Abstract
Cluster root (CR) formation contributes much to the adaptation to phosphorus (P) deficiency. CR formation by white lupin (Lupinus albus L.) is affected by the P-limiting level in shoots, but not in roots. Thus, shoot-derived signals have been expected to transmit the message of P-deficiency to stimulate CR formation. In this study, it is shown that sugars are required for a response to P starvation including CR formation and the expression of P starvation-induced genes. White lupin plants were grown in vitro on P-sufficient or P-deficient media supplemented with sucrose for 4 weeks. Sucrose supply stimulated CR formation in plants on both P-sufficient and P-deficient media, but no CR appeared on the P-sufficient medium without sucrose. Glucose and fructose also stimulated CR formation on the P-sufficient medium. On the medium with sucrose, a high concentration of inorganic phosphate in leaves did not suppress CR formation. Because sorbitol or organic acid in the media did not stimulate CR formation, the sucrose effect was not due to increased osmotic pressure or enriched energy source, that is, sucrose acted as a signal. Gene transcription induced by P starvation, LaPT1 and LaPEPC3, was magnified by the combination of P limitation and sucrose feeding, and that of LaSAP was stimulated by sucrose supply independently of P supply. These results suggest that at least two sugar-signalling mediating systems control P starvation responses in white lupin roots. One system regulates CR formation and LaSAP expression, which acts even when P is sufficient if roots receive sugar as a signal. The other system controls LaPT1 and LaPEPC3 expression, which acts when P is insufficient.
Keywords: Hexose, Lupinus albus L., phosphorus deficiency, proteoid root, sucrose
Introduction
White lupin (Lupinus albus L.) plants have a great ability to adapt to a phosphorus (P)-deficient environment (Vance et al., 2003). This adaptation may be attributed mainly to the formation of special roots with closely spaced lateral roots (rootlets) of determinate growth, called cluster (or proteoid) roots (Watt and Evans, 1999b). Cluster roots (CR) increase P uptake from the soil by expanding the root surface area and increasing the release of proton, acid phosphatase, and citrate (Johnson et al., 1994, 1996a, b; Keerthisinghe et al., 1998; Newmann et al., 1999; Watt and Evans, 1999a, b; Yan et al., 2002). The released acid phosphatase and citrate effectively change P compounds in the rhizosphere into an absorbable form. The P-deficient condition activates phosphate transporters in CR (Liu et al., 2001).
The P deficiency level in plants is the predominant inducer of CR formation, the release of acid phosphatase and citrate (Johnson et al., 1994, 1996b; Keerthisinghe et al., 1998; Newmann et al., 1999; Yan et al., 2002), and the expression of genes, such as the secreted acid phosphatase, LaSAP (Wasaki et al., 1999; Miller et al., 2001), the phosphate transporter, LaPT (Liu et al., 2001), the multidrug and toxin efflux, LaMATE (Uhde-Stone et al., 2005), and the phosphoenolpyruvate carboxylase, LaPEPC (Uhde-Stone et al., 2003a; Peñaloza et al., 2005). PEPC activity was thought to be essential for citrate biosynthesis (Johnson et al., 1994; 1996a, b). Iron deficiency also induces CR formation (Hagström et al., 2001). In studies on the effect of P level in plants on CR formation and citrate exudation, foliar P application and high internal P concentration in shoots suppressed CR formation and citrate exudation (Keerthisinghe et al., 1998; Shane et al., 2003). Split-root system studies indicated that induction of CR is suppressed in that half of the root grown in the nutrient solution without P (–P) by the high P supply to the other root half, and P concentrations in roots show no correlation with CR formation and citrate exudation (Shane et al., 2003; Shen et al., 2005). These studies indicate that induction of CR formation is systemic, that is, the physiological condition in shoots leads to CR formation and to organic acid and acid phosphatase exudation. Therefore, some signals and signalling cascades are expected to transport the message of P deficiency from the shoots to the roots to stimulate CR formation and organic acid and acid phosphatase release. However, such signalling mechanisms are not fully understood. Auxin is one candidate that participates in such signalling cascades. Auxin applied to leaves stimulates CR formation but does not stimulate PEPC and malate dehydrogenase activities (Gilbert et al., 2000; Newmann et al., 2000). Exogenously supplied cytokinin suppresses CR formation (Newmann et al., 2000), suggesting cytokinin negatively correlates with such signalling pathways.
Sugars act as signals that mediate plant metabolism, growth, development, ion transport, and responses to biotic and abiotic stresses. Many sugar signal sensors and sugar signal transduction pathways have been investigated (Gibson, 2005; Rolland et al., 2006), among which the hexokinase sugar sensor (Moore et al., 2003) and the SnRK/SNF4-dependent signal transduction pathway (Gissot et al., 2006; Lu et al., 2007) have been well investigated. In white lupin, exogenously supplied sugars and photosynthates stimulate the transcription of LaSAP, LaPT1, and LaMATE, indicating that sugar signalling mediates the expression of P starvation-induced genes (Liu et al., 2005). Liu's study predicted that sugar signalling should participate in other P starvation responses, such as CR formation in white lupin, but we believe the effect of sugar on CR formation has not been examined. In this study, the effect of sugars on the formation of cluster roots was examined to determine the signal that stimulates CR formation. CR formation and the expression of P starvation-induced genes, LaSAP, LaPT1, and LaPEPC3, were examined.
Materials and methods
Plant growth
The following culture media were used. 1P medium consisted of 2 mM KNO3, 3 mM Ca(NO3)2.4H2O, 1 mM KH2PO4, 1 mM MgSO4.7H2O, 0.01 mM Fe(III)-EDTA, 0.023 mM H3BO3, 4 μM MnCl2.4H2O, 0.46 μM ZnSO4, 0.1 μM CuSO4, 0.1 μM NaMoO4, and 0.6% agarose (Agarose L03, Takara, Japan) at pH 5.5. –P medium contained 1 mM KCl instead of 1 mM KH2PO4. To 2P, 5P or 10P media, 1, 4, or 9 mM NaH2PO4, respectively, was added in addition to 1 mM KH2PO4. To avoid precipitation, these phosphate solutions were autoclaved separately from other medium components and were then mixed just before pouring onto culture plates. Sucrose, glucose, fructose, sorbitol, or organic acid at 25 mM concentration were added to the medium. Seeds of white lupin (Lupinus albus L. cv. Kievskij mutant) were sterilized by chlorine gas exposure for 16 h by using the same method as that applied to soybean tissue culture (Olhoft et al., 2003). Sterilized seeds were pre-cultured on a –P medium for 2 or 3 d. Germinated seeds were transplanted onto 240×240 mm plates (20 mm depth) containing 250 ml medium. The plates were sealed with Parafilm M (Alcan Packaging, WI), and about two-thirds of each plate was covered with aluminium foil to shade the roots. They were kept in a growth incubator at 20 °C under a 16/8 h light/dark condition. The position of the plates was changed daily to minimize any surrounding influences.
Plants were harvested 4 weeks after the transplanting and were divided into CR, normal roots (non-cluster roots), and shoots. Their fresh weight (FW) was measured and the number of CR was counted. CR was defined as the parts of primary lateral roots bearing clusters of rootlets having a density of 10 rootlets or more per cm (Johnson et al., 1994, 1996a). The roots and leaves were frozen at –80 °C until the inorganic phosphate determination and RNA extraction.
Fluorescence microscopy
Whole cluster rootlets and the secondary lateral roots generated on the 5P medium with sucrose were fixed in an ethanol-acetic acid solution (3:1 v:v) at 4 °C overnight, were rinsed with tap water, and were stained with 0.1 μg ml−1 4′,6-diamino-2-phenylindole (DAPI) solution for more than 1 h. They were examined by using a microscope with epi-fluorescence optics.
Inorganic phosphate determination
The method for assaying inorganic phosphate was modified from Ames (1966). About 1 g FW leaves was weighed and was homogenized with 4 ml of 10% perchloride acid solution by using a mortar and pestle. After centrifugation at 15 000 rpm, 400 μl of supernatant was mixed with 4 ml solution consisting of 15 mM ammonium molybdate and 100 mM zinc acetate (pH 5.0) and then with 1 ml of fresh ascorbic acid (10%, pH 5.0). The mixture was incubated at 30 °C in a water bath for 15 min. Absorbance at 850 nm was measured.
Statistics
The number of CR and the inorganic phosphate concentration are shown as averages and standard errors (SE) of three to eight replications (most were six). Differences between treatments were tested by using the Tukey compromise test at the 5% probability level.
Expression analysis of P starvation-induced genes (quantitative RT-PCR)
Total RNA from CR and normal roots was extracted and purified by using the RNeasy Plant Mini Kit in combination with RNase-free DNase (Quagen, Germany). cDNA was synthesized by using Super Script III reverse transcriptase (Invitrogen) and the oligo dT primer. In quantitative PCR, SYBR Premix Ex Taq (Takara, Japan) was used to intercalate SYBR Green I in amplified products, and signals were monitored by using the Chromo4 real time PCR system (Bio-Rad Japan). The amount of LaUbiquitin (CA410752, Uhde-Stone et al., 2003b) mRNA in each sample was determined to normalize the differences in mRNA amount of LaSAP (AF309552, Miller et al., 2001), LaPT1 (AF305623, Liu et al., 2001), and LaPEPC3 (AY663387, Peñaloza et al., 2005). Primer sequences were: LaUbiquitin, 5′-TCTTTGTGAAGACCCTCACC-3′ and 5′-CTGCTGGTCCGGAGGAATG-3′; LaSAP, 5′-TCCACTCGTTACCATACTCC-3′ and 5′-CCTTCTAGGTTTCCTCCATCC-3′; LaPT1, 5′-ATAGTCCAAATTCTGTGTTGGC-3′ and 5′-ATGGTTTTCCCTGCGCCTCTTC-3′; LaPEPC3, 5′-TCGTGACCCGAACTTTAATGTG-3′ and 5′-TTTTGGTGAGTGCAACTATGAT-3′.
Results
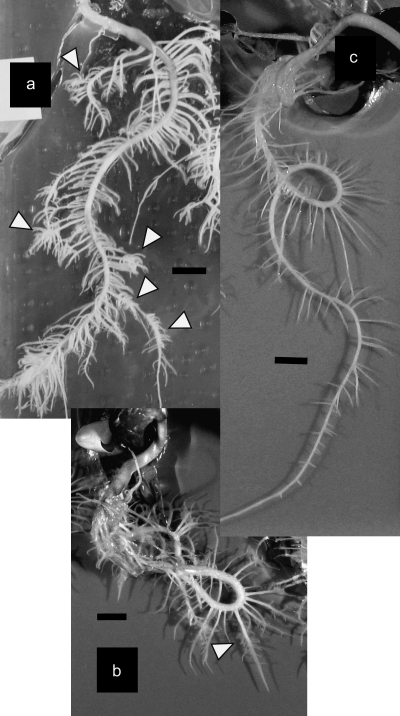
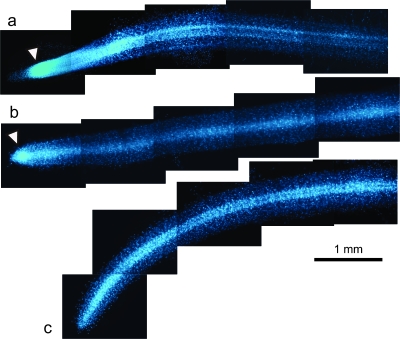
To examine the effect of sucrose on CR formation, white lupin plants were grown for 4 weeks on medium with or without sucrose in vitro. CR appeared on some of the primary lateral roots on the medium containing sucrose. Rootlet density and rootlet length of the CR were the same as those grown on the –P medium without sucrose (Fig. 1a, b). No CR appeared on the 1P medium without sucrose (Fig. 1c). To clarify if the CR grown on the medium containing sucrose was typical, rootlets of CR and secondary lateral roots were stained with DAPI and were observed under a fluorescence microscope. The meristem is not present in rootlets of mature CR, which is a typical feature of CR rootlets with determinate elongation (Watt and Evans, 1999a, b). The secondary lateral roots (i.e. normal root) had a dense region of nuclei near the root tips, which indicated a meristematic zone (Fig. 2a) that indicated indeterminate growth. Relatively young CR rootlets that were approaching their final length showed a smaller dense region of nuclei near the root tips (Fig. 2b). Mature rootlets that reached the final length no longer showed a dense region of nuclei (Fig. 2c), that is, the apical meristem was not present. These results indicate that CR grown on the medium containing sucrose were typical CR with determinate elongation of rootlets.
Fig. 1.
White lupin roots cultured on media with 1 mM P and 25 mM sucrose (a), without P or sucrose (b), and with 1 mM P without sucrose (c) for 4 weeks. Arrowheads indicate CR (bar=1 cm).
Fig. 2.
Whole mounts of CR rootlets and a secondary lateral root of white lupin stained with DAPI. A secondary lateral root, i.e. a normal root (a), a young CR rootlet that was approaching its final length (b), and a mature rootlet that had reached its final length (c) were collected from plants cultured on a medium containing both P and sucrose. Arrowheads indicate a dense region of nuclei (bar=1 mm).
In addition to relatively thick and long primary lateral roots, short (about 5 mm) and thin primary lateral roots emerged from the taproot on medium containing sucrose, resulting in a higher density of primary lateral roots than by plants on the medium without sucrose (Fig. 1). Short and thin primary lateral roots were stained with DAPI and were observed under a fluorescence microscope. These roots showed no dense region of nuclei near the root tips, indicating determinate growth (data not shown). That is, these short primary lateral roots were rootlet-like, but these CR-like taproot portions with rootlet-like lateral roots were not included when the number of CR was counted.
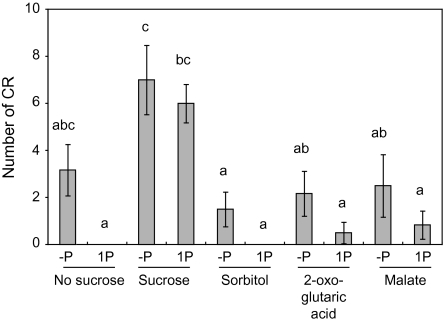
The effect of 25 mM sucrose, sorbitol and organic acid on CR number was examined. Fresh weight of roots and shoots was not changed significantly by the supply of these organic compounds (Table 1). Sucrose on media stimulated CR formation at seven and six CR under –P and 1P conditions, respectively, but no CR appeared on 1P medium without sucrose (Fig. 3). On –P medium without sucrose, 3.2 CR grew, but it was not significantly different from the no CR on the 1P medium without sucrose due to extensive variability in CR number from zero to seven. Sorbitol with or without P did not stimulate CR formation, indicating that the effect of sucrose on CR formation is not due to an increase in osmotic pressure. Sucrose acts as an energy source, so the effect of organic acids, 2-oxo-glutaric acid and malate, which can act as an energy source, on CR formation was investigated. CR numbers in plants on media containing 2-oxo-glutaric acid were almost the same as those on media without sucrose (Fig. 3). Although the primary lateral roots on –P and 1P media with malate elongated well (data not shown) resulting in a somewhat increased root fresh weight (Table 1), malate showed no effect on CR number (Fig. 3). These results indicate that stimulation of CR formation by sucrose supply is not due to an enriched energy source.
Table 1.
Fresh weight (average ±standard error) of white lupin plant cultured on media with (1P) or without (–P) P supplemented with sucrose, sorbitol, 2-oxo-glutaric acid or malate
| Culture condition | Root fresh weight (g)a | Shoot fresh weight (g)a |
| –P | 0.91±0.11 | 2.88±0.27 |
| 1P | 0.85±0.12 | 2.71±0.25 |
| –P, Sucrose | 1.01±0.12 | 2.31±0.20 |
| 1P, Sucrose | 0.74±0.15 | 2.26±0.17 |
| –P, Sorbitol | 0.92±0.22 | 2.52±0.35 |
| 1P, Sorbitol | 0.87±0.25 | 2.45±0.29 |
| –P, 2–oxo–glutaric acid | 0.66±0.20 | 1.95±0.23 |
| 1P, 2–oxo–glutaric acid | 0.68±0.02 | 1.95±0.14 |
| –P, Malate | 1.20±0.31 | 2.51±0.29 |
| 1P, Malate | 1.17±0.08 | 2.45±0.13 |
After ANOVA, no significant difference was detected at the 5% level.
Fig. 3.
Effect of sucrose, sorbitol, and organic acid on CR formation by white lupin. White lupin plants were cultured on solid media containing zero (–P) or 1 mM (1P) phosphate supplemented with 25 mM sucrose, sorbitol, 2-oxo-glutaric acid, or malate for 4 weeks. Vertical bars indicate the standard error. Histograms with different letters are significantly different at the 5% level.
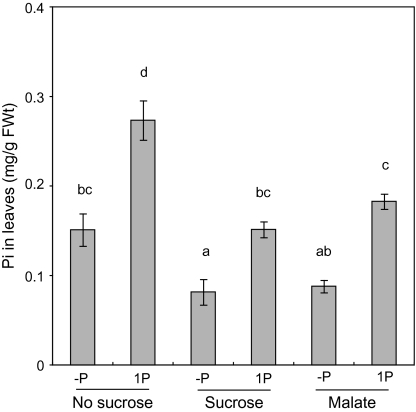
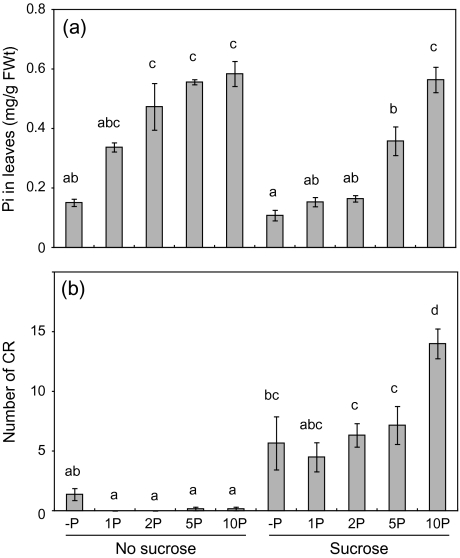
Because sugars can sequester inorganic phosphate during phosphorylation reaction, it was thought that the inorganic phosphate concentration in shoots should be low when sucrose is supplied. Therefore, the inorganic phosphate concentration in leaves was determined. On both 1P-sucrose and 1P-malate media, the inorganic phosphate concentration in leaves was almost at the same level as that on the –P medium without sucrose (Fig. 4). Therefore, there was a possibility that the low level of inorganic phosphate in shoots should stimulate CR formation on medium containing sucrose. To exclude this possibility, it was investigated whether CR formation is stimulated by sucrose with a high inorganic phosphate concentration in leaves. Phosphate (0, 1, 2, 5, or 10 mM) was added to both the medium with and without sucrose, and the CR number and inorganic phosphate concentration in leaves were determined. When 5 or 10 mM phosphate was added to the medium that contained sucrose, the inorganic phosphate concentration in leaves was the same as or higher than that in leaves of plants grown on 1P medium without sucrose (Fig. 5a). In these plants, the number of CR was greater than in plants on media without sucrose (Fig. 5b), indicating that sucrose stimulated CR formation independently of inorganic phosphate level in leaves. These results indicate that sucrose stimulates CR formation as a signal, not as indirect effects.
Fig. 4.
Inorganic phosphate (Pi) concentration in leaves of white lupin cultured on solid medium containing zero (–P), or 1 mM (1P) phosphate supplemented with 25 mM sucrose or malate for 4 weeks. Vertical bars indicate the standard error. Histograms with different letters are significantly different at the 5% level.
Fig. 5.
Effects of sucrose on inorganic phosphate (Pi) concentration in leaves (a) and cluster root number (b) of white lupin on culture media containing zero (–P), 1 (1P), 2 (2P), 5 (5P), or 10 (10P) mM phosphate. White lupin plants were cultured on the solid media for 4 weeks. Vertical bars indicate the standard error. Histograms with different letters are significantly different at the 5% level.
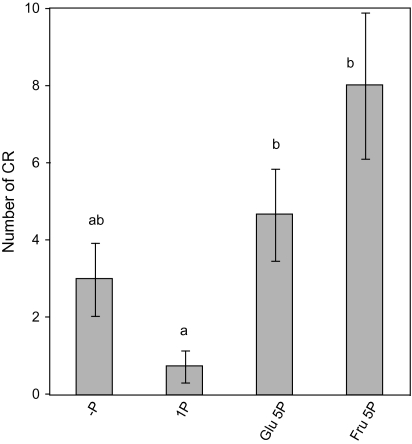
Sucrose can be readily hydrolysed in glucose and fructose in sink organs. The effect of glucose and fructose on CR formation was examined. Supplementation of 25 mM glucose or fructose stimulated CR formation on 5 mM P containing medium (Fig. 6), indicating that these hexoses are as valuable as signal molecules as sucrose is.
Fig. 6.
Effect of 0 mM P (–P), 1 mM P (1P), 25 mM glucose (Glu) plus 5 mM P, and 25 mM fructose (Fru) plus 5 mM P on CR formation by white lupin. Vertical bars indicate the standard error. Histograms with different letters are significantly different at the 5% level.
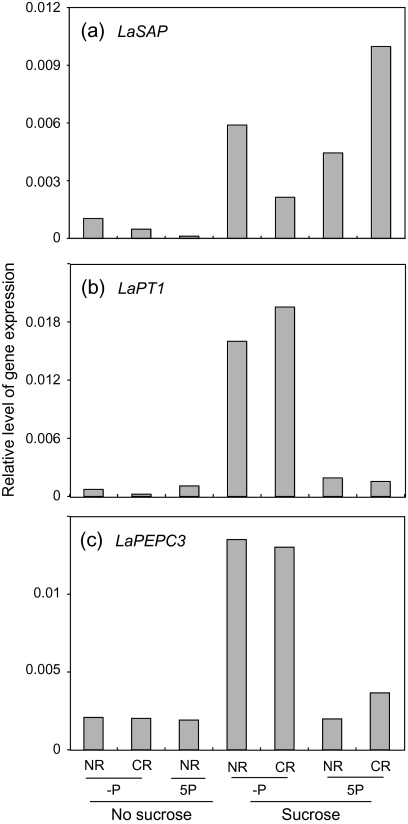
The transcript accumulation of P starvation-induced genes in white lupin roots was estimated by using quantitative RT-PCR. White lupin roots secrete acid phosphatase, and the expression of the secreted acid phosphatase gene LaSAP can be detected in roots only under the P starvation condition (Miller et al., 2001). In white lupin roots in vitro, transcription of LaSAP was nearly zero on the 5P medium without sucrose and was detected more on the –P medium without sucrose. Sucrose, however, stimulated LaSAP expression independently of the presence or absence of P in the medium (Fig. 7a). Both LaPT1 and PEPC3 are expressed weakly under normal conditions in white lupin roots, and their expression increases by P limitation (Liu et al., 2001; Peñaloza et al., 2005). In vitro white lupin in this study showed that the expression of LaPT1 and PEPC3 was high on the –P medium containing 25 mM sucrose, but the expression was weak in the other media, indicating that both a P-limiting condition and sucrose are necessary to increase the expression of LaPT1 and LaPEPC3 (Fig. 7b, c).
Fig. 7.
Effect of sucrose and 5 mM P (5P) in media on the relative expressions of LaSAP (a), LaPT1 (b), and LaPEPC3 (c) genes in white lupin roots. LaUbiquitin was used to normalize the expression of these genes. NR, normal roots; CR, cluster roots.
Discussion
Sugars act as signal molecules that regulate gene expression, growth and development in plants (Gibson, 2005; Rolland et al., 2006). P starvation responses are mediated by sugar signalling, that is, sugar signalling participates in the expression of P starvation-induced genes and in the changes in the root system architecture caused by P limitation (Jain et al., 2007; Karthikeyan et al., 2007; Hammond and White, 2008). In white lupin, the expression of P starvation-induced genes is stimulated by endogenously supplied sugars and is suppressed under photosynthate-limiting conditions (Liu et al., 2005). This study showed that endogenously supplied sucrose stimulated CR formation as a signal, not as an energy source or secondary effects (Figs 3, 5). Sucrose supply induced typical CR with determinate elongation of rootlets (Fig. 2) and with increased expression of LaPT1, LaSAP, and LaPEPC3 (Fig. 6). This sucrose effect on CR formation was independent of the presence or absence of P on the medium and of inorganic phosphate concentration in leaves (Fig. 5). The root system architecture in Arabidopsis is modulated by P limitation: suppressed taproot elongation and increased lateral root density. Exogenously supplied sugars stimulate Arabidopsis roots to increase lateral root density independently of the presence or absence of P in media and, however, have no effect on taproot length (Jain et al., 2007; Karthikeyan et al., 2007). Although CR is the special root architecture in white lupin, the regulation system to stimulate CR formation mediated by sugar signalling should be similar to that to increase lateral root density in Arabidopsis.
Although both hexose and sucrose are usually valuable as a signal, sucrose-specific induction of gene expression is reported in some sugar signalling pathways and glucose and fructose are less effective in them (Smeekens, 2000; Rolland et al., 2006). In this study, glucose and fructose showed an effect on CR formation (Fig. 6). The expression of P starvation-induced genes, LaPT1 and LaSAP, is enhanced by the supply of sucrose, glucose, and fructose in white lupin seedlings (Liu et al., 2005). This evidence suggests that hexose acts as the signal molecules in the signalling of P starvation responses in white lupin roots. There are at least three systems for hexose sensing; a hexokinase-dependent system, a glycolysis-dependent system and a hexokinase-independent system (Xiao et al., 2000; Rolland et al., 2006). Further studies to determine the sugar-sensing system are necessary in white lupin roots. Exogenously supplied sugars stimulate Arabidopsis roots to increase lateral root density, and Karthikeyan et al. (2007) showed by using the gin2 hexokinese mutant that a hexokinese sugar sensor participates in some aspects of the P deficiency response mechanism in lateral roots.
In this study, sugar signalling of the expression of P starvation-induced genes was investigated. The expression of LaSAP increased on the sucrose-containing media with or without P (Fig. 7). Liu et al. (2005) showed in their northern blot analysis that LaSAP gene expression in white lupin seedlings is induced by sugar supply even with sufficient P. In this study, in contrast to LaSAP, both P deficiency and sucrose supply were necessary to increase LaPT1 and LaPEPC3 expressions, but the presence of P and sucrose together had no or little effect on the increase (Fig. 7). Liu et al. (2005) showed that the expressions of LaPT1 and LaMATE in seedlings are stimulated by the combination of P limitation and sugars. That is, regulations of LaSAP expression and LaPT1 and LaPEPC3 expressions differ but sugar signalling participates in both. Many genes induced by P starvation in Arabidopsis have been examined. Among them, the transcription of some genes, such as phosphate transporters Pht1;1 and Pht1;4 and purple acid phosphatase PAP, increases by sugar treatment when P is insufficient (Müller et al., 2005; Karthikeyan et al., 2007). However, sugar supply stimulates the expression of the acid phosphatase ACP5, even when P is sufficient (Müller et al., 2005). That is, several pathways mediated by a sugar signal exsist that regulate P starvation responses. DNA microarray analysis of Arabidopsis showed that transcription of 187 of 21500 genes changes more than 2-fold by P starvation: 171 were induced and 16 were repressed (Müller et al., 2007). In that DNA microarray analysis, 149 genes that were synergistically or antagonistically regulated by P limitation and sugar supply were selected and categorized into three clusters (clusters 1, 2, and 3) according to the expression profile, and possible cis-regulatory elements in these genes were identified. P starvation stimulated the transcription of the cluster 3 genes, which was magnified by sucrose feeding, and 43% (20 of 47) of the cluster 3 genes contained the PHR1 binding site in their 3′ upstream region (Müller et al., 2007). PHR1 is the MYB-CC transcription factor that regulates P starvation responses (Rubio et al., 2001). This observation indicates that, in addition to sugar signals, many kinds of transcription factors as well as other signals and sensors participate in the regulation of gene expression induced by P deficiency.
DNA microarray and metabolome analyses in rice, common bean, and Arabidopsis reveal that gene transcription and enzyme activities in carbohydrate synthesis are increased in P-starved tissues that accumulate sucrose, hexoses, and starch (Wasaki et al., 2003, 2006; Hernández et al., 2007; Morcuende et al., 2007). Allocation of dry matter and 13C changes when P is insufficient, and roots receive increased allocation of photosynthates in common bean, Arabidopsis and tomato (Nielsen et al., 2001; López-Bucio et al., 2002; Fujita et al., 2003). The PHO3 gene in Arabidopsis encodes SUC2, the sucrose transporter that participates in phloem loading in shoots, and the pho3 mutant has limited ability to transport sucrose from leaves to roots (Lloyd and Zakhleniuk, 2004). Interestingly, the pho3 mutant is thought to be impaired in the expression of P starvation-induced genes in roots because this mutant was originally identified as a mutant that suppresses root phosphatase (Hammond and White, 2008). Thus, sugars have been proposed to be a long-range signal molecule synthesized mainly in the shoot and transported from the shoot to the roots to trigger plant responses to P limitation (Hermans et al., 2006; Hammond and White, 2008). Therefore, it is postulated that white lupin uses sugars as a systemic signal exported from shoots. This idea was supported by the stem girdling experiment using white lupin in which the expression of P starvation-induced genes in roots was repressed 24 h after the girdling (Liu et al., 2005). At least two sugar signal-mediating regulation systems control P starvation responses in white lupin roots. One system regulates CR formation and LaSAP expression and acts even when P is sufficient if roots receive a sugar signal. The other system regulates LaPT1 and LaPEPC3 gene expression and acts when P is limited, suggesting that sugar signalling interacts with other signalling networks of signals such as a local P-starvation signal or another systemic signal or both. Both the mechanism that senses low P in the rhizosphere (Svistoonoff et al., 2007) and the microRNA399-mediating signalling cascade that regulates P starvation responses (Bari et al., 2006; Chiou, 2007) have been clarified for Arabidopsis. Further analysis is necessary to examine how sugar signalling interacts with other signalling networks.
Acknowledgments
This work was supported by a Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 17658032).
Glossary
Abbreviations
- CR
cluster root
- P
phosphorus
- LaSAP
secreted acid phosphatase in Lupinus albus
- LaPT
phosphate transporter in Lupinus albus
- LaMATE
multidrug and toxin efflux in Lupinus albus
- LaPEPC
phosphoenolpyruvate carboxylase in Lupinus albus
References
- Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology. 1966;8:115–118. [Google Scholar]
- Bari R, Pant BD, Stitt M, Scheible W-R. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiology. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou T-J. The role of microRNAs in sensing nutrient stress. Plant. Cell and Environment. 2007;30:323–332. doi: 10.1111/j.1365-3040.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- Fujita K, Okada M, Lei J, Ito J, Ohkura K, Adu-Gyamfi JJ, Mohapatra PK. Effect of P-deficiency on photoassimilate partitioning and rhythmic changes in fruit and stem diameter of tomato (Lycopersicon esculentum) during fruit growth. Journal of Experimental Botany. 2003;54:2519–2528. doi: 10.1093/jxb/erg273. [DOI] [PubMed] [Google Scholar]
- Gibson SI. Control of plant development and gene expression by sugar signaling. Current Opinion in Plant Biology. 2005;8:93–102. doi: 10.1016/j.pbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany. 2000;85:921–928. [Google Scholar]
- Gissot L, Polge C, Jossier M, Girin T, Bouly JP, Kreis M, Thomas M. AKINβγ contributes to SnRK1 heterotrimeric complexes and interacts with two proteins implicated in plant pathogen resistance through its KIS/GBD sequence. Plant Physiology. 2006;142:931–944. doi: 10.1104/pp.106.087718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström J, James WM, Skene KR. A comparison of structure, development and function in cluster roots of Lupinus albus L. under phosphate and iron stress. Plant and Soil. 2001;232:81–90. [Google Scholar]
- Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hernández G, Ramírez M, Valdés-López O, et al. Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiology. 2007;144:752–767. doi: 10.1104/pp.107.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiology. 2007;144:232–247. doi: 10.1104/pp.106.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JF, Allan DL, Vance CP. Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiology. 1994;104:657–665. doi: 10.1104/pp.104.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JF, Allan DL, Vance CP, Weiblen G. Root carbon dioxide fixation by phosphorus-deficient Lupinus albus. Contribution to organic acid exudation by proteoid roots. Plant Physiology. 1996a;112:19–30. doi: 10.1104/pp.112.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JF, Allan DL, Vance CP. Phosphorus deficiency in Lupinus albus. Altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiology. 1996b;112:31–41. doi: 10.1104/pp.112.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta. 2007;225:907–918. doi: 10.1007/s00425-006-0408-8. [DOI] [PubMed] [Google Scholar]
- Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant. Cell and Environment. 1998;21:467–478. [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DL, Vance CP. Signalling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. The Plant Journal. 2005;41:257–268. doi: 10.1111/j.1365-313X.2004.02289.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Uhde-Stone C, Li A, Vance C, Allan D. A phosphate transporter with enhanced expression in proteoid roots of white lupin (Lupinus albus L.) Plant and Soil. 2001;237:257–266. [Google Scholar]
- Lloyd JC, Zakhleniuk OV. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. Journal of Experimental Botany. 2004;55:1221–1230. doi: 10.1093/jxb/erh143. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology. 2002;129:244–256. doi: 10.1104/pp.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Lin C, Lee K, Chen J, Huang L, Ho S, Liu H, Hsing Y, Yu S. The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. The Plant Cell. 2007;19:2484–2499. doi: 10.1105/tpc.105.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Liu J, Allan DL, Menzhuber CJ, Fedorova M, Vance CP. Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin. Plant Physiology. 2001;127:594–606. [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng W, Liu Y, Hwang I, Jones T, Sheen J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science. 2003;300:332–336. doi: 10.1126/science.1080585. [DOI] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant. Cell and Environment. 2007;30:85–112. doi: 10.1111/j.1365-3040.2006.01608.x. [DOI] [PubMed] [Google Scholar]
- Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiology. 2007;143:156–171. doi: 10.1104/pp.106.090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Nilsson L, Nielsen LK, Nielsen TH. Interaction between phosphate starvation signalling and hexokinase-independent sugar sensing in Arabidopsis leaves. Physiologia Plantarum. 2005;124:81–90. [Google Scholar]
- Newmann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.) Annals of Botany. 2000;85:909–919. [Google Scholar]
- Newmann G, Massonneau A, Martinoia E, Römheld V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta. 1999;208:373–382. [Google Scholar]
- Nielsen KL, Eshel A, Lynch JP. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. Journal of Experimental Botany. 2001;52:329–339. [PubMed] [Google Scholar]
- Olhoft P, Flagel LE, Donovan CM, Somers DA. Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta. 2003;216:723–735. doi: 10.1007/s00425-002-0922-2. [DOI] [PubMed] [Google Scholar]
- Peñaloza E, Muñoz G, Salvo-Garrido H, Silva H, Corcuera LJ. Phosphate deficiency regulates phosphoenolpyruvate carboxylase expression in proteoid root clusters of white lupin. Journal of Experimental Botany. 2005;56:145–153. doi: 10.1093/jxb/eri008. [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes and Development. 2001;15:2122–2133. doi: 10.1101/gad.204401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, De Vos M, De Roock S, Lambers H. Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant Cell and Environment. 2003;26:265–273. [Google Scholar]
- Shen J, Li H, Neumann G, Zhang F. Nutrient uptake, cluster root formation and exudation of protons and citrates in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Science. 2005;168:837–845. [Google Scholar]
- Smeekens S. Sugar-induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T. Root tip contact with low-phosphate media reprograms plant root architecture. Nature Genetics. 2007;39:792–796. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- Uhde-Stone C, Gilbert G, Johnson JMF, Litjens R, Zinn KE, Temple SJ, Vance CP, Allan DL. Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant and Soil. 2003a;248:99–116. [Google Scholar]
- Uhde-Stone C, Liu J, Zinn KE, Allan DL, Vance CP. Transgenic proteoid roots of white lupin: a vehicle for characterizing and silencing root genes involved in adaptation to P stress. The Plant Journal. 2005;44:840–853. doi: 10.1111/j.1365-313X.2005.02573.x. [DOI] [PubMed] [Google Scholar]
- Uhde-Stone C, Zinn KE, Ramirez-Yáñez M, Li A, Vance CP, Allan DL. Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiology. 2003b;131:1064–1079. doi: 10.1104/pp.102.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Wasaki J, Omura M, Osaki M, Ito H, Matsui H, Shinano T, Tadano T. Structure of a cDNA for an acid phosphatase from phosphate-deficient lupin (Lupinus albus L.) roots. Soil Science and Plant Nutrition. 1999;45:439–449. [Google Scholar]
- Wasaki J, Shinano T, Onishi K, et al. Transcriptomic analysis indicates putative metabolic changes caused by manipulation of phosphorus availability in rice leaves. Journal of Experimental Botany. 2006;57:2049–2059. doi: 10.1093/jxb/erj158. [DOI] [PubMed] [Google Scholar]
- Wasaki J, Yonetani R, Kuroda S, et al. Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant, Cell and Environment. 2003;26:1515–1523. [Google Scholar]
- Watt M, Evans JR. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiology. 1999a;120:705–716. doi: 10.1104/pp.120.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M, Evans JR. Proteoid roots. Physiology and development. Plant Physiology. 1999b;121:317–323. doi: 10.1104/pp.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Sheen J, Jang J-C. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Molecular Biology. 2000;44:451–461. doi: 10.1023/a:1026501430422. [DOI] [PubMed] [Google Scholar]
- Yan F, Zhu Y, Muller C, Zorb C, Schubert S. Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiology. 2002;129:50–63. doi: 10.1104/pp.010869. [DOI] [PMC free article] [PubMed] [Google Scholar]