, similar levels of μNS expression were observed regardless of the treatment, consistent with the fact that none of the siRNAs targeted T2J M3. In the case of T3D infection, all three siRNAs reduced μNS expression when compared to the two types of control-treated cells, consistent with the fact that they all targeted T3D M3. The relative efficacies of the three siRNAs at reducing μNS expression by T3D appeared to be M3-si01 > M3-si03 > M3-si02. By demonstrating the expected strain specificities with the three different siRNAs, the results show that the primary effect of each siRNA was on the respective M3 [+]RNA.
Fig. 2.
Viral protein expression and dsRNA production in siRNA-transfected cells. CV-1 cells were transfected by electroporation with either no siRNA, control siRNA, M3-si01, M3-si02, or M3-si03 as indicated in each panel. The cells were infected with MRV strain T1L, T2J, or T3D at 24 h p.t. at an MOI of 5. (a–c) Cells were harvested at 24 h p.i. by washing with PBS and then lysing in 2× sample buffer. Lysates were boiled for 5 min, separated by SDS/PAGE, transferred to nitrocellulose, and immunoblotted with polyclonal μNS antiserum (a) or polyclonal T1L virion antiserum (b). GAPDH immunoblot (c) was included as a loading control. (d) Cells were harvested at 24 h p.i. by washing with PBS and then purifying total RNA by TrizolLS extraction. Samples were heated to 60°C for 5 min, and dsRNAs (large, L; medium, M; and small, S as indicated) were resolved by SDS/PAGE. The gel was stained with 0.5% ethidium bromide.
M3-targeting siRNAs reduce expression of other viral proteins and production of viral dsRNAs
To investigate the effect of reduced μNS expression on other MRV proteins, the whole-cell lysates from the same representative experiment as described in the preceding section were also examined by immunoblotting with anti-virion polyclonal serum. In each of the samples that had been treated with siRNA that targeted M3 in a strain-dependent manner, there was also a substantial corresponding reduction in the expression of other viral proteins (Fig. 2b and data not shown). To determine the effect of M3 [+]RNA silencing on MRV genome replication, we examined viral dsRNA levels. RNA was extracted from cells of the same representative knockdown experiment, separated by SDS/PAGE, and visualized by ethidium bromide staining. Viral dsRNA production was inhibited by treatment with siRNA in a strain-dependent manner: M3-si01 and M3-si02 both effectively targeted T1L and T3D, whereas M3-si03 effectively targeted T3D only (Fig. 2d). The most effective siRNA at reducing levels of both viral proteins and viral dsRNAs in cells was M3-si01. From these experiments, we conclude that efficient expression of μNS is required for both efficient expression of other MRV proteins and efficient replication of the MRV genome. Due to the amplification of MRV protein and genome synthesis that results from transcript production by nascent cores (Murray and Nibert, 2007; Zarbl and Millward, 1983), we cannot yet identify whether μNS knockdown primarily affects the function of entering (i.e., parental) cores and their transcripts and/or the assembly and function of nascent (i.e., progeny) cores; however, we favor the hypothesis that μNS provides important functions for both entering and nascent cores (see Discussion).
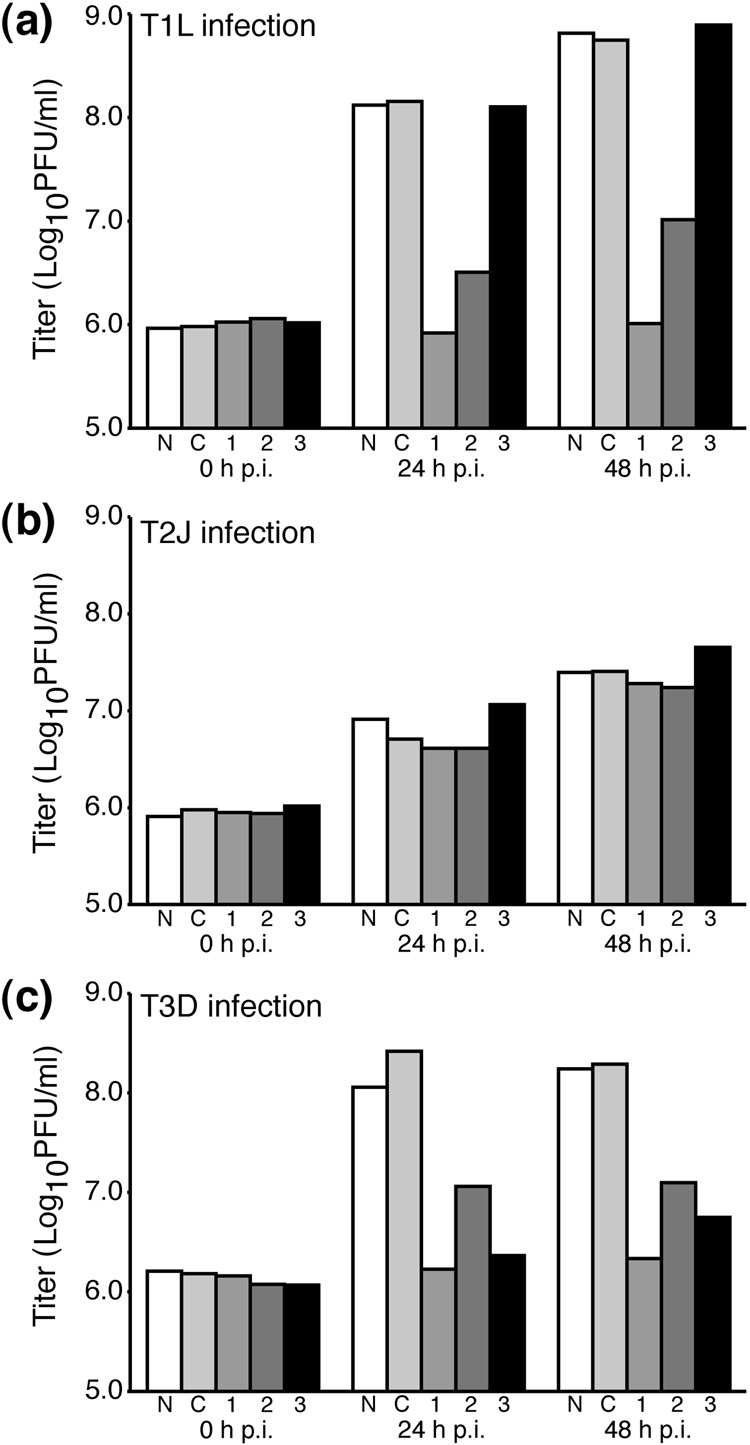
M3-targeting siRNAs reduce infectious viral titers
To test for their abilities to knock down viral titers, the siRNAs were individually transfected by electroporation into CV-1 cells. At 24 h p.t., the cells were infected with purified virions of MRV T1L, T2J, or T3D at an MOI of 5 PFU/cell. At 0, 24, and 48 h p.i., infectious viral titers were then determined by plaque assay. In the case of T1L infection (Fig. 3a), a >2-log10 increase in viral titer was seen at 24 h p.i. in control cells that were electroporated without siRNA, as well as in control cells that received an irrelevant siRNA. A similar increase in viral titer was also seen in cells that received the M3-specific siRNA that did not target T1L, M3-si03. In contrast, the two M3-specific siRNAs that targeted T1L, M3-si01 and M3-si02, reduced viral titers to near input (0-h) levels. Similar, respective effects of the different treatments on titers of T1L were seen at 48 h p.i. In the case of T2J infection (Fig. 3b), a similar increase in viral titer (>1 log10 at 48 h p.i.) was seen regardless of the treatment, as expected since none of the three M3-specific siRNAs target T2J. (Note that T2J is known to have lower infectious yields than T1L or T3D (Ahmed et al., 1981).) In the case of T3D infection (Fig. 3c), all three M3-specific siRNAs reduced viral titers to near input levels, as expected since all three siRNAs target T3D. The most effective siRNA at reducing the titers of both T1L and T3D was M3-si01, which in multiple experiments restricted viral titers to near input levels.
Fig. 3.
Viral titers in siRNA-transfected cells. CV-1 cells were transfected by electroporation with either no siRNA (N), control siRNA (C), M3-si01 (1), M3-si02 (2), or M3-si03 (3) as indicated in each panel. The cells were infected with MRV strain T1L (a), T2J (b), or T3D (c) at 24 h p.t. at an MOI of 5. Cells were then harvested by freeze-thaw at the times specified in each panel, and infectious viral titers were determined by plaque assays on L929 cells. This figure is representative of three independent experiments.
In summary, the results of these experiments with siRNAs indicate strong knockdown of infectious yields of MRVs T1L and T3D in a strain-dependent manner consistent with sequence-specific targeting of the viral M3 [+]RNA. In the case of M3-specific siRNAs that did not target particular strains (T1L by M3-si03 and T2J by M3-si01, M3-si02, M3-si03), as well as the irrelevant siRNA, the lack of effect on infectious yields provide evidence that any off-target effects of these siRNAs have little impact on MRV growth.
Complementation of μNS expression and rescue of viral growth
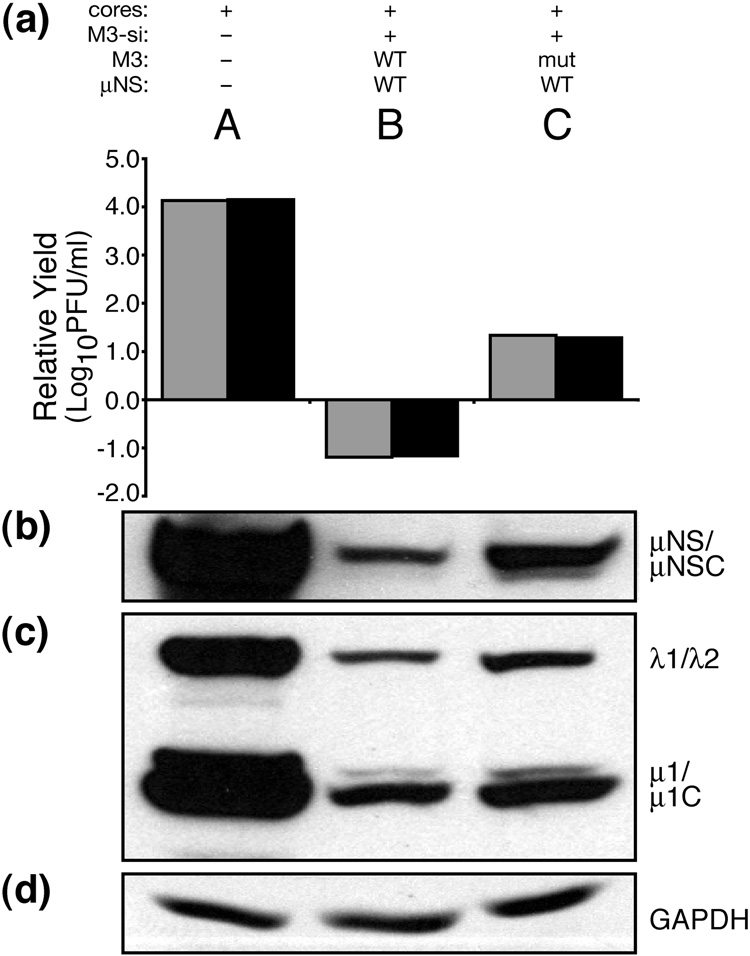
For both T1L and T3D infection, M3-si01 showed stronger effects than the other siRNAs. For this reason, we performed subsequent experiments with M3-si01 only. We endeavored to study properties of μNS in the context of MRV infection by designing a system to rescue viral growth upon expression of μNS in trans from M3 [+]RNA that would not be targeted by M3-si01.
A previous report has shown that transfection of MRV cores can be a useful method to infect cells, bypassing the entry steps (Jiang and Coombs, 2005). When a cell line derived from baby hamster kidney cells, BSR-T7 (Buchholz et al., 1999), was transfected with cores alone, the virus replicated to higher titers than in CV-1 cells (Fig. 4a, lane A, and data not shown). When M3-si01 was cotransfected with T1L cores, infection was inhibited in a similar manner to that seen when siRNA-pretreated cells were subsequently infected with virions (compare Fig. 4a, lane B, with Fig. 2a, bar 1). Following core transfection in BSR-T7 cells, however, knockdown of infectious yields in the presence of M3-si01 was even more substantial, resulting in an ~4-log10 decrease in viral titer. We next generated in vitro-transcribed M3 [+]RNA containing authentic 5′ and 3′ ends and three silent nucleotide changes in the siRNA target sequence (see Materials and Methods). Cotransfections with cores, M3-si01, and this siRNA-resistant rescue RNA (henceforth called mutRNA) resulted in increased expression of μNS (Fig. 4b, lane C), increased expression of other viral proteins (Fig. 4c, lane C), and ~2-log10 rescue of infectious yields (Fig. 4a, lane C). In contrast, cotransfections including instead the siRNA-sensitive wild-type M3 [+]RNA did not rescue infectious yields (Fig. 4a, lane B). Increasing the transfected amount of mutRNA did not increase μNS expression or viral titers (data not shown). Though we were unable to rescue the full 4 log10 values of infectious yield that were lost with siRNA treatment, we conclude that the level of rescue we do observe with mutRNA is sufficient to ascertain the effects of mutations in μNS.
Fig. 4.
Complementation of μNS with in vitro-transcribed M3 [+]RNA. BSRT7 cells were transfected using Lipofectamine 2000. Lanes reflect different combinations of transfected materials as follows: (A) T1L cores alone; (B) T1L cores, M3-si01, and in vitro-transcribed M3 [+]RNA expressing wild-type T1L μNS; and (C) T1L cores, M3-si01, and in vitro-transcribed M3 [+]RNA expressing wild-type T1L μNS but also containing silent mutations in the siRNA target sequence (mutRNA). Cells were then harvested at 24 h p.i. for viral titers and protein analyses. For titers (a), cells were harvested by freeze-thaw, and plaque assays were performed on L929 cells. Titers were expressed as infectious yields relative to that of the cores+M3-si01 sample (not shown in this figure) within each replicate experiment, and the relative yields from two such experiments are shown as adjacent bars for each type of sample. For protein analyses (b–d), cells were harvested by washing with PBS and then lysing in 2× sample buffer. Lysates were boiled for 5 min, separated by SDS/PAGE, transferred to nitrocellulose, and immunoblotted with polyclonal μNS antiserum (b) or T1L virion antiserum (c). GAPDH immunoblot (d) was included as a loading control. The blots are representative of the two experiments.
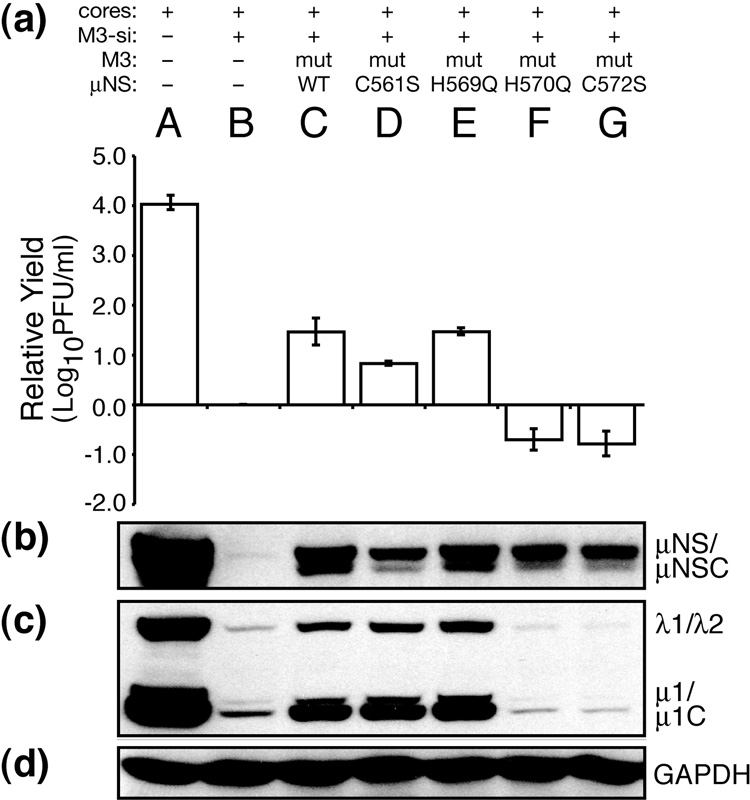
Putative zinc-hook residues His570 and Cys572 are important for rescuing viral growth
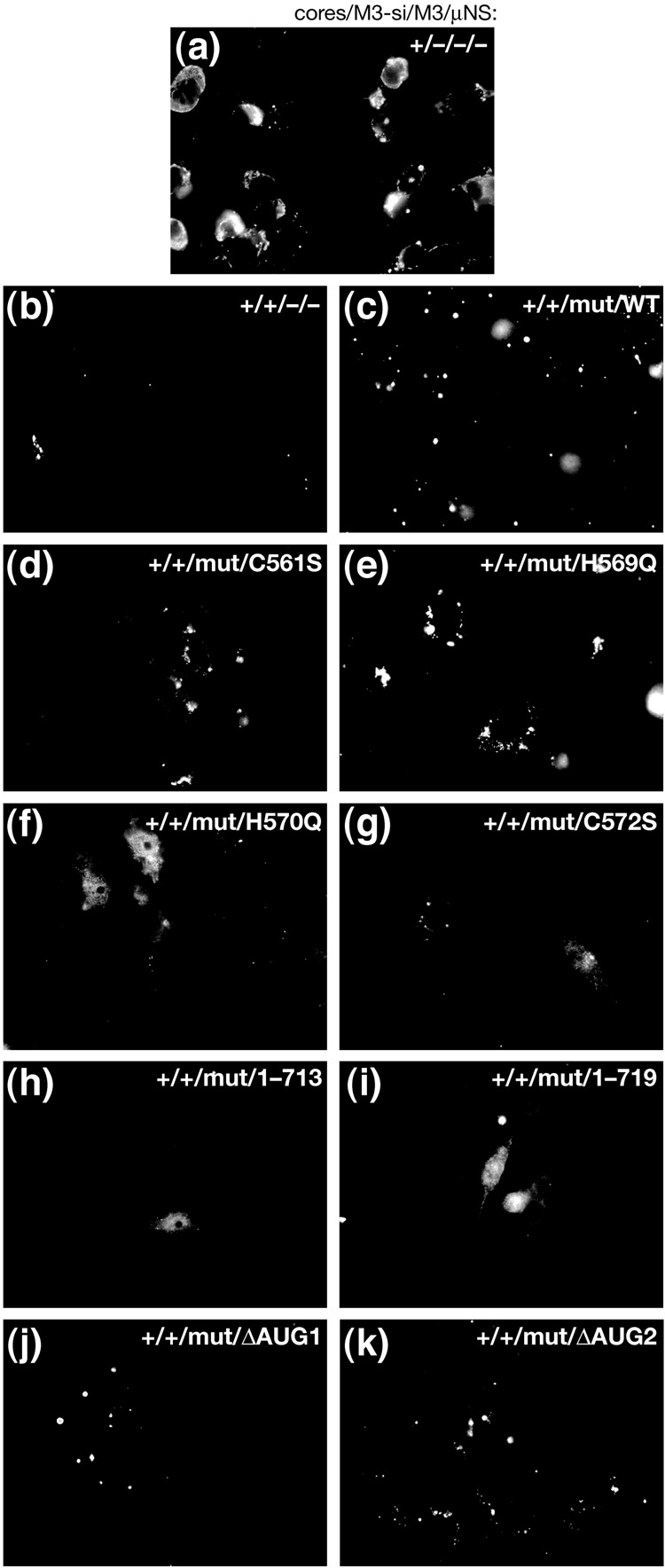
A previous report from our lab has shown that μNS residues His570 and Cys572 are each important for formation of factory-matrix structures; when these two residues are individually mutated and the μNS proteins are then transiently expressed in cells, μNS is diffusely distributed throughout the cell when observed by immunofluorescence microscopy (Broering et al., 2005). In contrast, mutation of other Cys and His residues flanking this region does not disrupt formation of these structures. To test the importance of these Cys and His residues in the context of MRV infection, we used our siRNA-complementation system. We generated constructs encoding single mutations at residues Cys561 (to Ser), His569 (to Gln), His570 (to Gln), or Cys572 (to Ser) within the μNS rescue plasmid, which were then used to generate in vitro-transcribed M3 [+]RNAs (mutRNAs). Point mutations C561S and H569Q still allow μNS to form factory-matrix structures when transiently expressed (Broering et al., 2005). These same two mutants tested in our siRNA-complementation system rescued infectious yields similarly to wild-type μNS (Fig. 5a, lanes C–E). The transfected mutRNAs expressed similar levels of μNS as shown by immunoblot (Fig. 5b, lanes C–E) and also rescued expression of other viral proteins to similar levels (Fig. 5c, lanes C–E). We furthermore confirmed by immunofluorescence microscopy that the factories formed in rescued cells were similar to the factories in wild-type infection, although smaller in size (Fig. 6d and e). On the other hand, point mutations H570Q and C572S disrupted formation of factory-matrix structures as expected (Broering et al., 2005), causing μNS to be diffuse in cells (Fig. 6f and g). Neither of these two mutants was able to rescue infectious yields (Fig. 5a, lanes F and G) or expression of other viral proteins (Fig. 5c, lanes F and G), even though the transfected mutRNAs expressed similar levels of μNS as compared to those in other rescue conditions (Fig. 5b, lanes C–G). These data provide direct evidence that the ability of μNS to form the factory matrix is important for MRV growth.
Fig. 5.
Rescue of viral growth with μNS mutants, part 1. BSRT7 cells were transfected using Lipofectamine 2000. Lanes reflect different combinations of transfected materials as follows: (A) T1L cores alone; (B) T1L cores and M3-si01; (C) T1L cores, M3-si01, and mutRNA expressing wild-type μNS as in Fig. 4; (D) T1L cores, M3-si01, and mutRNA with point mutaion C561S; (E) T1L cores, M3-si01, and mutRNA with point mutation H569Q; (F) T1L cores, M3-si01, and mutRNA with point mutation H570Q; and (G) T1L cores, M3-si01, and mutRNA with point mutation C572S. Cells were harvested at 24 h p.i. for viral titers (a) and protein analyses (b–d) as described for Fig. 4. Titers were expressed as infectious yields relative to that of the cores+M3-si01 sample within each replicate experiment, and the relative yields from three such experiments are shown as the mean ± standard deviation for each type of sample. The blots are representative of the three experiments.
Fig. 6.
Immunofluorescence microscopy of viral factories during rescue of viral growth with mutants of μNS. BSRT7 cells were transfected using Lipofectamine 2000 and then fixed at 24 h p.t. for immunostaining. The cells were immunostained with anti-μNS IgG followed by goat anti-rabbit IgG conjugated to Alexa 488. Images reflect different combinations of transfected materials as follows: (a) T1L cores alone; (b) T1L cores and M3-si01; (c) T1L cores, M3-si01, and mutRNA expressing wild-type μNS as in Fig. 4; (d) T1L cores, M3-si01, and mutRNA with point mutation C561S; (e) T1L cores, M3-si01, and mutRNA with point mutation H569Q; (f) T1L cores, M3-si01, and mutRNA with point mutation H570Q; (g) T1L cores, M3-si01, and mutRNA with point mutation C572S; (h) T1L cores, M3-si01, and mutRNA expressing μNS(1–713); (i) T1L cores, M3-si01, and mutRNA expressing μNS(1–719); (j) T1L cores, M3-si01, and mutRNA expressing μNS(41–721) (μNSC alone); and (k) T1L cores, M3-si01, and mutRNA expressing μNS(1–721) (μNS alone).
Within the minimal contiguous region of μNS sufficient for forming factory-matrix structures in transfected cells (residues 471–721) (Broering et al., 2005), there are five additional Cys and His residues (Cys488, His489, Cys491, Cys608, and Cys619) that previously had not been examined by mutagenesis. We therefore examined these other sites as a part of the current study, making a Ser substitution for each Cys and a Gln substitution for each His. When each of these mutants was expressed in transfected cells, factory-matrix structures formed that were indistinguishable from those formed by wild-type μNS (data not shown). These results further highlight the specific importance of Cys570 and His572 in formation of the factory matrix.
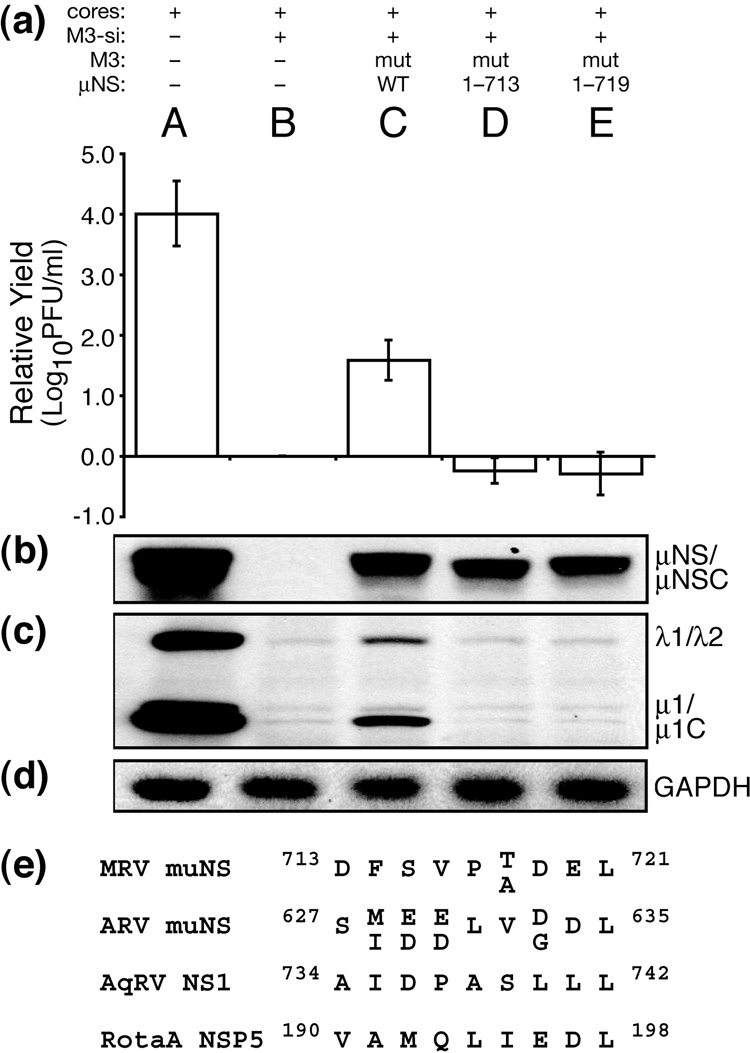
Extreme C terminus of μNS is important for rescuing viral growth
C-terminally truncated μNS proteins are also diffusely distributed in the cytoplasm of transfected cells (Broering et al., 2005). We have shown that some portion of residues 714–721 is important for formation of factory-matrix structures. To examine further our hypothesis that this function of μNS is required for MRV growth, we generated rescue plasmids to express μNS residues 1–713 or 1–719. We then used these plasmids to generate in vitro-transcribed M3 [+]RNAs (mutRNAs) as in preceding experiments. Neither of these C-terminal truncation mutants, when tested in our siRNA-complementation system, rescued infectious yields (Fig. 7a, lanes D and E) or expression of other viral proteins (Fig. 7c, lanes D and E), even though the transfected mutRNAs expressed similar levels of μNS as that expressing wild-type μNS (Fig. 7b, lanes C–E). We also confirmed by immunofluorescence microscopy that μNS(1–713) and μNS(1–719) expressed during rescue was diffuse in cells (Fig. 6h and i). These findings further support our hypothesis that the ability of μNS to form the factory matrix is important for MRV growth. The inability of μNS(1–719) to form factory-matrix structures and to rescue MRV growth indicates that one or both of the last two residues of μNS, Glu720 and Leu721, are important for formation of the factory matrix. A C-terminal Leu residue is conserved among the μNS proteins of mammalian and avian reoviruses (McCutcheon et al., 1999; Su et al., 2006; Zhang et al., 2007) as well as the μNS sequence homolog, NS1, of aquareoviruses (Attoui et al., 2002) (Fig. 7e), suggesting that it may be particularly important.
Fig. 7.
Rescue of viral growth with μNS mutants, part 2. (a–d) BSRT7 cells were transfected using Lipofectamine 2000. Lanes reflect different combinations of transfected materials as follows: (A) T1L cores alone; (B) T1L cores and M3-si01; (C) T1L cores, M3-si01, and mutRNA expressing wild-type μNS as in Fig. 4; (D) T1L cores, M3-si01, and mutRNA expressing μNS(1–713); and (E) T1L cores, M3-si01, and mutRNA expressing μNS(1–719). Cells were harvested at 24 h p.i. for viral titers (a) and protein analyses (b–d) as described for Fig. 4. Titers were expressed as relative yields as described for Fig. 5. The blots are representative of the three replicate experiments. (e) The C-terminal eight residues of μNS homologs from mammalian reoviruses (MRV), avian reoviruses (ARV), and aquareoviruses (AqRV NS1). Sequence variation between strains within each group of viruses is indicated by stacked letters. The C-terminal eight residues of viroplasm-forming protein NSP5 from group A rotavirus strains SA11 and RRV are also shown.
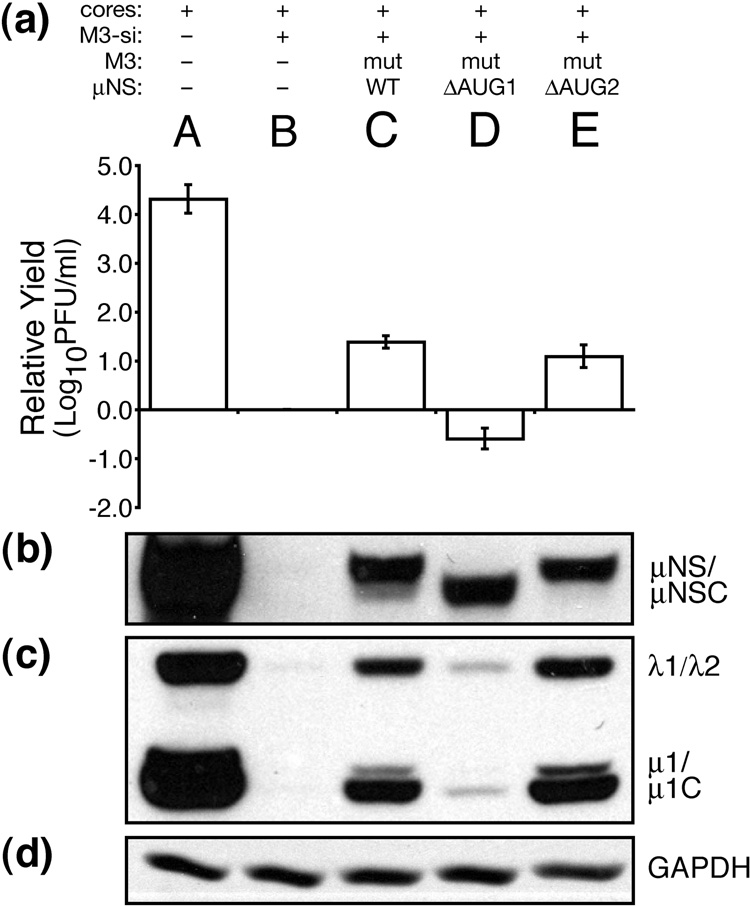
μNSC in the absence of μNS is unable to rescue viral growth
We additionally used our siRNA-complementation system to examine the role of the μNS N terminus in MRV infection. N-terminal truncations of μNS retain the ability to form factory-matrix structures but no longer associate with certain other MRV proteins (Broering et al., 2002, 2005; Miller et al., 2003, 2007). We generated a construct to express μNS residues 41–721, designed to represent μNSC (Lee et al., 1981; McCutcheon et al., 1999; Wiener et al., 1989), by changing the start codon of full-length μNS, Met1, to Lys (ΔAUG1). We also generated a construct to express full-length μNS alone, by changing the apparent start codon of μNSC, Met41, to Leu (ΔAUG2). These single point mutations were made in the siRNA-resistant rescue plasmid, from which we then generated in vitro-transcribed M3 [+]RNAs (mutRNAs). The expected translation products of these constructs were confirmed by immunoblot (Fig. 8b). We furthermore confirmed by immunofluorescence microscopy that both μNS(ΔAUG1) (i.e., μNSC alone) and μNS(ΔAUG2) (i.e., μNS alone), expressed during rescue, formed morphologically similar factories or factory-matrix structures (Fig. 6j and k). Expression of μNSC alone, however, was unable to mediate rescue of MRV growth, as both infectious yields and expression of other MRV proteins remained as low as in the control M3-si01 knockdown (Fig. 8a and c, lanes B and D). Thus, ability to form the factory matrix is not a sufficient function of μNS for rescue in this system. In contrast, expression of full-length μNS alone rescued both infectious yields and expression of other viral proteins as well as did μNS/μNSC expression from the wild-type μNS rescue RNA (mutRNA) (Fig. 8a and c, lanes C and E), providing evidence that μNSC is not required for rescue of viral growth in this system.
Fig. 8.
Expression of μNSC in the absence of μNS is unable to rescue viral growth. BSRT7 cells were transfected using Lipofectamine 2000. Lanes reflect different combinations of transfected materials as follows: (A) T1L cores alone; (B) T1L cores and M3-si01; (C) T1L cores, M3-si01, and mutRNA expressing wild-type μNS (and μNSC) as in Fig. 4; (D) T1L cores, M3-si01, and mutRNA expressing μNSC only (ΔAUG1); and (E) T1L cores, M3-si01, and mutRNA expressing full-length μNS only (ΔAUG2). Cells were harvested at 24 h p.i. for viral titers (a) and protein analyses (b–d) as described for Fig. 4. Titers were expressed as relative yields as described for Fig. 5. The blots are representative of the three replicate experiments.
Discussion
Since M3 [+]RNAs with authentic 5′ and 3′ ends, as well as a 5′ cap, were used for rescue in the described experiments, it is possible that these RNAs were packaged into nascent MRV core particles, and then infectious virions. Screening for in vitro-derived, mutant M3 RNA in rescued clones is underway; however, since knockdown by siRNA was not 100% efficient, the background of virus-derived, wild-type M3 RNA may be high in comparison to any potential packaging of rescue RNA. In addition, the MRV factories (and rotavirus viroplasms) are proposed to protect virus-derived [+]RNAs from degradation by the RNA-interference pathway (Arias et al., 2004; Carvalho et al., 2007; Silvestri et al., 2004), giving virus-derived RNAs an advantage for replication and packaging. To date, of the rescued clones that we have screened by restriction digest after cDNA amplification of the M3 gene, all have had wild-type M3 (M.M.A. and M.L.N., unpublished data). Thus, rescue in this system appears largely to occur through gene-specific complementation of viral protein expression, as also suggested by previous related studies with MRV and rotavirus (Carvalho et al., 2007; Kobayashi et al., 2006; Taraporewala et al., 2006).
By testing numerous variables when establishing the siRNA-complementation system to study μNS functions, we identified an optimal approach that involved cotransfection of MRV cores, siRNA targeting core-transcribed M3 [+]RNA, and in vitro-transcribed M3 [+]RNA containing silent mutations that render them resistant to the siRNA (mutRNA). Attempts to rescue MRV growth with a cotransfected μNS-expression plasmid were not as successful; we repeatedly observed an increase in infectious yields of only ~10-fold in those experiments (data not shown), versus ~100-fold with our optimal protocol. Notably, μNS expression from cotransfected plasmid was consistently lower than that from cotransfected M3 [+]RNA (data not shown). Increasing the amount of cotransfected plasmid did not much improve μNS expression, as more plasmid appeared toxic to the cells. We interpret these findings to indicate that the level of rescue correlates with the level of μNS expression, and that plasmid-based expression was less efficient in our hands. M3 is the second most efficiently translated reovirus mRNA, after S4 (Gaillard and Joklik, 1985), so it is perhaps not surprising that the extent of rescue with this general approach depends on the level of μNS expression. Kobayashi et al. (2006) also report only ~40-fold rescue of infectious yields upon trans-expression of μNS, albeit from transfected plasmid in their system.
M3-targeting siRNAs in these experiments were able to reduce infectious yields by as much as 4 log10 values. So why was only 10- to 100-fold rescue of yields then achieved by μNS trans-expression? Optimal levels of μNS expression achieved even with cotransfected M3 [+]RNA in our hands was still lower than that in control infections (see Fig. 4–Fig. 8). Thus, inefficient rescue could simply reflect that insufficient μNS was expressed. Another interesting explanation, however, could relate to the specific localization of μNS expression within the cell, especially as pertains to early steps in infection. Entering MRV core particles may need to be embedded within a μNS matrix soon after infection in order for (i) newly core-transcribed [+]RNAs to be retained in the viral factory and made properly available for genome replication and assembly of nascent cores and/or (ii) entering cores not to be prematurely recoated by newly synthesized outer-capsid proteins and thereby transcriptionally inhibited (Broering et al., 2000, 2004; Miller et al., 2003). If μNS from M3 transcripts made by an entering core is poorly expressed due to siRNA knockdown, there may be insufficient μNS in close proximity to the core to embed it effectively and thereby to trap the newly core-transcribed [+]RNAs. Moreover, complementing μNS from trans-expressing M3 [+]RNA molecules may not be able to reproduce this localized expression as effectively as core-made M3 transcripts. This type of spatial requirement for trans protein to be produced near entering virus particles has been convincingly shown for flock house virus (Venter et al., 2005), and we have also provided some related evidence in this regard in a recent study of MRV μ2 protein (Carvalho et al., 2007).
Some toxicity of transfected [+]RNA may also contribute to limiting the extent of rescue. This effect is evident in Fig. 4, Fig. 5, Fig. 7, and Fig. 8, in which noncomplementing M3 [+]RNAs produced yields below those of the standardization control (transfection with cores+M3-si01, but no M3 [+]RNA). These results might alternatively indicate that the μNS mutants expressed from noncomplementing mutRNAs in Fig. 5, Fig. 7, and Fig. 8 had a dominant-negative effect on viral growth; however, because the same effect was seen with noncomplementing wild-type M3 [+]RNA in Fig. 4, we favor RNA toxicity as the probable explanation.
Transfecting with cores versus infecting with virions is another distinctive part of our optimal protocol. We believe that the benefit in this case was to reduce the background of infectious particles present at early times of infection (e.g., in time-0 samples), so that a greater growth differential could then be seen over a single infectious cycle. Thus, though not an essential part of the protocol (data not shown), the use of transfected cores to initiate infection helped to improve the extent of both knockdown and rescue that we could recognize.
Having established our siRNA-complementation system for rescue of infectious yields, we then used it to study specific properties of μNS that had been identified and localized in previous studies (Becker et al., 2003; Broering et al., 2000, 2002, 2004, 2005; Miller et al., 2003, 2007), but not yet directly correlated with effects during MRV infection. First, we addressed whether ability to form the factory matrix is an important function of μNS for MRV growth. Our model based on previous results has held this to be the case, but direct evidence from our studies has been lacking. Neither do the results of Kobayashi et al. (2006) address this question directly through the use of pertinent μNS mutants. In the current study, we used μNS mutants which, according to our previous characterizations (Broering et al., 2005) and confirmed in this study (see Fig. 6), have specific defects in forming the factory matrix. By showing that these mutants were unable to rescue infectious yields in our siRNA-complementation system, while nonetheless being expressed to levels similar to wild-type μNS (see Fig. 5), we provided strong evidence that formation of the factory matrix per se is an important function of μNS for MRV growth. As suggested in Results, our findings do not yet identify when in the viral infectious cycle the factory matrix is playing its important role(s), whether on the function of entering cores and their transcripts and/or on the assembly and function of nascent cores; thus, future studies will be needed to address our hypothesis that the μNS-formed factory matrix provides important functions for both entering and nascent cores (Broering et al., 2000, 2004; Miller et al., 2003).
The preceding evidence that formation of the factory matrix per se is an important function of μNS for MRV growth extends to two separate small regions of μNS that have been implicated in matrix formation: (i) a putative zinc-hook motif at residues His570 and Cys572 and (ii) the extreme C terminus, now further localized to the last two residues, Asp720 and/or Leu721 (Broering et al., 2005; this study). We expect that a number of different types of μNS-μNS interactions are needed to build the large three-dimensional matrix of the viral factories and that these two small regions of μNS (570/572, 720/721) may each be directly involved in one or more of these interactions. In any case, the rescue results presented here provide evidence that both of these regions are important for forming a factory matrix that is functional to support MRV growth. A potentially related observation is that the C-terminal residues of viroplasm-forming protein NSP5 of many rotavirus strains (e.g., Fabbretti et al., 1999; Sen et al., 2007) are quite similar to those of mammalian and avian reoviruses, including the terminal Leu (see Fig. 7e).
Using our siRNA-complementation system to study specific properties of μNS that had been identified and localized in previous studies (Becker et al., 2003; Broering et al., 2000, 2002, 2004, 2005; Miller et al., 2003, 2007), but not yet directly correlated with effects during MRV infection, we addressed, too, whether any of the MRV protein-association activities of μNS is also an important function for MRV growth. Our model based on previous results has again held this to be the case, but evidence has again been limited. In this case, though, the results of Kobayashi et al. (2006) have addressed this question directly; trans-expression of μNSC was unable to rescue infectious yields even while being expressed to levels similar to wild-type μNS. We have now been able to reproduce this finding in our system (see Fig. 8). Our previous results have shown that two other MRV proteins, μ2 and σNS, associate with μNS, but not μNSC; thus, though μNSC forms factory-matrix structures, it cannot recruit these two viral proteins to them (Broering et al., 2002; Miller et al., 2003). Based on the current results, we therefore conclude that during infection there is a specific importance for one or both of these proteins, μ2 and σNS, to be recruited to the viral factories through association(s) with μNS. Our findings do not yet identify when in the viral infectious cycle either of these two recruitment activities of μNS is playing its important role(s); thus, future studies will be needed to address the hypothesis that recruitment of μ2 and/or σNS is important for core-transcribed [+]RNAs to be retained in the viral factory and made properly available for genome replication and assembly of nascent cores (Broering et al., 2000, 2004; Carvalho et al., 2007; Kobayashi et al., 2006; Miller et al., 2003). Indeed we further hypothesize that other MRV protein-association activities of μNS, which have been mapped to μNS regions C-terminal to Met41 (Broering et al., 2000, 2004; Miller et al., 2007; C.L.M., M.M.A., and M.L.N., manuscript in preparation), are also important functions of this multifunctional protein, and we expect to test this hypothesis in other future studies. In the meantime we can conclude that the function of μNS to form the factory matrix is not all that it does to support MRV growth; factory-matrix formation is an important, though not a sufficient function of μNS during MRV infection.
Materials and methods
Cells and viruses
BSR-T7 cells are derived from baby hamster kidney cells (Buchholz et al., 1999). BSR-T7 and CV-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) containing 10% fetal bovine serum (HyClone). Spinner-adapted mouse L929 cells were grown in Joklik’s modified minimal essential medium (Sigma-Aldrich) supplemented to contain 2% fetal bovine serum and 2% bovine calf serum (HyClone) in addition to 2 mM glutamine, 100 U of penicillin/ml, and 100 µg of streptomycin/ml (Mediatech). Reovirus strains T1L, T2J, and T3D were lab stocks originating from the Bernard N. Fields lab. Purified virions, grown in L929 cells and purified as described elsewhere (Nibert and Fields, 1992), were used for all infections. Cores were prepared as previously described (Chandran et al., 1999).
Antibodies and other reagents
Rabbit polyclonal antisera against μNS protein and T1L virions have been described previously (Broering et al., 2000; Virgin et al., 1988). GAPDH antibody was purchased from Santa Cruz Biotech. Horseradish peroxidase-conjugated goat anti-mouse IgG (KPL) or goat anti-rabbit IgG (Pierce) were used as secondary antibodies. Restriction enzymes were obtained from New England Biolabs.
siRNAs
All siRNAs were purchased from Dharmacon. The μNS siRNA target sequences are as follows: M3-si01 is 5′-UACCUUAUCAGCAUGUGAAdTdT-3′, M3-si02 is 5′-UGUCUCAA UCGCGUGAAUUdTdT-3′, and M3-si03 is 5′-AUGUGCUACUAUCAUCCUUdTdT – 3′. The control siCTRL is the non-targeting siRNA #1 from Dharmacon (siCONTROL). The siRNAs were resuspended according to the manufacturer’s instructions to a concentration of 40 µM, and used at a final concentration of 100 nM.
Mammalian expression vectors
Plasmid pBOS36 was constructed to serve as the transcription template for in vitro RNA synthesis. To generate a cDNA copy of the MRV T1L M3 gene, total RNA was obtained from MRV-infected L929 cells using TrizolLS reagent according to the manufacturer’s instructions (Invitrogen). Purified RNA was used as a template for M3 cDNA synthesis. M3 cDNA was generated using the Superscript First-Strand Synthesis System (Invitrogen) and the reverse primer 5′-GATGAATAGGGGTCGGG-3′. The first strand cDNA was PCR-amplified using PfuI Turbo (Stratagene) and the two primers M3-SacI(for) 5′-GGGGGGGAGCTCGCTAAAGTGACCGTGGTCATGGC-3′ and M3-MluI(rev) 5′-CCCCCCACGCGTGATGAATAGGGGTCGGG-3′, which added a 5′ SacI site and a 3′ MluI site onto the M3 cDNA. Following PCR amplification, the M3 cDNA was purified, SacI/MluI digested, and ligated into vector pET21B+ (Novagen) immediately downstream from the T7 promoter. A cDNA copy of the hepatitis delta virus ribozyme was PCR-amplified from a pCI-neo construct provided by Dr. Cathy L. Miller (C.L.M. and M.L.N., unpublished data) and ligated into the pET21B+ vector using the MluI site and a NotI site. To allow for the synthesis of M3 [+]RNAs possessing an authentic 5′ terminus, the SacI restriction site sequence was removed by site-directed mutagenesis using the two primers pBOS36-SacI(for) 5′-CGACTCACTATAGGCTAAAGTGACCGTGGTCATGGC-3′ and pBOS36-SacI(rev) 5′-CACGGTCACTTTAGCCTATAGTGAGTCGTATTAATTTCGCGG-3′, which juxtaposed the T7 promoter and the +1 nucleotide of M3. Likewise the MluI site was removed from the 3′ end of the M3 sequence by site-directed mutagenesis using primers pBOS36-MluI(for) 5′-GGCTAAGGGAGAGCTGCGGCCGCCTCGAGCACCACC-3′ and pBOS36-MluI(rev) 5′-GGTGCTCGAGGCGGCCGCAGCTCTCCCTTAGCCATCCG-3′, which allowed cleavage by the ribozyme to produce [+]RNA possessing the authentic MRV M3 3′ end. Correctness of the construct was verified by DNA sequencing.
The plasmid pBOS36 was used for site-directed mutagenesis to make the rescue plasmid pBOS36mut. The forward primer was 5′-GGGTCTATGTCTATACCATATCAACATGTAAATGTTCCAAAAGTTG-3′ and the reverse primer was 5′-CAACTTTTGGAACATTTACATGTTGATATGGTATAGACATAGACCC-3′. Underlines indicate positions where silent mutations were made. The region containing the desired mutation was excised with BlpI and NheI and then ligated to pBOS36 that had been cut with BlpI and NheI to remove the same region, generating pBOS36mut. Correctness of the subcloned region was verified by DNA sequencing.
To generate rescue constructs with mutations in μNS, pBOS36mut was used for site-directed mutagenesis, using the primers in Table 1 and Pfu polymerase (Stratagene) according to manufacturer’s instructions. For the constructs that expressed μNS(C561S), μNS(H569Q), μNS(H570Q), and μNS(C572S), the region containing the desired mutation was excised with HindIII and BsrGI and then ligated into pBOS36mut that had been cut with HindIII and BsrGI to remove the same region. For the constructs that expressed μNS(1–713) or μNS(1–719), the site-directed mutagenesis protocol was used to delete the nucleotides that encoded amino acids 714–721 or 720–721. The region containing the desired mutation was excised with XhoI and BsrGI and then ligated into pBOS36mut that had been cut with XhoI and BsrGI to remove the same region. The ΔAUG1 construct mutated the first Met codon, Met1, to Lys, and the ΔAUG2 construct mutated the second Met codon, Met41, to Leu. These constructs were subcloned by excising the region containing the desired mutation with MfeI and SphI followed by ligation into pBOS36mut that had been cut with MfeI and SphI to remove the same region. Correctness of each subcloned region was verified by DNA sequencing.
Table 1.
Forward and reverse primers used to generate M3/μNS mutants
| Mutationa | Forward mutagenesis primerb | Reverse mutagenesis primerb |
|---|---|---|
| C561S | GTCAGCTCAAGCATCTAGTCTGGATATGTATTTGAGACACC | TACATATCCAGACTAGATGCTTGAGCTGACTTAGCATCCGC |
| H569Q | GATATGTATTTGAGACAACACACCTGCATCAATGG | CCATTGATGCAGGTGTGTTGTCTCAAATACATATC |
| H570Q | GATATGTATTTGAGACACCAAACTTGCATCAATGG | CCATTGATGCAAGTTTGGTGTCTCAAATACATATC |
| C572S | GGATATGTATTTGAGGCACCACACCAGCATTAATGGTCATACAAAAGAAGATG | CTTCTTTTGTATGACCATTAATGCTGGTGTGGTGCCTCAAATACATATCCAGAC |
| 1–713 | GCTGACCTGATTGATTAAATGAGCTGTGACGCAGTGTTGCCC | GTCACAGCTCATTTAATCAATCAGGTCAGCAGCGCCGTCGAC |
| 1–719 | CTGATTGATTTCTCCGTTCCAACTGATTAAATGAGCTGTGACGCAGTGTTGCC | GGGCAACACTGCGTCACAGCTCATTTAATCAGTTGGAACGGAGAAATCAATCA |
| ΔAUG1 | AAAGTGACCGTGGTCCTAGCTTCATTCAAGGGATTCTCCGTC | CCCTTGAATGAAGCTAGGACCACGGTCACTTTAGCCTATAG |
| ΔAUG2 | CACTCCGTCTGTAGACTTGTCTCAATCGCGTGAATTCCTTACAAAGGC | GTAAGGAATTCACGCGATTGAGACAAGTCTACAGACGGAGTGAAGGG |
Mutation is named relative to μNS.
Primers are written in 5′ to 3′ orientation. Underlined nucleotides indicate changes that lead to a change in the encoded amino acid, and double underlines indicate silent changes made in some cases to add a restriction enzyme site for screening clones.
In vitro transcriptions
Following confirmation of the sequence, pBOS36 was linearized with NotI and used to template MRV M3 [+]RNA synthesis in in vitro run-off transcription reactions. Reactions were performed using the AmpliCap-Max T7 High Yield Message Maker Kit (Epicentre). The protocol for addition of 5′ caps was according to the manufacturer’s instructions. RNA was precipitated by addition of one volume of 5 M ammonium acetate. The pelleted RNA was washed in 70% ethanol, dried, and resuspended in 40 µl of diethylpyrocarbonate-treated water.
Transfections and infections
For electroporations, cells were trypsinized, pelleted, and resuspended in 400 µl OptiMEM (Invitrogen) along with siRNA and/or plasmid as described. The suspension was placed in a 4-mm cuvette and treated at 220 V, 950 µF, and 0 ohms with a BioRad Gene Pulser II electoporator. Time constants were routinely between 25 and 30 msec. Electroporated cells were resuspended in DMEM containing 10% fetal bovine serum and plated in 6-well plates for recovery at 37°C. At 24 h p.t., cells were infected with purified virions at an MOI of 5 or 10 PFU/cell diluted in phosphate-buffered saline (PBS) (137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1 mM KH2PO4). The virus was adsorbed at room temperature for 1 h and removed before fresh medium was added. Cells were further incubated at 37°C until harvested at specified times p.i. For lipofections, cells were transfected with reovirus cores, siRNA, and/or DNA or RNA in 6-well plates with 7 µl of Lipofectamine 2000 (Invitrogen) per well according to the manufacturer’s instructions. Based on the extent to which infectious yields could be optimally reduced in these experiments, we estimated that siRNAs were routinely transfected at >99% efficiency. Based on the numbers of cells expressing viral proteins as observed by microscopy, we estimated that cores and in vitro-transcribed M3 [+]RNAs were routinely transfected at >50% efficiency.
Immunoblot analysis
Whole-cell lysates were collected at specified times p.i. or p.t. Cells were washed once in PBS. Addition of 2× sample buffer (250 mM Tris pH 8.0, 20% glycerol, 2% SDS, 4% β-mercaptoethanol, 0.002% bromophenol blue) containing 1× protease inhibitors (Roche) was used to collect and lyse cells. Samples were boiled for 5 min and resolved by 10% SDS/PAGE. Proteins were electroblotted from the gels to nitrocellulose in 25 mM Tris and 192 mM glycine (pH 8.3). Binding of primary antibodies was detected with horseradish peroxidase-conjugated antibodies and enhanced chemiluminescence reagents (PerkinElmer).
dsRNA analysis
Cell lysates were collected at specified times p.i. using TrizolLS reagent (Invitrogen) according to the manufacturer’s instructions. Samples were heated to 60°C for 5 min, and reovirus dsRNAs were separated by 10% SDS/PAGE. The gel was stained with 0.5 µg/ml ethidium bromide in TAE buffer (40 mM Tris acetate, pH 8.3, 1 mM EDTA).
Immunofluorescence microscopy
Cells were fixed for 10 min at room temperature in 2% paraformaldehyde in PBS. Fixed cells were washed three times in PBS, then permeabilized and blocked in PBS containing 1% bovine serum albumin and 0.1% Triton X-100 (PBSAT). Primary antibodies were diluted in PBSAT and incubated with cells for 45 min at 37°C. After three washes in PBS, secondary antibodies diluted in PBSAT were added and incubated with cells for 30 min at 37°C. Cover slips were incubated with 300 nM 4,6-diamidino-2-phenylindole (Molecular Probes) in PBS for 10 min to counterstain cell nuclei, washed once in PBS, and mounted on glass slides with ProLong (Molecular Probes). Samples were examined with a 60×, 1.4 NA objective using a Nikon TE-2000U inverted microscope equipped with phase and fluorescence optics. Images were collected digitally using a cooled, charge-coupled device camera (Hamamatsu) and analyzed with Metamorph 6.1 (Molecular Devices). All images were processed and prepared for presentation using Photoshop 5.5 (Adobe Systems).
Acknowledgments
We express our sincere gratitude to Elaine Freimont for lab maintenance and technical assistance and to other members of our lab for helpful discussions. We also thank Melina Agosto, Tijana Ivanovic, Cathy Miller, John Parker, and Megan Talkington for comments on the manuscript.
This study was supported in part by NIH grants R01 AI47904 and R56 AI067445 to M.L.N.M.M.A. was additionally supported by NIH grant T32 AI07245 to the Viral Infectivity Training Program and by the Albert J. Ryan Foundation. K.E.M. was additionally supported by the Life Sciences Research Foundation and the Schering Plough Corp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Canning WM, Kauffman RS, Sharpe AH, Hallum JV, Fields BN. Role of the host cell in persistent viral infection: coevolution of L cells and reovirus during persistent infection. Cell. 1981;25:325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Arias CF, Dector MA, Segovia L, Lopez T, Camacho M, Isa P, Espinosa R, Lopez S. RNA silencing of rotavirus gene expression. Virus Res. 2004;102:43–51. doi: 10.1016/j.virusres.2004.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C, Silverstein SC, Levin DH, Acs G. Regulation of the reovirus RNA transcriptase by a viral capsomere protein. Virology. 1972;48:648–654. doi: 10.1016/0042-6822(72)90149-3. [DOI] [PubMed] [Google Scholar]
- Attoui H, Fang Q, Mohd Jaafar F, Cantaloube JF, Biagini P, de Micco P, de Lamballerie X. Common evolutionary origin of aquareoviruses and orthoreoviruses revealed by genome characterization of Golden shiner reovirus, Grass carp reovirus, Striped bass reovirus and golden ide reovirus (genus Aquareovirus, family Reoviridae) J. Gen. Virol. 2002;83:1941–1951. doi: 10.1099/0022-1317-83-8-1941. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Shatkin AJ. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J. Virol. 1970;6:1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MM, Peters TR, Dermody TS. Reovirus σNS and μNS proteins form cytoplasmic inclusion structures in the absence of viral infection. J. Virol. 2003;77:5948–5963. doi: 10.1128/JVI.77.10.5948-5963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, McCutcheon AM, Centonze VE, Nibert ML. Reovirus nonstructural protein μNS binds to reovirus cores, but does not inhibit their transcription activity. J. Virol. 2000;74:5516–5524. doi: 10.1128/jvi.74.12.5516-5524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Parker JSL, Joyce PL, Kim J, Nibert ML. Mammalian reovirus nonstructural protein μNS forms large inclusions and colocalizes with reovirus microtubule-associated protein μ2 in transfected cells. J. Virol. 2002;76:8285–8297. doi: 10.1128/JVI.76.16.8285-8297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Kim J, Miller CL, Piggott CD, Dinoso JB, Nibert ML, Parker JSL. Reovirus nonstructural protein μNS recruits viral core surface proteins and entering core particles to factory-like inclusions. J. Virol. 2004;78:1882–1892. doi: 10.1128/JVI.78.4.1882-1892.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Arnold MM, Miller CL, Hurt JA, Joyce PL, Nibert ML. Carboxyl-proximal regions of reovirus nonstructural protein μNS necessary and sufficient for forming factory-like inclusions. J. Virol. 2005;79:6194–6206. doi: 10.1128/JVI.79.10.6194-6206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz UJ, Finke S, Conzelmann KK. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna M, Eichwald C, Vascotto F, Burrone OR. RNA interference of rotavirus segment 11 mRNA reveals the essential role of NSP5 in the virus replicative cycle. J. Gen. Virol. 2005;86:1481–1487. doi: 10.1099/vir.0.80598-0. [DOI] [PubMed] [Google Scholar]
- Carvalho J, Arnold MM, Nibert ML. Silencing and complementation of reovirus core protein μ2: Functional correlations with μ2-microtubule association and differences between virus- and plasmid-derived μ2. Virology. 2007;364:301–316. doi: 10.1016/j.virol.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran K, Walker SB, Chen Y, Contreras CM, Schiff LA, Baker TS, Nibert ML. In vitro recoating of reovirus cores with baculovirus-expressed outer-capsid proteins μ1 and σ3. J. Virol. 1999;73:3941–3950. doi: 10.1128/jvi.73.5.3941-3950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Association between the spindle apparatus and reovirus. Proc. Natl. Acad. Sci. U.S.A. 1963;50:268–275. doi: 10.1073/pnas.50.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S, Gomatos P, Hsu KC. The uptake and development of reovirus in strain L cells followed with labelled viral ribonucleic acid and ferritin-antibody conjugates. Virology. 1965;25:193–211. doi: 10.1016/0042-6822(65)90199-6. [DOI] [PubMed] [Google Scholar]
- Drayna D, Fields BN. Activation and characterization of the reovirus transcriptase: genetic analysis. J. Virol. 1982;41:110–118. doi: 10.1128/jvi.41.1.110-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbretti E, Afrikanova I, Vascotto F, Burrone OR. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J. Gen. Virol. 1999;80:333–339. doi: 10.1099/0022-1317-80-2-333. [DOI] [PubMed] [Google Scholar]
- Fields BN, Raine CS, Baum SG. Temperature-sensitive mutants of reovirus type 3: defects in viral maturation as studied by immunofluorescence and electron microscopy. Virology. 1971;43:569–578. doi: 10.1016/0042-6822(71)90282-0. [DOI] [PubMed] [Google Scholar]
- Gaillard RK, Jr, Joklik WK. The relative translation efficiencies of reovirus messenger RNAs. Virology. 1985;147:336–348. doi: 10.1016/0042-6822(85)90136-9. [DOI] [PubMed] [Google Scholar]
- Gomatos PJ. RNA synthesis in reovirus-infected L929 mouse fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 1967;58:1798–1805. doi: 10.1073/pnas.58.4.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Coombs KM. Infectious entry of reovirus cores into mammalian cells enhanced by transfection. J. Virol. Methods. 2005;128:88–92. doi: 10.1016/j.jviromet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Joklik WK. Studies on the effect of chymotrypsin on reovirions. Virology. 1972;49:700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Chappell JD, Danthi P, Dermody TS. Gene-specific inhibition of reovirus replication by RNA interference. J. Virol. 2006;80:9053–9063. doi: 10.1128/JVI.00276-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PWK, Hayes EC, Joklik WK. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981;108:134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Levin DH, Acs G, Silverstein SC. Chain initiation by reovirus RNA transcriptase in vitro. Nature. 1970;227:603–604. doi: 10.1038/227603a0. [DOI] [PubMed] [Google Scholar]
- Lopez T, Rojas M, Ayala-Breton C, Lopez S, Arias CF. Reduced expression of the rotavirus NSP5 gene has a pleiotropic effect on virus replication. J. Gen. Virol. 2005;86:1609–1617. doi: 10.1099/vir.0.80827-0. [DOI] [PubMed] [Google Scholar]
- McCrae MA, Joklik WK. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978;892:578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- McCutcheon AM, Broering TJ, Nibert ML. Mammalian reovirus M3 gene sequences and conservation of coiled-coil motifs near the carboxyl terminus of the μNS protein. Virology. 1999;264:16–24. doi: 10.1006/viro.1999.9990. [DOI] [PubMed] [Google Scholar]
- Miller CL, Broering TJ, Parker JSL, Arnold MM, Nibert ML. Reovirus σNS protein localizes to inclusions through an association requiring the μNS amino-terminus. J. Virol. 2003;77:4566–4576. doi: 10.1128/JVI.77.8.4566-4576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Arnold MM, Broering TJ, Eichwald C, Kim J, Dinoso JD, Nibert ML. A cytoplasmic platform for easily visualizing protein-protein associations inside cells. Mol. Cell. Proteomics. 2007;6:1027–1038. doi: 10.1074/mcp.M700056-MCP200. [DOI] [PubMed] [Google Scholar]
- Murray KE, Nibert ML. Guanidine hydrochloride inhibits mammalian orthoreovirus growth by reversibly blocking the synthesis of double-stranded RNA. J. Virol. 2007;81:4572–4584. doi: 10.1128/JVI.02106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe TA, Ramig RF, Sharpe AH, Fields BN. Genetics of reovirus: identification of the dsRNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978;89:594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Nibert ML, Fields BN. A carboxy-terminal fragment of protein μ1/μ1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J. Virol. 1992;66:6408–6418. doi: 10.1128/jvi.66.11.6408-6418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JSL, Broering TJ, Kim J, Higgins DE, Nibert ML. Reovirus core protein μ2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J. Virol. 2002;76:4483–4496. doi: 10.1128/JVI.76.9.4483-4496.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie BL, Greenberg HL, Graham DY, Estes MK. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1984;1:133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Rhim JS, Jordan LE, Mayor HD. Cytochemical, fluorescent-antibody and electron microscopic studies on the growth of reovirus (ECHO 10) in tissue culture. Virology. 1962;17:342–355. doi: 10.1016/0042-6822(62)90125-3. [DOI] [PubMed] [Google Scholar]
- Schiff LA, Nibert ML, Tyler KL. Orthoreoviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1853–1915. [Google Scholar]
- Sen A, Sen N, Mackow ER. The formation of viroplasm-like structures by the rotavirus NSP5 protein is calcium regulated and directed by a C-terminal helical domain. J. Virol. 2007;81:11758–11767. doi: 10.1128/JVI.01124-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Chen LB, Fields BN. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV-1 cells. Virology. 1982;120:399–411. doi: 10.1016/0042-6822(82)90040-x. [DOI] [PubMed] [Google Scholar]
- Silvestri LS, Taraporewala ZF, Patton JT. Rotavirus replication: plus-sense templates for double-stranded RNA synthesis are made in viroplasms. J. Virol. 2004;78:7763–7774. doi: 10.1128/JVI.78.14.7763-7774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Joklik WK. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969;39:822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Spendlove RS, Lennette EH, John AC. The role of the mitotic apparatus in the intracellular location of reovirus antigen. J. Immunol. 1963;90:554–560. [PubMed] [Google Scholar]
- Su YP, Su BS, Shien JH, Liu HJ, Lee LH. The sequence and phylogenetic analysis of avian reovirus genome segments M1, M2, and M3 encoding the minor core protein μA, the major outer capsid protein μB, and the nonstructural protein μNS. J. Virol. Methods. 2006;133:146–157. doi: 10.1016/j.jviromet.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Taraporewala ZF, Jiang X, Vasquez-Del Carpio R, Jayaram H, Prasad BVV, Patton JT. Structure-function analysis of rotavirus NSP2 octamer by using a novel complementation system. J. Virol. 2006;80:7984–7994. doi: 10.1128/JVI.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier P, Plissier M. The intracellular development of the reovirus studied with the electron microscope. Presse Med. 1960;68:683–688. [PubMed] [Google Scholar]
- Venter PA, Krishna NK, Schneemann A. Capsid protein synthesis from replicating RNA directs specific packaging of the genome of a multipartite, positive-strand RNA virus. J. Virol. 2005;79:6239–6248. doi: 10.1128/JVI.79.10.6239-6248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, 4th, Bassel-Duby R, Fields BN, Tyler KL. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J. Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener JR, Bartlett JA, Joklik WK. The sequences of reovirus serotype 3 genome segments M1 and M3 encoding the minor protein μ2 and the major nonstructural protein μNS, respectively. Virology. 1989;169:293–304. doi: 10.1016/0042-6822(89)90154-2. [DOI] [PubMed] [Google Scholar]
- Yamakawa M, Furuichi Y, Shatkin AJ. Reovirus transcriptase and capping enzymes are active in intact virions. Virology. 1982;118:157–168. doi: 10.1016/0042-6822(82)90329-4. [DOI] [PubMed] [Google Scholar]
- Zarbl HJ, Millward S. The reovirus multiplication cycle. In: Joklik WK, editor. The Reoviridae. New York: Plenum Press; 1983. pp. 107–196. [Google Scholar]
- Zhang Y, Guo D, Geng H, Liu M, Hu Q, Wang J, Tong G, Kong X, Liu N, Liu C. Characterization of M-class genome segments of muscovy duck reovirus S14. Virus Res. 2007;125:42–53. doi: 10.1016/j.virusres.2006.12.004. [DOI] [PubMed] [Google Scholar]