Abstract
In an effort to characterize fruit ripening-related genes functionally, two glucosyltransferases, FaGT6 and FaGT7, were cloned from a strawberry (Fragaria×ananassa) cDNA library and the full-length open reading frames were amplified by rapid amplification of cDNA ends. FaGT6 and FaGT7 were expressed heterologously as fusion proteins in Escherichia coli and target protein was purified using affinity chromatography. Both recombinant enzymes exhibited a broad substrate tolerance in vitro, accepting numerous flavonoids, hydroxycoumarins, and naphthols. FaGT6 formed 3-O-glucosides and minor amounts of 7-O-, 4′-O-, and 3′-O-monoglucosides and one diglucoside from flavonols such as quercetin. FaGT7 converted quercetin to the 3-O-glucoside and 4′-O-glucoside and minor levels of the 7- and 3′-isomers but formed no diglucoside. Gene expression studies showed that both genes are strongly expressed in achenes of small-sized green fruits, while the expression levels were generally lower in the receptacle. Significant levels of quercetin 3-O-, 7-O-, and 4′-O-glucosides, kaempferol 3-O- and 7-O-glucosides, as well as isorhamnetin 7-O-glucoside, were identified in achenes and the receptacle. In the receptacle, the expression of both genes is negatively controlled by auxin which correlates with the ripening-related gene expression in this tissue. Salicylic acid, a known signal molecule in plant defence, induces the expression of both genes. Thus, it appears that FaGT6 and FaGT7 are involved in the glucosylation of flavonols and may also participate in xenobiotic metabolism. The latter function is supported by the proven ability of strawberries to glucosylate selected unnatural substrates injected in ripe fruits. This report presents the first biochemical characterization of enzymes mainly expressed in strawberry achenes and provides the foundation of flavonoid metabolism in the seeds.
Keywords: Achenes, detoxification, flavonols, Fragaria×ananassa, multisubstrate glucosyltransferase, plant defence, salicylic acid, strawberry, xenobiotics
Introduction
Plant glycosyltransferases transfer activated sugar molecules to a broad range of endogenous and xenobiotic substrates. The enzymes exhibit a rather broad acceptor tolerance, but have been shown to display strict regioselectivity in many cases (Vogt and Jones, 2000). Glycosylation is an essential modification contributing to the complexity of secondary metabolites in plants, which is reflected in the fact that more than 300 different glycosides of quercetin have been detected so far (Jones and Vogt, 2001).
Strawberry (Fragaria×ananassa) is one of the most popular fruit crops worldwide. The so-called fruit is actually an aggregate composed of the swollen receptacle and numerous achenes (Hancock, 1999). Fruit ripening is triggered by a decline in the concentration of auxin which is released by the achenes in early developmental stages (Perkins-Veazie, 1995). Quercetin and kaempferol (Fig. 1) have been reported to be the most important flavonols in strawberry fruits (Häkkinen et al., 1999). The glycosylation pattern of strawberry flavonols is quite complex. Seven different glycosides of kaempferol alone have been identified. It was reported that 3-O-glucosides and 3-O-glucuronides of quercetin and kaempferol, as well as kaempferol 7-O-glucoside, occur in strawberry fruits (Ryan, 1971). Additionally, 3-O-xylosylglucuronides and minor amounts of 3,7-O-diglucosides were identified (Henning, 1981). Achenes and green fruits contain high amounts of total phenolics (Wang and Lin, 2000; Aaby et al., 2005), but besides anthocyanin profiles no detailed study about the polyphenolic composition of the achenes has been performed so far.
Fig. 1.
Flavonols and hydroxycoumarins are substrates of FaGT6 and FaGT7 in vitro. Glycosides of quercetin and kaempferol are the main flavonols in strawberries (Ryan, 1971; Henning, 1981; Häkkinen et al., 1999) while hydroxycoumarins have not been detected in strawberry fruits.
Glycosyltransferases that transfer sugars to either hydroxy group 3 or 7 of flavonols in a regioselective way have been described in a number of plants including Petunia hybrida, Scutellaria baicalensis, and Arabidopsis thaliana (Miller et al., 1999; Hirotani et al., 2000; Jones et al., 2003). However, some glycosyltransferases from Allium cepa and Arabidopsis thaliana show no strict regioselectivity and glucosylate the hydroxy groups 3, 7, 3′, and 4′, and even form diglucosides in some cases (Kramer et al., 2003; Lim et al., 2004). In strawberries, flavonoid 3-O-glucosyltransferase activity was detected, but the respective protein was not purified (Given et al., 1988; Halbwirth et al., 2006). Although a transferase with broad substrate tolerance glucosylating quercetin at position 7 was purified from strawberry fruit (Cheng et al., 1994), little is known about the enzymes that form the complex array of glycosylated flavonoids or their spatial and temporal regulation in Fragaria.
As sedentary organisms, plants have evolved remarkable mechanisms to protect themselves from pathogens, herbivores, and xenobiotics. One strategy is the synthesis of preformed defence compounds such as cyanogenic glucosides and glucosinolates (Osbourn, 1996). Additionally, plants posses a flexible detoxification system. The conjugation with glucose leads to increased water solubility and reduced toxicity of most compounds. The respective reaction products can be subsequently stored in the plant vacuole (Jones and Vogt, 2001). Several glycosyltransferases that were cloned from cell cultures of Nicotiana tabacum exhibited broad substrate tolerance and were induced by salicylic acid (SA). They are assumed to be involved in the detoxification of xenobiotic compounds (Taguchi et al., 2001, 2003a, b). Other inducible glycosyltransferases from Arabidopsis thaliana were shown to form both N- and O-glucosides of 3,4-dichloroaniline and 2,4,5-trichlorophenol (Loutre et al., 2003) or to accept the Fusarium mycotoxin desoxynivalenol (Poppenberger et al., 2003). The glycosyltransferase with the broadest substrate tolerance reported so far was found in Rauwolfia serpentina. The enzyme accepts a total of 45 different substrates out of 74 tested. This includes simple phenolics, phenylpropanoids, flavonoids, and hydroxycoumarins (Hefner et al., 2002).
As a result of an ongoing project on strawberry glycosyltransferases, the cloning and biochemical characterization of the first two glucosyltransferases that are strongly expressed in achenes are reported here. Both enzymes show a rather broad substrate tolerance in vitro and glucosylate a range of endogenous and xenobiotic substrates. Gene expression studies combined with metabolite analyses suggest that both enzymes are involved in the biosynthesis of flavonols in achenes and the receptacle, while protein modelling studies revealed essential amino acids in the active sites of the enzymes.
Materials and methods
Plant material
The strawberry fruits were sampled from Fragaria×ananassa cv. Elsanta and Camarosa. Elsanta and Camarosa are octaploid cultivars that were grown under field conditions in Freising, Germany and Huelva, in the south-western part of Spain, respectively. Small-sized green and fully ripe red fruits were harvested and immediately frozen in liquid nitrogen and stored at –80 °C until used.
Chemicals
Except when otherwise stated, all chemicals, solvents, and reference compounds were obtained from Sigma, Aldrich, Fluka, Riedel de Haën (all Taufkirchen, Germany), Merck (Darmstadt, Germany) or Roth (Karlsruhe, Germany). Anthocyanidins were purchased from Polyphenols Laboratories (Sandnes, Norway). Quercetin 7-O-glucoside was a donation of Thilo Fischer (Chair for Ornamental Crops, Technical University Munich). Radiolabelled [6-3H]UDP–glucose (1 mCi ml−1, 60 Ci mmol−1) was obtained from American Radiolabelled Compounds (St Louis, MO, USA).
Rapid amplification of cDNA ends (RACE)
Two partial sequences obtained from a strawberry cDNA library were identified as putative glycosyltransferases and named FaGT6 and FaGT7. As both clones already contained the poly(A)-tail at the 3′ end of the open reading frame (ORF), only the 5′ end had to be amplified by RACE (Schaefer, 1995). RNA was extracted from strawberry fruits (Fragaria×ananassa) according to a protocol originally developed for banana fruit (Asif et al., 2000). cDNA was generated using Superscript III (Invitrogen, Karlsruhe, Germany) and gene-specific primers (5′-GTT CAA TAT GGG TCC CAC CGG ATA CA-3′ for FaGT6 and 5′-GTT CCA CCC ACA GTG AGT CAC AAA T-3′ for FaGT7). Following purification of the cDNA obtained with a commercial kit (Qiagen, Hilden, Germany), a new poly(A)-tail at the 5′ end of the sequence was added with terminal transferase (Promega, Madison, WI, USA). The following Touchdown-PCR (Don et al., 1991) was performed with Oligo(dT)18, GeneRacer 3′ (both Invitrogen, Karlsruhe, Germany) and a nested gene-specific primer (5′-CTT ACC GTC GGA ACT GAG AGA CTG AA-3′ for FaGT6 and 5′-CAC TTG AGG AGC CCA ATC TCT TAT-3′ for FaGT7). Because the first RACE did not yield the complete 5′ end of the ORFs for both FaGT6 and FaGT7, new primers were designed which are located closer to the 5′ end and the RACE was repeated as described before. For reverse transcription, 5′-ATC ACG TAA GGC TTG GAG GTG GA AC-3′ (FaGT6) and 5′-CCA TAG CTC CTT TCT TCA ATC TCT-3′ (FaGT7) were used as gene-specific primers. The final Touchdown-PCR was conducted with 5′-ATC CGC AGC TCC AGA GGT GTA GAA C-3′ (FaGT6) and 5′-CTG ACT CAT CAG GAA ACA CAG GTA-3′ (FaGT7) as gene-specific primers and the complete coding sequences were obtained.
Construction of expression plasmids
The full-length ORFs were subcloned to the pGEM-T vector (Promega, Madison, WI, USA). For the FaGT6 construct, 5′-TGA ATT CAT GAA GAA AGC TTC AGA GC-3′ was used as forward primer and 5′-TGC GGC CGC CGA GGT TTG AAT TTG ATC G-3′ as reverse primer introducing EcoRI and NotI restriction sites. The ORF of FaGT7 was amplified with 5′-TGG ATC CAT GGC CAT GGA AAC TAA ATC ATG C-3′ as forward primer and 5′-TCT CGA GTT CAA CTA AGC CTC CAA AAG CTA G-3′ as reverse primer with BamHI and XhoI restriction sites. Subsequently, the ORFs were cloned to the pGEX-4T-1 expression vector (Amersham Bioscience, Freiburg, Germany). The identity of the cloned gene was confirmed by sequencing the complete insert (MWG Biotech, Ebersberg, Germany).
Heterologous protein expression
Escherichia coli BL21 (DE3) pLysS cells (Novagen, Schwalbach, Germany) were transformed with the pGEX-4T-1 expression vector containing the complete ORFs of FaGT6 or FaGT7. Cultures were grown overnight at 37 °C in Luria-Bertani medium containing 100 μg ml−1 ampicillin and 34 μg ml−1 chloramphenicol. The next day, the OD600 was measured and the cultures were diluted with Luria-Bertani medium containing the appropriate antibiotics to an OD600 of 0.06 and a final volume of 800 ml. This culture was grown at 37 °C until the OD600 reached 0.4–0.6. After cooling down the culture to 16 °C, 1 mM IPTG was added to induce protein expression. After overnight incubation at 16–18 °C, the cells were harvested by centrifugation and stored at –20 °C.
Cell lysis and protein purification
The GST-fusion proteins were affinity-purified using the GST Bind resin (Novagen) as recommended by the manufacturer. Briefly, cells were resuspended in binding buffer and sonicated three times on ice. All buffers contained 10 mM β-mercaptoethanol and all steps were performed at 4 °C. The crude protein extract was incubated for 30 min with the GST Bind resin to bind GST fusion protein. The target protein was eluted with GST elution buffer containing glutathione and the protein concentration was determined (Bradford, 1976). The presence of recombinant protein was confirmed by SDS–PAGE and western blot using anti-GST antibodies (Sigma, Taufkirchen, Germany). As FaGT7 could only be partially purified, the amount of FaGT7 was quantified using the Alpha Imager 2200 Documentation and Analysis System and the Alpha Ease Software (Alpha Innotech, San Leandro, CA, USA).
Biochemical characterization
The optimization of the assay conditions was carried out with radioactively labelled UDP–glucose. The biochemical characterization was performed with McIllvaine buffer (citrate-phosphate buffer, 5 mM β-mercaptoethanol, 10% glycerol) using 0.6–0.8 μg affinity purified recombinant protein in a total volume of 200 μl. The UDP–glucose was a mixture of 1 μl 0.016 mM [6-3H]UDP–glucose (1 mCi ml−1) and 99 μl unlabelled 101 mM UDP–glucose. The specific reaction conditions for the determination of substrate preferences are given in the text. Assays were incubated for 30 min and extracted with 1 ml water-saturated n-butanol. The radioactivity of the product was determined by liquid scintillation counting (LKB Rackbeta 1219, Wallac, Turku, Finland) following the addition of 4 ml Ultima Gold XR LSC cocktail (Perkin-Elmer, Boston, MA, USA). The kinetic constants were calculated with SigmaPlot 8.0 software (Systat Software, Erkrath, Germany) using the regression wizard and presuming a one-site saturation binding.
Identification of formed products
Assays were performed in a buffer containing 100 mM TRIS–HCl (pH 7.0), 10% glycerol, and 10 mM β-mercaptoethanol. Standard assays contained 5 mM UDP–glucose, 200 μM substrate, and crude protein extract with 15–20 μg total protein in a volume of 250 μl. Enzyme assays were incubated for 30 min at 30 °C and stopped by the addition of 200 μl 5 % HCl (anthocyanidin substrates) or 50 μl acetic acid (all other substrates). As a control, BL21 (DE3) pLysS cells were transformed with an empty pGEX-4T-1 vector and the respective protein extract was assayed under the same conditions. Furthermore, assays were conducted with heat-inactivated enzyme solution (5 min at 95 °C). Enzyme activity was monitored by detecting the reaction products with LC-UV-ESI-MSn.
Metabolite analysis in the receptacle and achenes
Strawberry fruits (50 g) were harvested in the green and red ripening stages and were homogenized with 20 ml water using an Ultra Turrax (T18 basic; IKA Works Inc. Wilmington, NC, USA) followed by centrifugation (3500 g, 10 min). The supernatant was collected for metabolite analysis of the receptacle and sedimented achenes were separated from the residue. Achenes (5 g) were homogenized with 5 ml water using a mortar and pestle, centrifuged (3500 g, 10 min), and analysed by LC-UV-ESI-MSn. 4-Methyl umbelliferyl glucuronide was added as internal standard.
LC-UV-ESI-MSn
A Bruker Daltonics esquire 3000plus ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany) connected with an Agilent 1100 HPLC system (Agilent Technologies, Waldbronn, Germany) equipped with a quaternary pump and a variable wavelength detector was utilized. Components were separated with a Phenomenex (Aschaffenburg, Germany) Luna C-18 column (150 mm long×2.0 mm inner diameter, particle size 5 μm) which was held at 25 °C. Enzyme assays were analysed using a linear gradient from 100% A (0.1% formic acid in water) to 100% B (acetonitrile) in 30 min with a flow rate of 0.2 ml min−1. To distinguish between flavonoid glucoside isomers and for metabolite analysis in strawberry fruit extracts, the gradient went from 100% A to 40% B in 40 min and in a further 5 min to 100% B. The detection wavelength was either 360 nm for assays (520 nm for anthocyanidin substrates) or 280 nm for metabolite analysis. The electrospray ionization voltage of the capillary was set to –4000 V and the end plate to –500 V. Nitrogen was used as dry gas at a temperature of 300 °C and a flow rate of 10 l min−1. The full scan mass spectra were measured in a scan range from m/z 50 to 800 with a scan resolution of 13 000 m/z/s until the ICC target reached 20 000 ms or 200 ms, whatever was achieved first. Tandem mass spectrometry was carried out using helium as collision gas (3.56×10−6 mbar) with the collision voltage set at 1 V. Spectra were acquired in the positive and negative ionization mode. Data analysis was performed using the DataAnalysis 3.1 software (Bruker Daltonics, Bremen, Germany).
Auxin treatment
The hormonal treatment was performed as described previously (Medina-Escobar et al., 1997). Briefly, achenes from mid-sized green fruits were removed carefully with a scalpel blade. One set of fruits was treated with a lanolin paste containing 1 mM 1-naphthaleneacetic acid (NAA) in 1% (v/v) DMSO. The other set of deachened fruits was treated with the same paste but without the synthetic auxin NAA. Fruits were harvested 96 h after treatment and immediately frozen in liquid nitrogen and stored at –80 °C.
SA treatment of strawberry fruits
One millilitre of a sterile solution of 0.75 mM SA or sterile water (control) was carefully injected into white-stage fruit still attached to the plants using a hypodermic syringe with a needle gauge. Fruits were harvested at 4, 6, and 8 h, immediately frozen in liquid nitrogen, and stored at –80 °C until RNA isolation.
SA treatment of strawberry cell cultures
To initiate callus cultures, young leaves from micropropagated strawberry plants were removed and cut into strips (3–4 mm wide) with a sterile scalpel and forceps. These explants were transferred to Petri dishes on the surface of solid medium (Murashige and Skoog, 1962), supplemented with 2.5 mg 2,4-dichlorophenoxyacetic acid, with the lower epidermis downward. The dishes were sealed with parafilm and incubated at 25 °C in a growth chamber under diffuse light. When sufficient callus was developed from the explants (4–6 weeks), small pieces (0.3–0.5 cm diameter) were excised and transferred to fresh medium and cultured as before. In this way, callus stocks were maintained indefinitely by sub-culturing to fresh medium at monthly intervals. To initiate cell suspension cultures, 1 g of callus was excised, disaggregated, and transferred to 30 ml of liquid medium in a 100 ml Erlenmeyer flask. These cultures were incubated at 25 °C at 100 rpm under diffuse light. The cell cultures were maintained by sub-culturing 1 g of filtered cells in fresh liquid medium every 15 d. For the SA treatment, 0.5 l of cellular culture refreshed for 5 d was divided in two Erlenmeyer flasks. To 0.25 l of cellular culture, 0.75 mM SA was added, while the same volume of sterile distilled water was added to the other 0.25 l used as control. Both SA and control cellular cultures were incubated at 25 °C at 100 rpm under diffuse light. Cells were harvested by filtering after 4 h and 8 h, immediately frozen in liquid nitrogen, and stored at –80 °C until RNA isolation.
Quantitative PCR (qPCR)
Total RNA was isolated from a pool of six or seven strawberry fruits as described before (Asif et al., 2000). Subsequently, RNA was treated with DNase I (Amersham Bioscience, Barcelona, Spain) to remove any DNA contamination prior to cDNA synthesis. Reverse transcription was carried out using 1 μg of total RNA and the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) as recommended by the manufacturer. qPCR analysis was performed using the iCycler system (Bio-Rad) as described previously (Benítez-Burraco et al., 2003; Raab et al., 2006). Specific primers for FaGT6 (5′-CGC AGC GCA TCA GGT TCG TC-3′ and 5′-GAT CCG CAG CTC CAG AGG TGT AGA-3′) and FaGT7 (5′-CTC GCG GCC ACA GCA TAC-3′ and 5′-GTC GGC ACT TTC GCA ATC TT-3′) were designed. The results were normalized with an interspacer 26S-18S RNA gene used as the housekeeping gene (5′-ACC GTT GAT TCG CAC AAT TGG TCA TCG-3′ and 5′-TAC TGC GGG TCG GCA ATC GGA CG-3′). PCR reactions contained 2 mM MgCl2, 0.2 mM dNTPs, 0.2 μM of the respective primers, 2.5 μl SYBR Green I (diluted 1:15000), 2 μl of cDNA, and 0.5 U of Taq polymerase (Biotools, Madrid, Spain) in a total volume of 25 μl. The thermal cycling conditions were as follows: 2 min at 94 °C, followed by 40 cycles of 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. Subsequently, melting point analysis and agarose gel electrophoresis were applied to confirm that the desired amplicon had been generated. In qPCR analysis, quantification is based on the threshold cycle (Ct) when the fluorescence is first detectable above background level. The Ct-values are inversely proportional to the logarithm of the initial copy number. Each reverse transcription was repeated at least once and every PCR was performed at least in triplicate. All results were normalized in relation to the housekeeping gene. Relative expression values were calculated according to Livak and Schmittgen (2001), normalizing the results to a calibrator (receptacle from full-sized green fruits or untreated control).
Glucosylation of xenobiotics in planta
Ripe strawberry fruits were injected with 10 mM 3-hydroxycoumarin, 2-naphthol (both dissolved in 10% ethanol), or 3-hydroxyflavone (as alkaline aqueous solution) using a syringe with a needle (0.4×20 mm; Braun, Melsungen, Germany). In an independent experiment, 10 mM kaempferol and 2-naphthol were separately injected into fruits and in combination. As controls, the solvents without substrates were used. The fruits were harvested after 1 d and freeze-dried in a lyophilizer (ALPHA 1-4; Martin-Christ, Osterode, Germany). Fifty milligrams of the fine powder were extracted with 250 μl methanol. The solvent was removed in a rotary vacuum concentrator (RVC 2-18; Martin-Christ) and the extract was redissolved in 35 μl water and analysed by LC-UV-ESI-MSn.
Protein modelling
DeepView (http://www.expasy.org/spdbv/) software was used for visualization of the amino acids in the protein models (Guex and Peitsch, 1997).
Results
RACE and phylogenetic analysis
FaGT6 and FaGT7 were identified in a strawberry (Fragaria×ananassa) cDNA library and annotated as glycosyltransferases due to their sequence similarities to other known enzymes. To obtain the full-length sequences, RACE was successfully applied to amplify the unknown 5′ end of the ORF (Schaefer, 1995). The resulting ORF of FaGT6 is 1440 bp long and codes for a protein with 479 amino acids. FaGT7 is a protein of 487 amino acids and is encoded by 1464 bp.
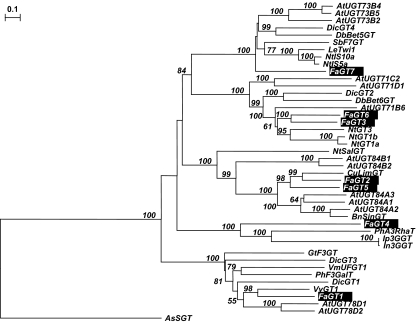
Phylogenetic analysis (Fig. 2) was carried out with the full-length protein sequences of FaGT6, FaGT7, and five other glycosyltransferases from strawberry (FaGT1–5; Griesser et al., 2008). FaGT6 shows highest homology to FaGT3, another uncharacterized glycosyltransferase from strawberry. Similarities were also found to several multi-substrate glycosyltransferases from Nicotiana tabacum acting on flavonols, hydroxycoumarins, naphthols, and hydroxycinnamic acids (Taguchi et al., 2001, 2003a, b) and to an enzyme from Dorotheanthus bellidiformis which accepts the betacyanin betanidin, but also anthocyanidins and flavonols (Vogt et al., 1997; Vogt, 2002). UGT71D1 and UGT71C2 from Arabidopsis thaliana glucosylate flavonols and hydroxycoumarins in vitro (Lim et al., 2003, 2004).
Fig. 2.
Phylogenetic analysis of selected plant secondary product glycosyltransferases. The neighbor-joining tree was calculated with the Treecon software package (Van de Peer and De Wachter, 1994). Distance calculation was performed with Poisson correction and insertions/deletions were not taken into account. The tree was rooted using a sterol glycosyltransferase from Avena sativa (AsSGT) as an outgroup. Branch lengths indicate the number of substitutions per site. Bootstrap analysis was performed with 100 replicates and only values above 50% are shown. GenBank accession numbers and sources for the respective protein sequences are: AtUGT73B4, AAD17393 (A. thaliana); AtUGT73B5, AAD17392 (A. thaliana); AtUGT73B2, AAR01231 (A. thaliana); DicGT4, BAD52006 (Dianthus caryophyllus); DbBet5GT, CAB56231 (Dorotheanthus bellidiformis); SbF7G, BAA83484 (Scutellaria baicalensis); Letwi1, CAA59450 (Lycopersicum esculentum); NtIS10a, AAB36652 (N. tabacum); NtIS5a, AAB36653 (N. tabacum); FaGT7, ABB 92749 (F.×ananassa); AtUGT71C2, AAC35238 (A. thaliana); AtUGT71D1, AAC35239 (A. thaliana); DicGT2, BAD52004 (D. caryophyllus); DbBet6GT, AAL57240 (D. bellidiformis); AtUGT71B6, BAB02840 (A. thaliana); FaGT6, ABB92748 (F.×ananassa); FaGT3, AAU09444 (F.×ananassa); NtGT3, BAB88934 (N. tabacum); NtGT1b, BAB60721 (N. tabacum); NtGT1a, BAB60720 (N. tabacum); NtSalGT, AAF61647 (N. tabacum); AtUGT84B1, AAB87119 (A. thaliana); AtUGT84B2, AAB87106 (A. thaliana); CuLimGT, BAA93039 (Citrus unshiu); FaGT2, AAU09443 (F.×ananassa); FaGT5, ABB92747 (F. x ananassa); AtUGT84A3, CAB10327 (A. thaliana); AtUGT84A1, CAB10326 (A. thaliana); AtUGT84A2, BAB02351 (A. thaliana); BnSinGT, AAF98390 (Brassica napus); FaGT4, AAU09445 (F.×ananassa); PhA3RhaT, CAA81057 (Petunia hybrida); Ip3GGT, BAD95882 (Ipomea purpurea); In3GGT, BAD95881 (I. nil); GtF3GT, BAA12737 (Gentiana triflora); DicGT3, BAD52005 (D. caryophyllus); VmUFGT1, BAA36972 (Vigna mungo); PhF3GalT, AAD55985 (P. hybrida); DicGT1, BAD52003 (D. caryophyllus); VvGT1, AAB81682 (Vitis vinifera); FaGT1, AAU09442 (F.×ananassa); AtUGT78D1, NP_564357 (A. thaliana); AtUGT78D2, NP_197207 (A. thaliana); AsSGT, CAB06081 (A. sativa).
FaGT7 belongs to a cluster of several inducible glycosyltransferases including two enzymes from Nicotiana tabacum accepting flavonols, hydroxycoumarins, hydroxycinnamic and hydroxybenzoic acids in vitro (Horvath and Chua, 1996; Fraissinet-Tachet et al., 1998; Vogt et al., 1999), and glycosyltransferases from Scutellaria baicalensis (Hirotani et al., 2000) and Dianthus caryophyllus (Ogata et al., 2004). The betanidin 5-glycosyltransferase from D. bellidiformis shows activity with certain flavonols, too (Vogt et al., 1999). UGT73B2, UGT73B4, and UGT73B5 from A. thaliana accept quercetin and hydroxycoumarins in vitro (Lim et al., 2003, 2004).
Although FaGT6 and FaGT7 are grouped in two different clusters, they can be assigned as putative multi-substrate glycosyltransferases based on their phylogenetic classification.
Enzymatic activity of the recombinant protein
The full-length ORFs of FaGT6 and FaGT7 were cloned in the expression vector pGEX-4T-1 and the recombinant enzymes were expressed as GST-fusion proteins in E. coli. FaGT6 and FaGT7 were affinity-purified using an immobilized reduced glutathione resin. The presence of target protein was confirmed by SDS–PAGE and western blot using antibodies against the GST domain at the N-terminus of the fusion enzyme (data not shown). Although GST-fusion proteins may not necessarily share all biochemical properties of the native enzymes, the use of recombinant proteins is widely accepted as a valuable tool to study plant secondary product glucosyltransferases (Vogt and Jones, 2000).
Initial activity tests were performed with [6-3H]UDP–glucose and various flavonols. FaGT6 readily showed activity with most flavonols tested (3-hydroxyflavone, 3,7-dihydroxyflavone, galangin, kaempferol, quercetin, myricetin, isorhamnetin, and morin), but not with 5-hydroxyflavone and 7-hydroxyflavone (Fig. 3A). Flavanones (taxifolin and naringenin) and some anthocyanidins (malvidin, peonidin, and petunidin) were also accepted, while delphinidin, cyanidin, and pelargonidin could not serve as substrates. Additionally, FaGT6 glucosylated several hydroxycoumarins (3-, 6-, and 7-hydroxycoumarin, esculetin, daphnetin, and scopoletin) as well as 1-naphthol and 2-naphthol. Other substrates such as coumarin, 4-hydroxycoumarin, 7-methoxycoumarin, catechin, epicatechin, p-coumaric acid, 4-hydroxybenzoic acid, and 4-hydroxy-2,5-dimethyl-3-(2H)-furanon were not converted. UDP–glucuronic acid and UDP–galactose were not accepted as donors with quercetin as acceptor.
Fig. 3.
Substrate preference of the recombinant enzymes. Enzymatic activity was measured with liquid scintillation counting testing a range of flavonoids (black columns), hydroxycoumarins (open columns), and naphthols (shaded columns) with radioactive donor substrate [6-3H]UDP–glucose as described in Materials and methods. FaGT6 (A) reactions were performed with 30 min incubation time in McIllvaine buffer (pH 7.5) at 30 °C. Relative activities refer to the best substrate 3-hydroxyflavone (100%). FaGT7 (B) reactions were performed with 30 min incubation time in McIllvaine buffer (pH 6.5) at 35 °C. Relative activities refer to the best substrate isorhamnetin (100%).
FaGT7 showed a similar substrate tolerance and accepted all flavonols tested (3-hydroxyflavone, 3,7-dihydroxyflavone, galangin, kaempferol, quercetin, myricetin, and isorhamnetin) as well as certain flavones (7-hydroxyflavone, chrysin), and flavanones (taxifolin), while anthocyanidins (pelargonidin and malvidin), catechin, naringenin, apigenin, and 5-hydroxyflavone were not accepted (Fig. 3B). Selected hydroxycoumarins (3- and 7-hydroxycoumarin, esculetin, daphnetin, and scopoletin) as well as 1-naphthol and 2-naphthol were also converted. However, 4-hydroxycoumarin, 6-hydroxycoumarin, and 7-methoxycoumarin could not serve as substrates. No activity could be detected with several hydroxybenzoic and hydroxycinnamic acids and the important flavour compound 4-hydroxy-2,5-dimethyl-3-(2H)-furanon. UDP–glucuronic acid and UDP–galactose were not substrates when assayed with quercetin and isorhamnetin.
A comparison of the substrate preferences of the two glucosyltransferases reveals that galangin, isorhamnetin, and 3-hydroxycoumarin are the preferred acceptors in both cases. FaGT6 also showed high activity with 3-hydroxyflavone and elevated activities with 2-naphtol and scopoletin. Both glycosyltransferases exhibited a rather low relative activity with the abundant natural substrates quercetin and kaempferol. In accordance with the phylogenetic assignment, FaGT6 and FaGT7 are multi-substrate glucosyltransferases accepting a broad range of natural and xenobiotic substrates in vitro and show high activity with substrates that have not been detected in strawberry fruits until now.
Biochemical characterization
The assay conditions for FaGT6 were optimized using the endogenous substrate quercetin and [6-3H]UDP–glucose. FaGT6 showed maximum activity at 35 °C and pH 7.5–8.0 in McIIIlvaine buffer, and product formation was linear for at least 30 min. The optimum reaction conditions for FaGT7 were determined with 3-hydroxycoumarin. The highest activity was monitored in McIllvaine buffer (pH 6.5) at 45 °C, and product formation was linear for at least 60 min.
Kinetic constants were determined for UDP–glucose and 3-hydroxyflavone (FaGT6) or isorhamnetin (FaGT7). Different concentrations of the flavonol substrate were assayed keeping the level of UDP–glucose constant and vice versa. The apparent Km and Vmax values were obtained from the hyperbolic Michaelis–Menten saturation curves. The Km and Vmax values for 3-hydroxyflavone were determined to be 5.3 μM and 1.4 nkat mg−1, respectively. As sugar donor for FaGT6, UDP–glucose showed a Km of 2.0 mM and a Vmax of 0.5 nkat mg−1. As acceptor substrate for FaGT7, isorhamnetin exhibited Km and Vmax values of 3.5 μM and 2.8 nkat mg−1, respectively. For UDP–glucose, a Km of 0.6 mM and a Vmax of 2.7 nkat mg−1 were determined.
Identification of reaction products
The substrate screening indicated already that FaGT6 and FaGT7 probably act on the hydroxyl group at position 3 of flavonols. Thus, the products formed from isorhamnetin, kaempferol, and quercetin with UDP–glucose as the donor were analysed by LC-UV-ESI-MSn. The ion traces indicated that besides one major product, two or three additional monoglucosides were produced by FaGT6 and FaGT7 while diglucosides were only formed by FaGT6 (Fig. 4A–C). Using authentic reference compounds, the major product formed from quercetin was identified as the 3-O-glucoside while the minor products were assigned as 7-O-, 3′-O-, and 4′-O-glucoside (Fig. 4C), according to the published elution order of the isomers (Lim et al., 2004; Shao et al., 2005) and their mass spectra (Ablajan et al., 2006). The fragmentation behaviour of flavonoid mono-O-glucosides can be used to distinguish between the different regioisomers. The relative abundance of the radical aglycone ion [Y-H]–• (compared with [Y]–) shows a close correlation with the glycosylation site. The product ion spectra of 3-O- and 7-O-glucosylated isomers of quercetin (kaempferol and isorhamnetin) show clear differences in the abundances of the fragment ions m/z 300 and 301 (m/z 284 and 285 as well as m/z 314 and 315), respectively (Fig. 4D–F). Similar to quercetin, the 3-O-glucoside constituted the major product when kaempferol and isorhamnetin were used as substrates for FaGT6, while the 7-O and 4′-O-glucosides of the flavonols were only produced in small amounts. The product ion spectra of the diglucosides revealed that both glucose moieties are located at different hydroxy groups of the flavonols. Only in this case, the daughter ions m/z 477, 447, and 463 can be detected, because a covalent linkage between two sugar molecules would not be cleaved effectively under the LC-UV-ESI-MS2 conditions applied (Fig. 4D–F insets; Giusti et al., 1999; Ablajan et al., 2006).
Fig. 4.
Identification of enzymatically formed products. Isorhamnetin (A), kaempferol (B), and quercetin (C) were incubated with UDP–glucose and recombinant FaGT6 (top) or FaGT7 (bottom) and subsequently analysed by LC-UV-ESI-MSn. Products were tentatively identified by means of authentic references or by comparison with published data (Giusti et al., 1999; Ablajan et al., 2006). Coeluting 3-O (top) and 7-O-glucosides (bottom) of isorhamnetin (D), kaempferol (E), and quercetin (F) were clearly distinguishable by their product ion spectra (MS2) according to Ablajan et al. (2006). The MS2 spectra of additional compounds formed by FaGT6 from isorhametin (inset in D), kaempferol (inset in E), and quercetin (inset in F) demonstrated the loss of two glucose molecules that are attached to different hydroxyl groups of the flavonols. They were tentatively identified as diglucoside, probably 3,7-O-diglucosides (Ablajan et al., 2006).
FaGT7 catalysed the formation of two major and two minor quercetin-monoglucosides as revealed by LC-UV-ESI-MSn (Fig. 4C). The 3-O- and 4′-O-glucosides were produced at almost equal amounts in addition to low levels of the 7-O- and 3′-O-isomers. Diglucosides were not detected. When kaempferol was used as the substrate the abundance of the 4′-O-glucoside was much lower and the 3′-O-glucoside could not be formed due to the missing 3′-hydroxyl group in kaempferol. The 4′-O-glucoside of isorhamnetin was only produced in trace amounts because the bulky 3′-methoxy group in the ortho position probably inhibits the attachment of a glucose molecule at the adjacent hydroxyl group. Instead, large amounts of the 3-O-glucoside were formed in addition to minor levels of the 7-O-isomer.
Metabolite profiling
Levels of the different flavonol (quercetin, kaempferol, and isorhamnetin) glucoside isomers were quantified by LC-UV-ESI-MS2 by means of the internal standard 4-methyl umbelliferyl glucuronide in extracts obtained from strawberry achenes and receptacles of small-sized green and ripe red fruits (Table 1). Kaempferol 3-O- and 7-O-, quercetin 3-O- and 4′-O-, and isorhamnetin 7-O-glucosides were detected in the achenes and receptacle of both ripening stages. In general, almost all potential products of the glucosyltransferases were found in the two tissues. Levels of 3-O-glucosides increased throughout ripening. Kaempferol 3-O-glucoside constituted the major flavonol glucoside in red achenes while almost equal amounts of kaempferol- and quercetin 3-O-glucoside were isolated from the red receptacle. Isorhamnetin occurred mainly as 7-O-glucoside.
Table 1.
Quantification of flavonol glucosides in Fragaria×ananassa cv. Elsanta achenes and receptacle of different ripening stages
| Achene | Receptacle | |||
| Green | Red | Green | Red | |
| Kaempferol 3-O-glucoside | 8.3 | 82.8 | 2.2 | 15.5 |
| Kaempferol 7-O-glucoside | 4.8 | 10.4 | 0.3 | 6.9 |
| Kaempferol 4′-O-glucoside | <0.1 | <0.1 | 0.2 | <0.1 |
| Quercetin 3-O-glucoside | 3.0 | 9.8 | 0.5 | 15.2 |
| Quercetin 7-O-glucoside | 1.0 | 2.4 | 0.2 | <0.1 |
| Quercetin 4′-O-glucoside | 14.2 | 7.3 | 0.3 | 3.4 |
| Isorhamnetin 3-O-glucoside | <0.1 | <0.1 | 0.6 | 3.1 |
| Isorhamnetin 7-O-glucoside | 7.2 | 8.0 | 1.3 | 0.4 |
| Isorhamnetin 4′-O-glucoside | <0.1 | <0.1 | <0.1 | 0.2 |
Values are expressed as mg-equiv. kg−1 4-methyl umbelliferyl glucuronide. Shown are the mean values of duplicates which differed by <20%.
Gene expression in achenes and receptacle
Transcript levels of FaGT6 and FaGT7 were determined by qPCR. RNA was extracted from the receptacle and achenes of small-sized green and red fruits. Following reverse transcription, the respective mRNA levels were quantified using sequence-specific primers. Transcript levels of FaGT6 are higher in achenes than in the receptacle of small-sized green and red fruits (Fig. 5A). The expression in achenes does not change in these two developmental stages while the expression in the receptacle is ripening-related. FaGT7 shows higher expression in achenes than in the receptacle of small-sized green fruits (Fig. 5B). In the receptacle, the expression is ripening-related and transcript levels in red fruits reach a level comparable to achenes of small-sized green fruit. FaGT6 and FaGT7 show similar relative expression levels in the receptacle. In conclusion, both glucosyltransferases display an achene-related gene expression different from other known strawberry glucosyltransferases (Lunkenbein et al., 2006; Griesser et al., 2008).
Fig. 5.
Gene expression of FaGT6 (A) and FaGT7 (B) in achenes (black columns) and the receptacle (open columns) of small-sized green (G) and red (R) fruits. Transcript levels were analysed by qPCR as described in Materials and methods. Gene expression is shown as relative expression normalized with receptacle tissue from small-sized green fruit (G). Hormonal control of FaGT6 (C) and FaGT7 (D) gene expression. The achenes were carefully removed at mid-sized green stage and the fruits were harvested after 5 d. Additionally, deachened green fruits were treated with a lanolin paste containing the synthetic auxin NAA. Gene expression was analysed by qPCR as described in Materials and methods and the data were normalized against untreated strawberries with the achenes still attached to the fruit.
Hormonal control of developmental expression
In early developmental stages, auxin is synthesized by the achenes and subsequently transported to the receptacle (Perkins-Veazie, 1995). It has been reported that a range of strawberry ripening-related genes are negatively regulated by auxin (Medina-Escobar et al., 1997; Manning, 1998; Aharoni et al., 2002; Lunkenbein et al., 2006; Raab et al., 2006; Griesser et al., 2008). Because expression levels of FaGT6 and FaGT7 increase during fruit ripening in the receptacle, the influence of auxin on the respective transcript levels was studied. Mid-sized green fruits were carefully de-achened and gene expression was analysed by qPCR after 5 d. Additionally, de-achened fruits were treated with a lanolin paste containing NAA, a synthetic auxin. Gene expression of both glycosyltransferases is induced after removing the achenes (Fig. 5C, D). Furthermore, the induction of FaGT6 and FaGT7 can be repressed by the subsequent application of a synthetic auxin. This indicates that the expression of both genes in the receptacle is indeed under the control of auxin.
SA-induced gene expression
To determine whether FaGT6 and FaGT7 could play a role in plant defence responses, their gene expression was studied by qPCR in strawberry cell cultures and strawberry fruits treated with SA. Treatment of strawberry cells with SA induced the accumulation of FaGT6 and FaGT7 transcripts by a factor of 5–6 already 4 h after treatment. Transcripts remained at this high level until 6 h and then declined to negative values (–5 to –4) in relation to a housekeeping gene (Fig. S1A, B in Supplementary data available at JXB online). Furthermore, in strawberry fruits injected with SA, the levels of both transcripts increased by a factor of 5–6 as soon as 4 h after injection, remained stable until 6 h, and fell below control levels until 8 h (Fig. S1C, D in Supplementary data available at JXB online). FaGT6 and FaGT7 genes show a similar expression profile both in SA-treated cells and SA-injected fruits, suggesting that these genes are co-regulated under these conditions.
Glucosylation of xenobiotics in planta
Both FaGT6 and FaGT7 were able to glucosylate a number of exogenous substrates in vitro. To test if the enzymes could exhibit the same functionality in vivo, selected substrates were injected into ripe fruits. 3-Hydroxyflavone and 3-hydroxycoumarin are both natural compounds which do not occur in strawberry fruits, while 2-naphthol is a synthetic chemical. One day after injection, the fruits were harvested, extracted with methanol, and analysed by LC-UV-ESI-MS2. Fruits injected with solvents served as controls. The substrates and their glucosylated products were not detected in any of the controls. By contrast, in all fruits injected with one of the selected substrates, the respective glucoside could be detected and its formation was confirmed by product ion experiments (Fig. S2 in Supplementary data available at JXB online). Thus, it could be shown that strawberry fruit has the ability to glucosylate selected exogenously applied substrates of FaGT6 and FaGT7 in vivo. To demonstrate that xenobiotics and natural substrates compete for glucosylation, equal amounts of kaempferol and 2-naphthol were injected into strawberry fruits separately and in combination. Quantification of the levels of produced glucosides showed that lower amounts of 2-naphthol glucoside and especially kaempferol glucosides were produced when both substrates were offered at once, indicating a competition of the substrates for the glucosyltransferases (Fig. S2 in Supplementary data available at JXB online).
Protein modelling
Although FaGT6 and FaGT7 display similar substrate donor and acceptor preference and comparable kinetic data, they show low amino acid identities and can be clearly distinguished by the products they form (Fig. 6). To understand the structural basis for the different product tolerance of FaGT6 and FaGT7, their amino acid sequences and the sequences of VvGT1 from grape and UGT71G1 from Medicago truncatula were aligned. The 3D structures of the latter enzymes have already been elucidated and these proteins also glucosylate kaempferol and quercetin. VvGT1 forms regioselectively only 3-O-glucosides, while UGT71G1 represents a multi-product enzyme (Shao et al., 2005; Offen et al., 2006). Conserved amino acids identified by the ClustalX program in the four sequences were visualized by DeepView (http://www.expasy.org/spdbv/) in the Michaelis complex of VvGT1 (Guex and Peitsch 1997; Offen et al.; 2006; Fig. S3 in Supplementary data available at JXB online). Although kaempferol is used as glucose acceptor by all four glucosyltransferases, only the catalytically active His23 and Asp128 as well as Phe130 and Leu156 are conserved in the flavonol binding pocket. By contrast, the amino acids assumed to be responsible for UDP–glucose binding are mainly residues of the UGT signature PSPG motif (residues 370–413) and are well conserved, e.g. Trp370, Ala371, Gln373, His388, Trp391, Asn392, Ser393, Glu396, and Gln413 (Shao et al., 2005; Offen et al., 2006). Next, the amino acids that lie within 5 Å from kaempferol in the Michaelis complex of VvGT1 were visualized (Fig. S4 in Supplementary data available at JXB online). To compare the binding pockets of the four glycosyltransferases, the amino acids in close vicinity of kaempferol in the VvGT1 model were substituted by amino acids that were aligned in the same positions of FaGT6 and FaGT7 according to the ClustalX alignment. Although this simple procedure does not exactly describe the 3D structures of the active sites, the models obtained demonstrate significant differences for FaGT6 and FaGT7 (Fig. S4 in Supplementary data available at JXB online). The binding pockets in FaGT7 and VvGT1 show several consensuses while FaGT6 groups with MtUGT71G1. In FaGT7 and VvGT1, kaempferol stands in a narrow, deep pocket built by five non-polar amino acids (Phe18, Phe130, Phe210, Leu214, and Phe410). The general base His23 responsible for the deprotonation of the flavonol and the acceptor UDP–glucose are located on the bottom of the channel. Hydrogen bonds between 4′-OH of the flavonol and His159 and Gln198 (VvGT1) as well as Gln198 (FaGT7) further stabilize the transition states. Comparison with the models of FaGT6 and MtUGT71G1 shows that only Phe130 is conserved in these enzymes. Phe410 which forms π-stacking interactions with the A ring of the flavonol in VvGT1 and FaGT7 is replaced by Tyr410 that swings over the channel due to its polar character and enlarges the binding pocket. The rigid non-polar channel in the 3-O-glucosyltransferases formed by one Leu and four Phe residues becomes wider and more polar in FaGT6 and MtUGT71G1 due to substitutions with short-chain and polar amino acids and may explain the ability of FaGT6 to form diglucosides.
Fig. 6.
Sequence alignment of quercetin and kaempferol O-glucosyltransferases, including two already crystallized proteins from Vitis vinifera (VvGT1) and Medicago truncatula (UGT71G1) as well as Fragaria×ananassa GT6 (FaGT6), and Fragaria×ananassa GT7 (FaGT7). The alignment was performed using ClustalX (Thompson et al., 1997). Conserved amino acids are shaded, amino acids within 5 Å to kaempferol in the protein model of VvGT are boxed.
Discussion
Biochemical characterization
Plant secondary product glycosyltransferases have been reported to exhibit a rather strict regioselectivity towards the position of the sugar attachment (Vogt and Jones, 2000). FaGT6 and FaGT7 accepted a number of substrates and formed multiple products when assayed with quercetin, kaempferol, and isorhamnetin, which are potential substrates that occur naturally in strawberry. While FaGT6 produced mainly quercetin 3- and 7-O-glucoside, FaGT7 converted quercetin to almost equal amounts of quercetin 3-O-glucoside and quercetin 4′-O-glucoside in addition to small amounts of the 7-O- and 3′-O-isomers. The monoglucoside patterns of FaGT6 and FaGT7 with kaempferol as well as with isorhamnetin were almost indistinguishable because the ratios of the levels of 3-O- and 4′-O-glucoside were equal. Glycosyltransferases from Allium cepa and Arabidopsis thaliana show a comparable regioselectivity forming several monoglucosides and diglucosides of flavonol substrates but their expression pattern and regulation have not been investigated (Kramer et al., 2003; Lim et al., 2004). More recently, it was reported that the hydroxylation pattern in ring B of the acceptor molecule can influence product specificity. UGT73A4 from Beta vulgaris accepts the positions 4′ and 7 of flavonols. If a hydroxy group is present at position 3′ (e.g. quercetin), 4′-O-glucosides are preferentially formed. If the hydroxy group is missing (e.g. kaempferol), the enzyme produced 7-O-glucosides (Isayenkova et al., 2006). Likewise FaGT7 formed less kaempferol-4′-O-glucoside than quercetin-4′-O-glucoside. RUGT-5, the first recombinant glycosyltransferase from Oryza sativa showed a similar behaviour. The presence of hydroxy group 3′ led to the formation of 4′-O-glucosides, but its absence directed the reaction towards the formation of 3-O-glucosides (Ko et al., 2006). However, in the case of FaGT6, such an effect was not observed.
FaGT6 and FaGT7 also accepted a series of secondary metabolites not occurring in strawberry fruits and unnatural substrates such as 2-naphthol. This indicates that these glycosyltransferases may not only function in flavonol biosynthesis, but perhaps play a role in xenobiotic metabolism as well. Surprisingly, FaGT7 exhibited a relatively high temperature optimum (45 °C). A similar effect was observed when a multifunctional glycosyltransferase from Rauwolfia serpentina was characterized showing maximum activity at 50 °C (Hefner et al., 2002). This could indicate that these enzymes maintain their activity even under stress conditions, as required for enzymes involved in plant protection.
Gene expression studies
FaGT6 showed higher expression values in achenes than in the receptacle of small-sized green and ripe red fruits. In microarray experiments, 441 sequences were identified showing a different expression in achenes and the receptacle. Surprisingly, 259 genes (59%) were predominantly expressed in achenes (Aharoni and O'Connell, 2002). However, up to now mainly receptacle- and ripening-related strawberry genes were identified and characterized (Medina-Escobar et al., 1997; Lunkenbein et al., 2006; Raab et al., 2006; Griesser et al., 2008). Genes showing higher expression in achenes were not heterologously expressed and further characterized (Medina-Escobar et al., 1998; Blanco-Portales et al., 2004). Both FaGT6 and FaGT7 displayed a different expression pattern in achenes and the receptacle. This complies with earlier assumptions that gene expression is regulated differently in these tissues (Aharoni and O'Connell, 2002). Transcription of FaGT6 and FaGT7 in the receptacle is under the negative control of auxin, as it has been shown for several ripening-related genes (Medina-Escobar et al., 1997; Manning, 1998; Lunkenbein et al., 2006; Raab et al., 2006; Griesser et al., 2008).
Additionally, gene expression of both glycosyltransferases can be induced by SA, an important signal molecule in plant defence (Reymond and Farmer, 1998). Many glycosyltransferases including UGT73B3 and UGT73B5 from Arabidopsis thaliana were induced in an SA-dependent way after infection with Pseudomonas syringae pv. tomato (Langlois-Meurinne et al., 2005). UGT73C5 which glucosylates the Fusarium mycotoxin desoxynivalenol is induced by SA as well (Poppenberger et al., 2003). Furthermore, several multifunctional glycosyltransferases from Nicotiana tabacum that are putatively involved in plant defence are induced by SA (Taguchi et al., 2001). Similarly FaGT6 and FaGT7 might be part of a defence mechanism.
Function of FaGT6 and FaGT7 in planta
Although quercetin and kaempferol are moderate substrates for FaGT6 and FaGT7 in vitro, it is reasonable to propose that both enzymes are involved, at least in part, in the glucosylation of phenolics in achenes and the receptacle (Table 1). The gene expression patterns correlate with high amounts of phenolic compounds in achenes (Aaby et al., 2005) and fruits (Wang and Lin, 2000). It was shown that FaGT6 and FaGT7 gene expression in the receptacle (Fig. 5A, B) correlates with higher levels of kaempferol, quercetin, and isorhamnetin 3-O-glucosides determined in the red receptacle compared with the green stage (Table 1). Achenes generally accumulate the same flavonoid glucosides as the receptacle. Interestingly mainly isorhamnetin 7-O-glucosid was detected, although the 3-O-glucoside was the major product formed by FaGT6 and FaGT7. Significant levels of the 3-isomer were only determined in the receptacle of the late-ripening stage consistent with the increased transcript levels. The metabolite analyses combined with gene expression data point towards a possible role of both glucosyltransferases in flavonol biosynthesis in the strawberry receptacle and achene during ripening. This is supported by the fact that it was possible to identify for the first time quercetin 4′-O-glucoside in strawberry achenes and receptacles. It is noteworthy to mention that it was the product specificity of FaGT7 and its high expression in achenes which led to the identification of this novel strawberry flavonoid.
Achenes of strawberry accumulate a series of storage and defence compounds to ensure survival of the seeds for prolonged periods. In microarray experiments a large set of achene-related genes was identified belonging to a group of stress-associated genes (Aharoni and O'Connell, 2002). Accordingly, a heat-shock protein was reported to be mainly expressed in strawberry achenes (Medina-Escobar et al., 1998). Furthermore, transcripts of a lipid-transfer protein with a putative function in plant defence were localized in strawberry achenes using in situ hybridization (Yubero-Serrano et al., 2003). It was assumed that some of the stress-associated genes from achenes code for proteins involved in xenobiotic metabolism. This included a putative glutathione-S-transferase with the ability to conjugate xenobiotic substances in a phase-II reaction. However, a glycosyltransferase was not identified in this experiment (Aharoni and O'Connell, 2002). FaGT6 and FaGT7 are multifunctional glycosyltransferases that are mainly expressed in strawberry achenes and are induced by SA. This indicates that they may take over an additional role in the detoxification of xenobiotics, contributing to the defence capacity of strawberry achenes. A role for FaGT6 and FaGT7 in the glucosylation of xenobiotics was supported by the capability of strawberry fruits to glucosylate several unnatural compounds including 2-naphthol when injected in ripe fruits.
Similar to FaGT6 and FaGT7 there are several glycosyltransferases that act on endogenous and exogenous substrates at the same time. The best-studied enzymes were isolated from Arabidopsis thaliana. In vitro, UGT73C5 glycosylated the flavonol quercetin (Lim et al., 2004) and the mycotoxin desoxynivalenol. Plants with constitutively overexpressed UGT73C5 showed increased tolerance against desoxynivalenol, but the concentrations of flavonols were not analysed (Poppenberger et al., 2003). UGT72B1 glycosylated the xenobiotic compounds 2,4,5-trichlorophenol (TCP) and 3,4-dichloroanilin (DCA) (Loutre et al., 2003). T-DNA insertion lines with disrupted UGT72B1 showed strongly reduced conjugating activity towards DCA and TCP while activities towards natural phenolics were not affected (Brazier-Hicks and Edwards, 2005). In another experiment, it has been shown that endogenous substrates can inhibit the conversion of xenobiotics (Meßner et al., 2003). Similarly, we have shown that the xenobiotic compound 2-naphthol and natural kaempferol compete for glucosylation. Together this indicates that the detoxification of xenobiotics is probably a side-reaction of glycosyltransferases involved in secondary metabolism rather than the task of specialized plant defence enzymes.
Protein modelling
To investigate the active sites of FaGT6 and FaGT7, the Michaelis complex of the recently crystallized VvGT1 was used (Offen et al., 2006) with the kaempferol acceptor and the non-transferable donor UDP-2-fluoroglucose as template and the positions of the conserved amino acids of FaGT6, FaGT7, and MtUGT71G1 compared (Shao et al., 2005) in the 3D structure. The red grape enzyme VvGT1 is responsible for the formation of anthocyanins but also accepts a number of flavonols and transfers the hexose to the 3-O position (Offen et al., 2006). MtUGT71G1 is a triterpene glucosyltransferase that glucosylates certain isoflavones and the flavonol quercetin with higher efficiency than triterpenes (Achnine et al., 2005). UDP–glucose is almost completely buried in a long, narrow channel within the C-terminal domain and interacts through residues in the highly conserved PSPG motif (residues 370–413) (Fig. S3 in Supplementary data available at JXB online). In contrast to the numerous conserved amino acids in the UDP–glucose recognition site of the four flavonol glucosyltransferases, there is an apparent lack of consensus for amino acids except for Phe130 and Leu156 in the acceptor binding site (Fig. S3 in Supplementary data available at JXB online). A closer look at the amino acids in the vicinity of the acceptor molecule showed that FaGT6 is more closely related to MtUGT71G1 while FaGT7 groups with VvGT1, although the Fragaria proteins exhibit similar catalytic activities (Fig. S4 in Supplementary data available at JXB online). Because FaGT6 produced higher levels of quercetin-3′-O-glucoside than FaGT7 and the enzyme from M. truncatula glucosylated quercetin primarily at the 3′-O-position, it is conceivable that their active sites show some similarities. Secondly, it has been suggested that the acceptor binding pocket of MtUGT71G1 is much longer and larger than quercetin, and quercetin fits in easily with any of its hydroxyl groups which may explain the formation of multiple products. Such a large pocket would also account for the formation of diglucosides, which has been shown in the case of FaGT6. These findings nicely confirm the hypotheses that glucosyltransferases are two-domain enzymes that were produced during evolution, with one domain recognizing similar donors and another adapting to acceptors (Shao et al., 2005).
The first multi-substrate, multi-product glucosyltransferases that are strongly expressed in strawberry achenes have been isolated and characterized. Metabolite profiling suggested that they are involved in the glucosylation of flavonols in achenes and the receptacle but they probably also function in the metabolism of xenobiotics. As glucosides are no longer regarded as useless end products but rather serve as precursors for diverse biosynthetic pathways and have important physiological roles, the interest in their metabolism has increased in recent years. The data reported in this study provide a basis for the exploration of their formation and their functions in achenes and the receptacle during strawberry fruit development.
Supplementary data
Gene expression data, mass spectra of glucosylated xenobiotics, and protein models are presented in the Supplementary Figs S1–S4.
Supplementary Material
Acknowledgments
We thank T Hofmann for helpful discussions and T Fischer for technical support and the donation of quercetin 7-O-glucoside. This work was supported by Degussa AG.
References
- Aaby K, Skrede G, Wrolstad RE. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa) Journal of Agricultural and Food Chemistry. 2005;53:4032–4040. doi: 10.1021/jf048001o. [DOI] [PubMed] [Google Scholar]
- Ablajan K, Abliz Z, Shang X-Y, He J-M, Zhang R-P, Shi J-G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. Journal of Mass Spectrometry. 2006;41:352–360. doi: 10.1002/jms.995. [DOI] [PubMed] [Google Scholar]
- Achnine L, Huhman DV, Farag MA, Sumner LW, Blount JW, Dixon RA. Genomics-based selection and functional characterization of terpene glycosyltransferases from the model legume Medicago truncatula. The Plant Journal. 2005;41:875–887. doi: 10.1111/j.1365-313X.2005.02344.x. [DOI] [PubMed] [Google Scholar]
- Aharoni A, Keizer LCP, van den Broeck HC, Blanco-Portales R, Munoz-Blanco J, Bois G, Smit P, de Vos RCH, O'Connell AP. Novel insights into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiology. 2002;129:1019–1031. doi: 10.1104/pp.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, O'Connell AP. Gene expression analysis of strawberry achene and receptacle maturation using DNA microarrays. Journal of Experimental Botany. 2002;53:2073–2087. doi: 10.1093/jxb/erf026. [DOI] [PubMed] [Google Scholar]
- Asif MH, Dhawan P, Nath P. A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Molecular Biology Reporter. 2000;18:109–115. [Google Scholar]
- Benítez-Burraco A, Blanco-Portales R, Redondo-Nevado J, Luz Bellido M, Moyano E, Caballero JL, Munoz-Blanco J. Cloning and characterization of two ripening-related strawberry (Fragaria x ananassa cv. Chandler) pectate lyase genes. Journal of Experimental Botany. 2003;54:633–645. doi: 10.1093/jxb/erg065. [DOI] [PubMed] [Google Scholar]
- Blanco-Portales R, López-Raéz JA, Bellido ML, Moyano E, Dorado G, González-Reyes JA, Caballero JL, Munoz-Blanco J. A strawberry fruit-specific and ripening-related gene codes for a HyPRP protein involved in polyphenol anchoring. Plant Molecular Biology. 2004;55:763–780. doi: 10.1007/s11103-004-1966-4. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brazier-Hicks M, Edwards R. Functional importance of the family 1 glucosyltransferase UGT72B1 in the metabolism of xenobiotics in Arabidopsis thaliana. The Plant Journal. 2005;42:556–566. doi: 10.1111/j.1365-313X.2005.02398.x. [DOI] [PubMed] [Google Scholar]
- Cheng GW, Malencik DA, Breen PJ. UDP-glucose:flavonoid O-glucosyltransferase from strawberry fruit. Phytochemistry. 1994;35:1435–1439. [Google Scholar]
- Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Research. 1991;19:4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraissinet-Tachet L, Baltz R, Chong J, Kauffmann S, Fritig B, Saindrenan P. Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid. FEBS Letters. 1998;437:319–323. doi: 10.1016/s0014-5793(98)01257-5. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Rodriguez-Saona LE, Griffin D, Wrolstadt RE. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. Journal of Agricultural Food Chemistry. 1999;47:4657–4664. doi: 10.1021/jf981242+. [DOI] [PubMed] [Google Scholar]
- Given NK, Venis MA, Grierson D. Phenylalanine ammonia-lyase activity and anthocyanin synthesis in ripening strawberry fruit. Journal of Plant Physiology. 1988;133:25–30. [Google Scholar]
- Griesser M, Hoffmann T, Bellido ML, Rosati C, Fink B, Kurtzer R, Aharoni A, Munoz-Blanco J, Schwab W. Redirection of flavonoid biosynthesis through the downregulation of an anthocyanidin glucosyltransferase in ripening strawberry (Fragaria×ananassa) fruit. Plant Physiology. 2008;146:1–12. doi: 10.1104/pp.107.114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Häkkinen SH, Kärenlampi SO, Heinonen M, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. Journal of Agricultural Food Chemistry. 1999;47:2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- Halbwirth H, Puhl I, Haas U, Jezik K, Treutter D, Stich K. Two-phase flavonoid formation in developing strawberry (Fragaria×ananassa) fruit. Journal of Agricultural Food Chemistry. 2006;54:1479–1485. doi: 10.1021/jf0524170. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Strawberries. New York, NY: CABI Publishing; 1999. [Google Scholar]
- Hefner T, Arend J, Warzecha H, Siems K, Stöckigt J. Arbutin synthase, a novel member of the NRD1β glycosyltransferase family, is a unique multifunctional enzyme converting various natural products and xenobiotics. Bioorganic and Medicinal Chemistry. 2002;10:1731–1741. doi: 10.1016/s0968-0896(02)00029-9. [DOI] [PubMed] [Google Scholar]
- Henning W. Flavonolglykoside der Erdbeeren (Fragaria×ananassa Duch.), Himbeeren (Rubus idaeus L.) und Brombeeren (Rubus fruticosus L. Zeitschrift für Lebensmitteluntersuchung und Forschung. 1981;173:180–187. [Google Scholar]
- Hirotani M, Kuroda R, Suzuki H, Yoshikawa T. Cloning and expression of UDP-glucose:flavonoid 7-O-glucosyltransferase from hairy root cultures of Scutellaria baicalensis. Planta. 2000;210:1006–1013. doi: 10.1007/PL00008158. [DOI] [PubMed] [Google Scholar]
- Horvath DM, Chua N-H. Identification of an immediate-early salicylic acid-inducible tobacco gene and characterization of induction by other compounds. Plant Molecular Biology. 1996;31:1061–1072. doi: 10.1007/BF00040724. [DOI] [PubMed] [Google Scholar]
- Isayenkova J, Wray V, Nimtz M, Strack D, Vogt T. Cloning and functional characterisation of two regioselective flavonoid glucosyltransferases from Beta vulgaris. Phytochemistry. 2006;67:1598–1612. doi: 10.1016/j.phytochem.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Jones P, Messner B, Nakajima J-I, Schäffner AR, Saito K. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. Journal of Biological Chemistry. 2003;278:43910–43918. doi: 10.1074/jbc.M303523200. [DOI] [PubMed] [Google Scholar]
- Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164–174. doi: 10.1007/s004250000492. [DOI] [PubMed] [Google Scholar]
- Ko JH, Kim BG, Hur H-G, Lim Y, Ahn J-H. Molecular cloning, expression and characterization of a glycosyltransferase from rice. Plant Cell Reporter. 2006;25:741–746. doi: 10.1007/s00299-006-0119-4. [DOI] [PubMed] [Google Scholar]
- Kramer CM, Prata RTN, Willits MG, de Luca V, Steffens JC, Graser G. Cloning and regiospecifity studies of two flavonoid glucosyltransferases from Allium cepa. Phytochemistry. 2003;64:1069–1076. doi: 10.1016/s0031-9422(03)00507-7. [DOI] [PubMed] [Google Scholar]
- Langlois-Meurinne M, Gachon CMM, Saindrenan P. Pathogen-responsive expression of glycosyltransferase genes UGT73B3 and UGT73B5 is necessary for resistance to Pseudomonas syringae pv tomato in Arabidopsis. Plant Physiology. 2005;139:1890–1901. doi: 10.1104/pp.105.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Baldauf S, Li Y, Elias L, Worrall D, Spencer SP, Jackson RG, Taguchi G, Ross J, Bowles DJ. Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology. 2003;13:139–145. doi: 10.1093/glycob/cwg017. [DOI] [PubMed] [Google Scholar]
- Lim E-K, Ashford DA, Hou B, Jackson RG, Bowles DJ. Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnology and Bioengineering. 2004;87:623–631. doi: 10.1002/bit.20154. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loutre C, Dixon DP, Brazier M, Slater M, Cole DJ, Edwards R. Isolation of a glucosyltransferase from Arabidopsis thaliana active in the metabolism of the persistant pollutant 3,4-dichloroanilin. The Plant Journal. 2003;34:485–493. doi: 10.1046/j.1365-313x.2003.01742.x. [DOI] [PubMed] [Google Scholar]
- Lunkenbein S, Bellido ML, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner HA, Munoz-Blanco J, Schwab W. Cinnamate metabolism in ripening fruit: characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiology. 2006;140:1047–1058. doi: 10.1104/pp.105.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K. Isolation of a set of ripening-related genes from strawberry: their identification and possible relationship to fruit quality traits. Planta. 1998;205:622–631. doi: 10.1007/s004250050365. [DOI] [PubMed] [Google Scholar]
- Medina-Escobar N, Cárdenas J, Moyano E, Caballero JL, Munoz-Blanco J. Cloning, molecular characterization and expression pattern of a strawberry ripening-specific cDNA with sequence homology to pectate lyase from higher plants. Plant Molecular Biology. 1997;34:867–877. doi: 10.1023/a:1005847326319. [DOI] [PubMed] [Google Scholar]
- Medina-Escobar N, Cárdenas J, Munoz-Blanco J, Caballero JL. Cloning and molecular characterization of a strawberry fruit ripening-related cDNA corresponding a mRNA for a low-molecular-weight heat-shock-protein. Plant Molecular Biology. 1998;36:33–42. doi: 10.1023/a:1005994800671. [DOI] [PubMed] [Google Scholar]
- Meßner B, Thulke O, Schäffner AR. Arabidopsis glucosyltransferases with activities toward both endogenous and xenobiotic substrates. Planta. 2003;217:138–146. doi: 10.1007/s00425-002-0969-0. [DOI] [PubMed] [Google Scholar]
- Miller KD, Guyon V, Evans JNS, Shuttleworth WA, Taylor LP. Purification, cloning, and heterologous expression of a catalytically efficient flavonol 3-O-galactosyltransferase expressed in the male gametophyte of Petunia hybrida. Journal of Biological Chemistry. 1999;274:34011–34019. doi: 10.1074/jbc.274.48.34011. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologa Plantarum. 1962;15:473–497. [Google Scholar]
- Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies G. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO Journal. 2006;25:1396–1405. doi: 10.1038/sj.emboj.7600970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata J, Itoh Y, Ispida M, Yoshida H, Ozeki Y. Cloning and heterologous expression cDNAs encoding flavonoid glucosyltransferases from Dianthus caryophyllus. Plant Biotechnology Journal. 2004;21:367–375. [Google Scholar]
- Osbourn AE. Preformed antimicrobial compounds and plant defense against fungal attack. The Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Veazie P. Growth and ripening of strawberry fruit. Horticultural Reviews. 1995;17:267–297. [Google Scholar]
- Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glössl J, Luschnig C, Adam G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry. 2003;278:47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- Raab T, López-Ráez JA, Klein D, Caballero JL, Moyano E, Schwab W, Munoz-Blanco J. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase. The Plant Cell. 2006;18:1023–1037. doi: 10.1105/tpc.105.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Current Opinion in Plant Biology. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Ryan JJ. Flavonol glycosides of the cultivated strawberry. Journal of Food Science. 1971;36:867–870. [Google Scholar]
- Schaefer BC. Revolutions in rapid amplification of cDNA ends: new strategies for polymerase chain reaction cloning of full-length cDNA ends. Analytical Biochemistry. 1995;227:255–273. doi: 10.1006/abio.1995.1279. [DOI] [PubMed] [Google Scholar]
- Shao H, He X, Achine L, Blount JW, Dixon RA, Wang X. Crystal structure of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula. The Plant Cell. 2005;17:3141–3154. doi: 10.1105/tpc.105.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi G, Nakamura M, Hayashida N, Okazaki M. Exogenously added naphthols induce three glycosyltransferases, and are accumulated as glucosides in tobacco cells. Plant Science. 2003a;164:231–240. [Google Scholar]
- Taguchi G, Ubukata T, Hayashida N, Yamamoto H, Okazaki M. Cloning and characterization of a glucosyltransferase that reacts on 7-hydroxyl group of coumarin from tobacco cells. Archives of Biochemistry and Biophysics. 2003b;420:95–102. doi: 10.1016/j.abb.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Taguchi G, Yazawa T, Hayashida N, Okazaki M. Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrate specificity and are induced by salicylic acid and auxin. European Journal of Biochemistry. 2001;268:4086–4094. doi: 10.1046/j.1432-1327.2001.02325.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for Microsoft Windows environment. Computer Applications in the Biosciences. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- Vogt T. Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis. Planta. 2002;214:492–495. doi: 10.1007/s00425-001-0685-1. [DOI] [PubMed] [Google Scholar]
- Vogt T, Grimm R, Strack D. Cloning and expression of a cDNA encoding betanidin 5-O-glucosyltransferase, a betanidin- and flavonoid-specific enzyme with high homology to inducible glucosyltransferases from the Solanaceae. The Plant Journal. 1999;19:509–519. doi: 10.1046/j.1365-313x.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- Vogt T, Jones P. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sciences. 2000;5:380–386. doi: 10.1016/s1360-1385(00)01720-9. [DOI] [PubMed] [Google Scholar]
- Vogt T, Zimmermann E, Grimm R, Meyer M, Strack D. Are the characteristics of betanidin glucosyltransferases from cell-suspension cultures of Dorotheanthus bellidiformis indicative of their phylogenetic relationship with flavonoid glucosyltransferases? Planta. 1997;203:349–361. doi: 10.1007/s004250050201. [DOI] [PubMed] [Google Scholar]
- Wang SY, Lin H-S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. Journal of Agricultural and Food Chemistry. 2000;48:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- Yubero-Serrano E-M, Moyano E, Medina-Escobar N, Munoz-Blanco J, Caballero J-L. Identification of a strawberry gene encoding a non-specific lipid transfer protein that responds to ABA, wounding and cold stress. Journal of Experimental Botany. 2003;54:1865–1877. doi: 10.1093/jxb/erg211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.