Abstract
Cytokinin deficiency causes pleiotropic developmental changes such as reduced shoot and increased root growth. It was investigated whether cytokinin-deficient tobacco plants, which overproduce different cytokinin oxidase/dehydrogenase enzymes, show changes in different sink and source parameters, which could be causally related to the establishment of the cytokinin deficiency syndrome. Ultrastructural analysis revealed distinct changes in differentiating shoot tissues, including an increased vacuolation and an earlier differentiation of plastids, which showed partially disorganized thylakoid structures later in development. A comparison of the ploidy levels revealed an increased population of cells with a 4C DNA content during early stages of leaf development, indicating an inhibited progression from G2 to mitosis. To compare physiological characteristics of sink leaves, source leaves and roots of wild-type and cytokinin-deficient plants, several photosynthetic parameters, content of soluble sugars, starch and adenylates, as well as activities of enzymes of carbon assimilation and dissimilation were determined. Leaves of cytokinin-deficient plants contained less chlorophyll and non-photochemical quenching of young leaves was increased. However, absorption rate, photosynthetic capacity (Fv/Fm and JCO2max) and efficiency (ΦCO2app), as well as the content of soluble sugars, were not strongly altered in source leaves, indicating that chlorophyll is not limiting for photoassimilation and suggesting that source strength did not restrict shoot growth. By contrast, shoot sink tissues showed drastically reduced contents of soluble sugars, decreased activities of vacuolar invertases, and a reduced ATP content. These results strongly support a function of cytokinin in regulating shoot sink strength and its reduction may be a cause of the altered shoot phenotype. Roots of cytokinin-deficient plants contained less sugar compared with wild-type. However, this did not negatively affect glycolysis, ATP content, or root development. It is suggested that cytokinin-mediated regulation of the sink strength differs between roots and shoots.
Keywords: Carbohydrates, cell cycle, cytokinin, cytokinin deficiency, differentiation, invertase, meristem, photosynthesis, sink, source
Introduction
Cytokinins are key regulators of numerous processes in plant development and growth (Mok, 1994). Essential steps in their metabolism and signal transduction have been elucidated recently (reviewed by Ferreira and Kieber, 2005; Heyl et al., 2006; Sakakibara, 2006; Werner et al., 2006). Overexpression of genes that code for cytokinin-degrading cytokinin oxidase/dehydrogenase (CKX) enzymes was used to produce tobacco and Arabidopsis plants with reduced cytokinin content (Werner et al., 2001, 2003; Yang et al., 2003). These plants show a compound phenotype called the cytokinin deficiency syndrome. The main features of this syndrome are the formation of slow-growing, stunted shoots with small leaves and an enhanced root system. The phenotypic consequences of cytokinin deficiency have largely been confirmed in loss-of-function mutants of cytokinin receptors (Higuchi et al., 2004; Nishimura et al., 2004; Riefler et al., 2006).
The analysis of cytokinin-deficient plants has shown that cytokinin is a positive regulator of shoot growth and a negative regulator of root growth. However, it is still unknown which cellular processes in growing tissues limit growth in the shoot or enhance growth in the root under conditions of cytokinin deficiency. It is known from previous work that cytokinin regulates several parameters, which determine source or sink strength of tissues—for example, carbon fixation, assimilation, and partitioning of primary metabolites and cell cycle activity—and which could thus be causally involved in the establishment of the cytokinin deficiency syndrome.
Cytokinin stimulates chloroplast biogenesis and chlorophyll synthesis (Mok, 1994; Reski, 1994) and increases the photosynthetic rate (Wareing et al., 1968). The abundance of various transcripts and proteins involved in photosynthetic reactions is affected by cytokinin, including chlorophyll a/b-binding protein of the light-harvesting complex (Flores and Tobin, 1988), Rubisco (Lerbs et al., 1984), and phosphoenolpyruvate carboxylase and carbonic anhydrase in maize (Sugiharto et al., 1992). Genome-wide transcript profiling in Arabidopsis revealed that genes of photosynthesis were over-represented among the genes up-regulated by cytokinin and several photosynthesis-related genes of the chloroplast genome are also induced (Brenner et al., 2005). It is thus presumed that cytokinin plays an important role in carbon assimilation in source organs.
Plant growth is modulated by the sink strength and cytokinin may regulate rate-limiting steps that determine the availability of nutrients. Indicative of this capacity is the ability of cytokinins to establish local metabolic sinks, which has been initially demonstrated by the mobilization of radiolabelled nutrients such as amino acids or sugars from other parts of the plant to cytokinin-treated areas (Mothes et al., 1961; Kuiper, 1993). Localized expression of the cytokinin-synthesizing ipt gene also caused local sink enhancement (Guivarc'h et al., 2002). Cytokinin supplied at physiological concentrations to Urtica dioica plants could change the direction of assimilate export from individual leaves, thus completely inverting the source–sink relationship in favour of the shoot. It was proposed that cytokinin may be a root-derived signal which, in response to nitrogen, controls uptake and utilization of assimilates and biomass distribution (Beck, 1999; Sakakibara et al., 2006).
Sucrose transported into the sink tissue can be cleaved by sucrose synthases or invertases, the activities of the latter being more dominant during sink initiation and expansion growth (reviewed by Koch, 2004). Plants contain neutral invertases localized to the cytosol and acidic invertase isoforms which are localized to vacuoles or are in an unsoluble form bound to the cell wall in the apoplast (Roitsch and González, 2004). It has been shown that cytokinin up-regulates the expression of genes encoding cell wall invertases (Ehneß and Roitsch, 1997) and it was shown recently that the activity of this type of invertase is essential for cytokinin-induced nutrient mobilization and delay of senescence (Balibrea Lara et al., 2004).
Cell division activity is also an important factor determining sink strength. Cytokinin is known to have regulatory roles during different cell cycle phases, including the G1/S transition, S phase, and the G2/M transition (Jacqmard et al., 1994; Dewitte and Murray, 2003). However, most of these results have been obtained in cell culture systems and it is unclear at which of these cell cycle stages cytokinin exerts its regulatory function(s) during different development processes in different tissues.
Despite a large body of experimental work exemplified above, very little is known about which processes may be rate-limiting for the regulation of growth by cytokinin. The analysis of sink and source parameters in plants with a lowered cytokinin content might advance our understanding and help to elucidate whether physiological changes might have an important role in the establishment of the cytokinin deficiency syndrome. Therefore, selected metabolic parameters were analysed in source leaves and two sink tissues—the vegetative shoot apex and roots—of cytokinin-deficient tobacco plants and compared with the respective wild-type tissues. In this study, two different transgenic tobacco lines which differ in the expressivity of the cytokinin-deficiency syndrome, depending on the overproduced CKX isoform, were used. 35S:CKX1-expressing plants exhibit a stronger reduction to shoot growth compared with 35S:CKX2 plants (Werner et al., 2001). In addition, the cell cycle activity and the cellular ultrastructure were analysed in the line with a stronger phenotype (35S:CKX1). This study identified the G2/M transition as a cytokinin-sensitive step of the cell cycle, and revealed premature differentiation and responses indicative of oxidative stress as part of the cytokinin deficiency syndrome.
Materials and methods
Plant material and growth conditions
Construction of transgenic tobacco (Nicotiana tabacum L. cv. Samsun NN) expressing 35S:CKX1 and 35S:CKX2 was reported previously (Werner et al., 2001). This study also contains a detailed morphological analysis and comparative growth kinetics of cytokinin-deficient and wild-type tobacco plants. Homozygous 35S:CKX1-50 and 35S:CKX2-38 tobacco lines were used in all experiments. Plants were cultured in vitro on MS medium under 16 h light/8 h dark cycles at 20 °C or grown in a glasshouse at 20–24 °C with 16 h light/8 h dark cycles.
Electron microscopy
Excised tobacco shoot apical meristems (SAMs) and pieces of developing leaves (third youngest visible leaf) were fixed in 4% paraformaldehyde, 1.5% glutaraldehyde, and 100 mM phosphate buffer, pH 7.4, for 6 h at 4 °C, then post-fixed with 1% osmium tetraoxide, dehydrated in an ethanol series, and embedded in Araldite resin. Ultra-thin sections were made and stained sequentially with a saturated solution of uranyl acetate in 50% ethanol, then with 3% lead citrate, for 15 min each (Reynolds, 1963). Sections were observed at 80 kV under a transmission electron microscope (model EM201, Philips, The Netherlands; platform of electron microscopy, UPMC, IFR 83, Paris, France).
DNA flow cytometry
Isolation of nuclei was carried out by mechanical chopping with a razor blade in Galbraith's buffer (Galbraith et al., 1983) supplemented with 5 μg ml−1 Hoechst 33342 (Sigma, St Louis, MO, USA). The extracted nuclei were filtered through 48 μm nylon and the analysis was performed on an EPICS Elite ESP (Coulter, Hialeath, FL, USA) with laser excitation (40 mV) at 351–364 nm (platform ‘Imagerie en Biologie Cellulaire’, IFR87, CNRS, Gif-sur-Yvette, France). Five to ten thousand nuclei were analysed per sample and five to ten samples were pooled for each analysis.
Photosynthetic parameters
Source leaves’ absorbance in the wavelength range of 400–700 nm was calculated by determining transmission and reflection using a Specord M500 (Carl Zeiss Jena, Germany) as described in Pörs et al. (2001).
Chlorophyll fluorescence parameters were measured using a PAM 2000 (Fa. Walz, Effeltrich, Germany). To determine the Fv/Fm=(Fm–Fo)/Fm ratio, the maximum quantum yield of photosystem II (PSII) photochemistry, the attached leaves were dark adapted for 30 min. After determining the basic fluorescence (Fo), an 800 ms saturating pulse was applied to determine the maximum fluorescence (Fm). The rate of electron transport (ETR) through PSII was calculated after Krall and Edwards (1992) by formula (1).
| (1) |
ΦPSII, the actual PSII quantum efficiency, was calculated as (Fm′–Fs)/Fm′ according to Genty et al. (1989) after determining the maximum fluorescence (Fm′) and the steady-state fluorescence (Fs) of light-adapted leaves by the PAM 2000. abs is the absorbance of the leaves determined as described above and PPFD the incident photosynthetic photon flux density under growth conditions determined with an LI-190 quantum sensor connected to an LI-189 light meter (LI-COR Biosciences, Inc., Lincoln, NB, USA) at the time of chlorophyll fluorescence measurements.
Gas exchange measurements were carried out on attached leaves in an open CO2/H2O gas exchange cuvette system (LI-6400, Fa. LI-COR Biosciences, Inc.) using an infrared gas analyser. The following settings were chosen: temperature in the leaf cuvette = 22 °C, relative humidity ≅50%, cuvette air flow rate = 500 μmol s−1, and external CO2 concentration = 360 ppm. Irradiance during the measurements was provided by an LED source (6400-02B) with 10% blue and 90% red light. Light-dependent CO2 exchange rates (light saturation curves) were surveyed and modelled as described in Mustroph et al. (2006b). From these curves, the actual CO2 uptake rates under growth PPFD (JCO2growth), the maximum apparent quantum yield of the CO2 uptake (ΦCO2app), and the dark respiration rate (RD) were calculated by the formula described previously (Mustroph et al., 2006b).
A/Ci curves were surveyed at light saturation (PPFD of 1000 μmol photons m−2 s−1) by a stepwise change of external CO2 concentrations between 50 ppm and 800 ppm in an up-and-down order. The A/Ci curves were fitted in analogy to the light saturation curves by using formula (2). The maximum CO2 uptake rate under light and CO2 saturation (the capacity of the CO2 uptake, JCO2max) was calculated from the A/Ci curves by formula (3).
| (2) |
| (3) |
After photosynthetic parameters of the leaves were measured, the plant material was frozen in liquid nitrogen. The in vivo 5-aminolevulinic acid (ALA)-synthesizing capacity of leaves was determined according to Yaronskaya et al. (2006). Chlorophyll was extracted with acetone and measured by HPLC as described in Pörs et al. (2001).
Determination of metabolite contents and enzyme activities
Material for analysis of carbohydrates, adenylates, and metabolic enzymes was collected from 8- to 9-week-old plants in the middle of the light period. Sink-tissue samples consisted of shoot apices including young leaves up to 1.5 cm in size; source-tissue material was sampled from the intercostal regions of fully developed leaves. Per extraction, 200 mg of tissue was used. Soluble sugars and starch were extracted and measured photometrically by a coupled enzymatic assay as described previously (Mustroph et al., 2006a). Adenylate contents were determined according to Mustroph et al. (2006a).
To determine activities of phosphofructokinase (PFK; EC 2.7.1.11), pyrophosphate-fructose-6-phosphate-phosphotransferase (PFP; EC 2.7.1.90), aldolase (EC 4.1.2.13), and enolase (EC 4.2.1.11), samples were ground in liquid nitrogen and extracted in 50 mM HEPES-KOH, pH 6.8, containing 5 mM Mg-acetate, 5 mM β-mercaptoethanol, 15% (v/v) glycerol, 1 mM EDTA, 1 mM EGTA, 5 mM dithiothreitol (DTT), and 0.1 mM Pefabloc proteinase inhibitor (Boehringer Mannheim, Germany). The homogenate was centrifuged at 13 000 g at 4 °C for 15 min and the supernatant was used for spectrophotometric determination of enzyme activities (Mustroph and Albrecht, 2003).
To assay activities of cytosolic, vacuolar, and cell wall invertases the extraction was carried out as described by Roitsch et al. (1995) with the following modifications: the homogenization buffer was 50 mM HEPES-KOH, pH 7.5, 3 mM MgCl2, 1 mM EDTA, 2% (v/v) glycerol, 0.1 mM PMSF, and 1 mM benzamidine. The homogenate was mixed for 30 min at 4 °C before centrifugation, and extracts were used without dialysis. The invertase reactions were carried out in K-phosphate/citrate buffer, pH 6.8 for cytosolic invertase, and pH 4.5 for vacuolar and cell wall invertases, respectively, with 125 mM sucrose as a substrate. The reaction was incubated for 1 h at 26 °C and stopped by 5 min incubation at 95 °C. The amount of glucose liberated in the reaction was determined as above. Protein concentration was determined according to Bradford (1976) using Bio-Rad reagents and bovine serum albumin as a standard.
Results
Cytokinin deficiency alters ultrastructural organization of cells in the SAM and developing leaf primordia
Cytokinin deficiency causes slow growth of the shoot and morphological changes such as dwarfism and smaller leaves. It has been shown previously that SAMs of cytokinin-deficient plants are smaller because of reduced cell numbers (Werner et al., 2001). Leaves of cytokinin-deficient tobacco plants contain fewer cells than leaves of wild-type plants, but these cells are larger (Werner et al., 2001). Here, transmission electron microscopy was used to analyse in more detail the consequences of cytokinin deficiency upon the organization of cellular structures in the SAM and developing leaves of 35S:CKX1 transgenic plants.
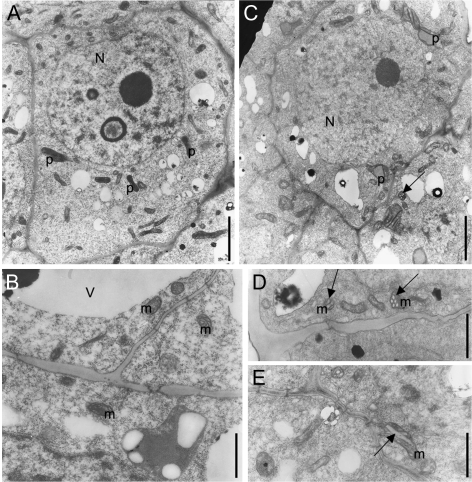
In the wild-type SAM, cells of the peripheral zone displayed typical meristematic structure, with few small vacuoles and fewer differentiated proplastids and mitochondria. Proplastids were small with electron-dense stroma and some initial primary thylakoid membranes (Fig. 1A). Mitochondria were mainly round-shaped (mean diameter ∼0.3 μm) with typical infolding of the inner membrane giving rise to short tubuli or microvilli typical of juvenile mitochondria (Fig. 1B). Nuclei were more or less spherical and displayed a clear network of electron-dense heterochromatin and euchromatin typical of the reticulate type of tobacco nuclei (Nagl and Fusenig, 1979). Electron microscopy of SAMs of 35S:CKX1 transgenic tobacco confirmed their reduced size (not shown) and revealed a range of distinct cytological changes indicative of a reduced meristematic character compared with cells of wild-type SAMs. Compared with wild type, the vacuolar system was increased and the heterochromatic network of the nuclei was attenuated (compare Fig. 1C, A, and Fig. S1 in Supplementary data available at JXB online). The mitochondria were enlarged and elongated (around 0.7 μm long and 0.4 μm thick) and showed specific changes: in particular, dilated internal tubuli were observed in cross-sections (Fig. 1C, D) and long and dilated internal tubuli in longitudinal sections (Fig. 1E). Plastids showed features of premature differentiation, such as the presence of some thylakoid stacks and electron-dense prethylakoidal inclusions (Fig. 1C).
Fig. 1.
Altered ultrastructure of cells in shoot meristems of cytokinin-deficient tobacco plants. Transmission electron micrographs of cells from the peripheral zone of wild-type (A, B) and 35S:CKX1 transgenic shoot meristems (C–E) are presented at a similar magnification. N, Nucleus; m, mitochondria; p, proplastid; V, vacuole. Arrows in C, D, and E indicate the unusually large tubuli arising from the inner membrane of mitochondria, sectioned transversally (C, D) and longitudinally (E). Scale bars = 2 μm (A, C) and 1 μm (B, D, E).
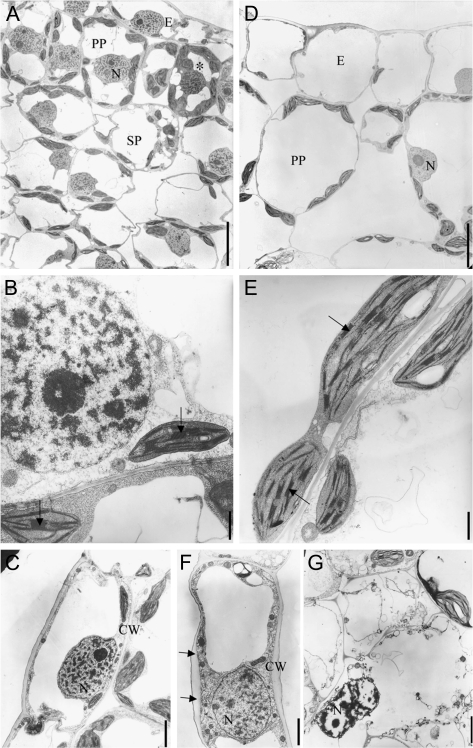
In the differentiating wild-type leaf primordia, the cells were more vacuolated than in the SAM and mitotic activity was still visible (Fig. 2A). Cytoplasm remained abundant around the vacuoles and the nuclei retained the reticulated appearance. The plastids in mesophyll cells were differentiated into lens-shaped chloroplasts, with a dense stroma and regular arrangements of thylakoids and thick grana stacks (Fig. 2B). Differentiated mitochondria were larger and showed more folds of the inner membrane compared with their juvenile stage. Few intercellular spaces existed between palisade parenchyma cells, whereas large air spaces separated the spongy cells (Fig. 2A). Nuclei in the palisade cells had a central position maintained by cytoplasmic bridges between vacuoles. By contrast, the developing leaf primordia of 35S:CKX1 transgenic plants had strongly enlarged cells and all cell types were highly vacuolated and the relative content of cytoplasm was reduced (Fig. 2D). The palisade parenchyma cells were round-shaped and often separated by large intercellular spaces (Fig. 2D) so that the distinction between the types of parenchyma cells was no longer obvious. Chloroplasts were longer and larger than in the wild type and contained smaller grana (compare E and B in Fig. 2). Additionally, irregular cell wall thickening was often observed in 35S:CKX1 cells (compare C and F in Fig. 2). Developing 35S:CKX1 transgenic leaves contained cells with disorganized cytoplasm and nuclei, displaying features of vacuolar autophagy, vesicle formation, and swelling of the endoplasmic reticulum. The plastids in these cells were swollen and contained dilated lumen and disorganized thylakoids (Fig. 2G and data not shown). These cells were mainly observed in the vicinity of the veins. These cellular changes may indicate that cell death was in progress.
Fig. 2.
Altered cellular ultrastructure in developing leaves of cytokinin-deficient plants. Transmission electron micrographs of young leaves (leaf 3; see Fig. 3) from wild-type (A–C) and 35S:CKX1-expressing (D–G) tobacco plants. Cross-section of wild-type (A) and 35S:CKX1 (D) leaf. The asterisk indicates a cell in preparation for mitosis with dense cytoplasm and condensed chromatin. (B, E) Comparison of chloroplast structure (arrows indicate grana). (C, F) Comparison of adaxial epidermal cells (arrows point to irregular cell wall thickness). (G) Necrotic cell with pycnotic nuclei and disorganized plastid and membrane. CW, Cell wall; E, epidermis; N, nucleus; PP, palisade parenchyma; SP, spongy parenchyma. Scale bars = 10 μm (A, D), 2 μm (B, E), and 1 μm (C, F, G).
Cytokinin deficiency alters cell cycle parameters in the shoot
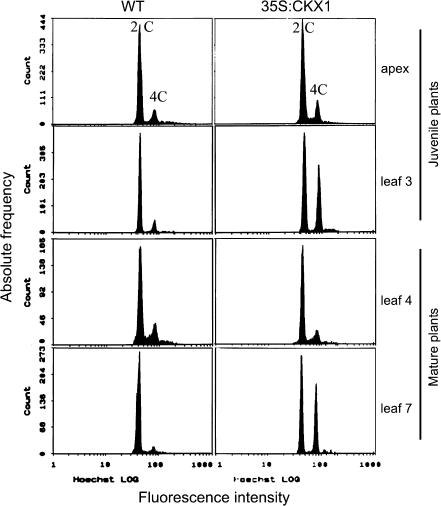
Because of the reduced cell number in shoots of cytokinin-deficient plants and because of the functions of cytokinin during several stages of the cell cycle it was of interest to see if cytokinin-deficient plants show differences in the nuclear DNA content during different developmental stages. Flow cytometry analysis showed that the nuclear DNA content of cells in dissected shoot apices of wild-type and 35S:CKX1 transgenic tobacco plants was similar. Apex cells of both genotypes contained mainly 2C nuclei and very few 4C nuclei. However, developing leaves of juvenile 35S:CKX1 tobacco plants contained a strongly increased population of cells with a 4C DNA content (Fig. 3). This increase was observed in the third youngest leaf (∼3 mm in size) as well as in older more expanded leaves. The enhanced 4C/2C ratio indicates a reduced progression from G2 phase to mitosis. In 15-week-old plants, no difference in DNA content was found from the apex to the sixth leaf, but all subsequent older leaves of transgenic plants showed a strong increase in the 4C population (Fig. 3 and data not shown). An increased 4C content of leaf cells was found also in 35S:CKX2 transgenic tobacco plants as well as in cytokinin-deficient Arabidopsis plants (data not shown).
Fig. 3.
Cell cycle parameters are altered in cytokinin-deficient shoot organs. Histograms of nuclear DNA distribution in wild-type and 35S:CKX1-expressing tobacco plants. Pooled apices and pooled leaves of different developmental stages (leaf 1 being the youngest visible leaf) were analysed using juvenile (4-week-old plants with 4–5 leaves) and mature (15-week-old plants with 20–22 leaves) plants. The x- and y-axis show DNA fluorescence (log scale) and the frequency of stained nuclei, respectively. For each sample, 5000–10 000 nuclei were analysed. Samples consisted of pooled tissues from 5 to 10 plants.
Photosynthetic activity of cytokinin-deficient plants
It is known that cytokinin can influence biosynthesis and content of chlorophyll (Chl) as well as photosynthesis (Yaronskaya et al., 2006). Therefore, the consequences of cytokinin-deficiency on Chl biosynthesis, Chl content, and photosynthetic activities were explored. The cytokinin-deficient plants had a lower Chl content in comparison with wild-type plants, but their Chl a/b ratio did not significantly differ (Table 1). The source leaves of 35S:CKX1 and 35S:CKX2 plants contained 63% and 85% of the wild-type Chl content related to leaf area, respectively. However, the Chl content of transgenic leaves differed only slightly, when Chl was related to protein content, indicating a higher water content per leaf area. Indeed, the dry weight of 35S:CKX1 and 35S:CKX2 source leaves was 8% and 12% of their fresh weight, respectively, compared with 20% of wild-type leaves. The capacity to synthesize ALA was analysed in source leaves to determine Chl biosynthesis as a possible factor for limitation of the Chl content. The synthesis rate of ALA was 75% and 28% lower in 35S:CKX1 and 35S:CKX2 plants, respectively (Table 1).
Table 1.
ALA-synthesizing capacity (ALA), Chl a+b content, and Chl a/b ratio in source leaves of wild-type and cytokinin-deficient tobacco plants
| Parameter | WT | 35S:CKX1 | 35S:CKX2 | |
| ALA | (nmol h−1 mg−1 FW) | 0.32±0.03a | 0.08±0.02b | 0.23±0.01c |
| Chl (a + b) | (μmol m−2) | 506±40a | 317±38b | 429±38c |
| Chl a/b | (rel. units) | 2.76±0.04a | 2.71±0.06a | 2.78±0.05a |
Data represent mean values ±SD (n=3–5). Values with the same superscript letter in one row do not significantly differ at P < 0.05 (calculated by the Student's t-test). FW, Fresh weight; WT, wild type.
Analysis of photosynthetic parameters was performed with source leaves. The transgenic lines showed a lower absorbance (90% of wild type for 35S:CKX1 and 95% for 35S:CKX2) of photosynthetic active radiation (Table 2), which may be explained by the lower Chl content and the higher water content in the leaves compared with wild type. As a consequence, the electron transfer rate (ETR) was reduced, whereas the maximum yield of PSII photochemistry showed slightly increased values (Fig. 4). Analysis of the photosynthetic efficiency of the CO2 uptake (ΦCO2app) revealed no differences between CKX-expressing and wild-type plants, but the mitochondrial respiration rate of source leaves was elevated in the transgenic lines (33% and 51% more in 35S:CKX1 and 35S:CKX2, respectively) (Table 2). The higher dark respiration correlated with the reduced carbohydrate content in cytokinin-deficient source leaves (see below).
Table 2.
Selected parameters of photosynthetic processes in source leaves of wild-type and cytokinin-deficient tobacco plants
| Parameter | WT | 35S:CKX1 | 35S:CKX2 | |
| abs | (%) | 84.0±0.5a | 75.3±0.5b | 80.1±2.9a |
| ETR | (μmol e– m−2 s−1) | 88.2±4.3a | 76.4±6.9b | 83.8±4.7c |
| ΦPSII | (rel. units) | 0.700±0.034a | 0.676±0.061a | 0.698±0.039a |
| JCO2growth | (μmol CO2 m−2 s−1) | 9.33±0.90a | 9.53±0.48a | 9.91±0.47a |
| JCO2max | (μmol CO2 m−2 s−1) | 34.2±14.6a | 38.9±12.1a | 39.1±6.3a |
| ΦCO2app | [mol CO2 (mol photons)−1] | 0.076±0.009a | 0.076±0.003a | 0.076±0.003a |
| RD | (μmol CO2 m−2 s−1) | 1.75±0.23a | 2.32±0.08b | 2.63±0.42b |
Data represent mean values ±SD (n=33 for ETR; n = 4–5 for other parameters). Values with the same superscript letter in one row do not significantly differ at P < 0.05 (calculated by the Student's t-test). abs, Rate of the absorption of photons in the range of 400–700 nm; ETR, electron transport rate; JCO2growth, CO2 uptake rate under growth conditions; JCO2max, CO2 uptake rate under light and CO2 saturation; ΦCO2app, maximum apparent quantum yield of the CO2 uptake; ΦPSII, quantum efficiency of PSII; RD, dark respiration rate; WT, wild type.
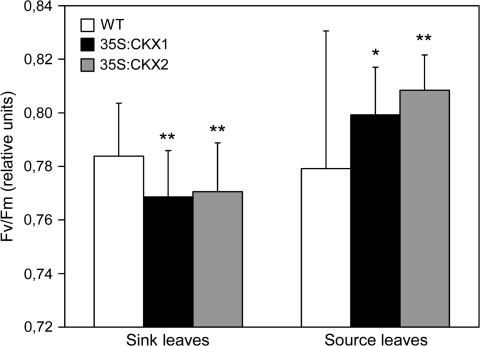
Fig. 4.
Maximum quantum yield (Fv/Fm) of PSII in source and sink leaves of wild-type and cytokinin-deficient tobacco plants. Data represent mean values ±SD (n ≥30). Representative results from two independent measurements are shown. Pairwise Student's t-test was used to compare values with the wild type. *, P < 0.05; **, P < 0.01. WT, Wild type.
Analysis of the Chl fluorescence parameters of sink and source leaves showed a higher Fv/Fm ratio in source leaves, while in sink leaves this parameter was slightly lower in both transgenic lines than in wild type (Fig. 4). This may indicate that transgenic leaves with low cytokinin content are able to adapt during their development and to increase their initially reduced maximum quantum yield.
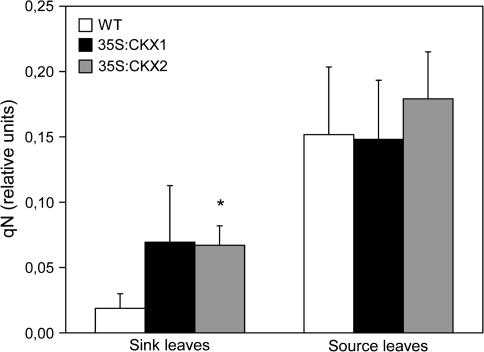
Non-photochemical quenching (qN) was similar in source leaves of cytokinin-deficient and control plants (Fig. 5). By contrast, the young sink leaves of CKX-expressing lines showed strongly increased qN values in comparison with the wild type. This difference could be due to an advanced developmental status of cytokinin-deficient sink leaves.
Fig. 5.
Non-photochemical quenching of chlorophyll fluorescence (qN) in source and sink leaves of wild-type and cytokinin-deficient tobacco plants. Data represent mean values ±SD (n=3–6). Representative results from two independent measurements are shown. Pairwise Student's t-test was used to compare values with the wild type. *, P < 0.05; **, P < 0.01. WT, Wild type.
Altered distribution of carbohydrates and ATP/ADP in sink and source tissues of cytokinin-deficient plants
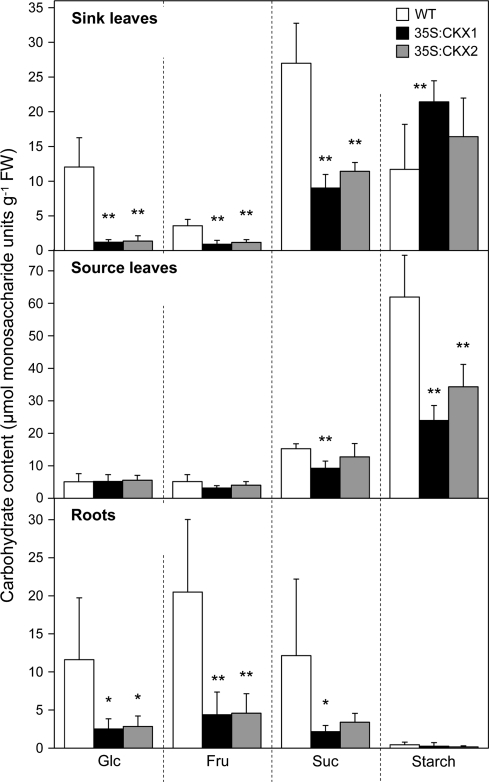
Next, the distribution of non-structural carbohydrates in different organs was analysed. Source leaves of cytokinin-deficient plants showed only moderate changes in soluble sugar content (Fig. 6). Only the Suc concentration was significantly reduced by ∼40% in source leaves of 35S:CKX1 transgenic plants in comparison with wild-type, while the more moderate reduction found in source leaves of 35S:CKX2 transgenic plants was not significant. The levels of hexoses in source leaves were not significantly changed. The accumulation of starch was reduced in source leaves of both CKX transgenic plants to ∼40–50% of the wild-type level (Fig. 6). In total, the content of soluble sugars (Suc, Glc, Fru) was reduced about 30% in 35S:CKX1 and 13% in 35S:CKX2 source leaves. The total non-structural carbohydrates including starch were reduced by 50% and 35%, respectively.
Fig. 6.
Contents of carbohydrates in sink and source tissues of wild-type and cytokinin-deficient tobacco plants. Data represent mean values ±SD (n=6). Representative results from three independent experiments are shown. Pairwise Student's t-test was used to compare values with the wild type. *, P < 0.05; **, P < 0.01. FW, Fresh weight; WT, wild type.
In contrast to source leaves, the contents of all measured soluble sugars per fresh weight were strongly reduced in shoot sink tissues of cytokinin-deficient plants. The content of Suc was decreased to ∼30%, Glc to 10%, and Fru to 25% of the wild-type values (Fig. 6). Surprisingly, cytokinin-deficient shoot sink tissues accumulated higher amounts of starch. In 35S:CKX1 shoot sink tissues, a significant increase of 80% compared with shoot sink tissues of wild type were found (Fig. 6).
A strong reduction in sugar content was also found in cytokinin-deficient roots. The contents of Suc, Glc, and Fru were reduced to 18–27% of the wild type (Fig. 6). The starch content of roots was, in general, very low and was even further decreased by ∼50% in cytokinin-deficient roots.
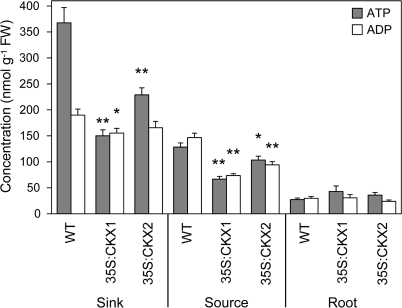
The ATP content was decreased in 35S:CKX1 and 35S:CKX2 source leaves by 50% and 20%, respectively, in comparison with wild type (Fig. 7). As the ADP content was reduced to a similar extent, the ATP/ADP ratio remained similar in transgenic and wild-type source leaves. The amount of ATP in 35S:CKX1 and 35S:CKX2 shoot sink tissues was reduced even more than in their source leaves (60% and 38% reduction, respectively). However, in this case the ADP contents were reduced only very slightly, leading to an almost 50% lower ATP/ADP ratio in shoot sink tissues of the cytokinin-deficient plants. The values for wild-type shoot sink tissues are typical for energized tissues with high metabolic rates, while the values for cytokinin-deficient tissues are probably due to an inhibited metabolism resulting in an intermediate state between source and sink. In contrast to the shoot tissues, the altered carbohydrate amount did not lead to significant changes of the ATP and ADP contents or ratios in the roots (Fig. 7).
Fig. 7.
ATP and ADP content in sink and source tissues of wild-type and cytokinin-deficient tobacco plants. Data represent mean values ±SE (n=6–9) of three independent experiments. Pairwise Student's t-test was used to compare values with the wild type. *, P < 0.05; **, P < 0.01. FW, Fresh weight; WT, wild type.
Activities of invertases and glycolytic enzymes in sink and source tissues of cytokinin-deficient plants
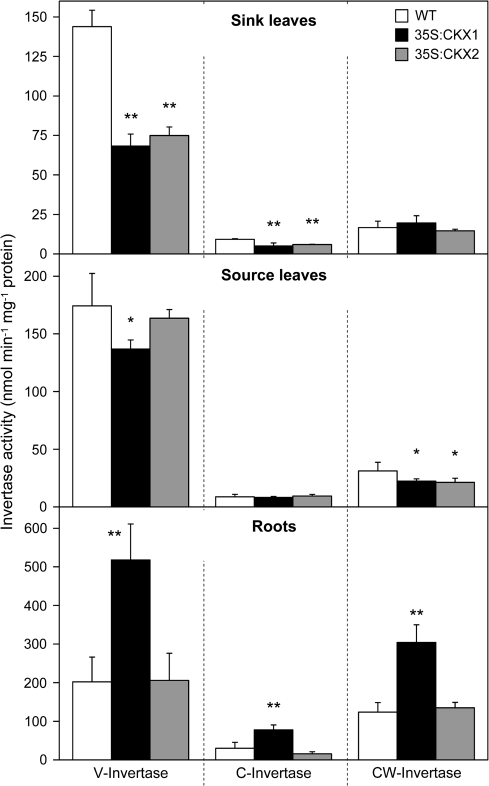
The decreased content of hexoses and the increased starch accumulation in shoot sink tissues could be caused by impaired metabolic utilization of Suc. Therefore, the activities of the vacuolar, cytosolic, and cell wall-bound isoforms of Suc-cleaving invertases were analysed. In each of the tissues analysed, the major portion of the total invertase activity was attributed to the vacuolar invertases, in particular in sink and source leaves (Fig. 8).
Fig. 8.
Invertase activity in sink and source tissues of wild-type and cytokinin-deficient tobacco plants. Data represent mean values ±SD (n=6). Representative results from three independent experiments are shown. Pairwise Student's t-test was used to compare values with the wild type. *, P < 0.05; **, P < 0.01. C, Cytosolic; CW, cell wall; V, vacuolar; WT, wild type.
In shoot sink tissues of cytokinin-deficient plants, the activities of vacuolar and cytosolic invertases were strongly reduced to ∼50% of wild type (Fig. 8). By contrast, the activities of cell wall invertases were not significantly altered in shoot sink tissues. Comparison of source leaves revealed no or only minor changes in the activities of vacuolar and cytosolic invertases, while the activity of cell wall invertases was reduced by about 30% in both transgenic lines (Fig. 8). Roots of 35S:CKX1 transgenic plants showed an about 2-fold increase in the different invertase activities, while no changes were found in the roots of 35S:CKX2 transgenic plants.
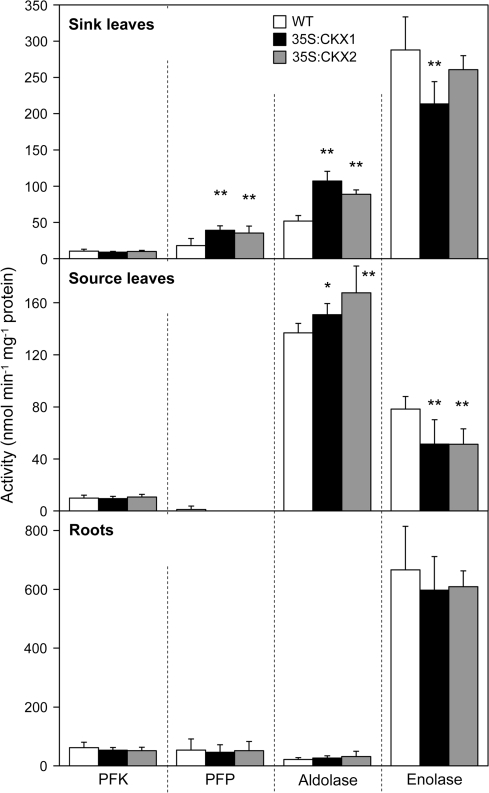
The activities of several glycolytic enzymes changed significantly in cytokinin-deficient shoot tissues. For shoot sink tissues of both transgenic lines about 2-fold higher PFP and aldolase activities were measured in comparison with wild type. By contrast, ATP-dependent PFK activity was not changed and enolase activity was slightly reduced (Fig. 9). Similarly, the activity of aldolase and enolase in source leaves was enhanced and reduced, respectively. In contrast to sink tissues, however, the activity of PFP was reduced in the source leaves of transgenic lines. No significant changes in activities of glycolytic enzymes were detected in cytokinin-deficient roots (Fig. 9).
Fig. 9.
Activity of PFK, PFP, aldolase, and enolase in sink and source tissues of wild-type and cytokinin-deficient tobacco plants. Data represent mean values ±SD (n=6). Representative results from three independent experiments are shown. Activity of PFP in CKX-expressing source leaves was reduced to detection limit in all replicate measurements. Pairwise Student's t-test was used to compare values with the wild type. *, P < 0.05; **, P < 0.01. WT, Wild type.
Discussion
Cytokinins affect cell differentiation during shoot development
The size, activity, and maintenance of the SAM are defined by a balanced rate of mitotic cell divisions and functional cell differentiation. Similarly, during leaf development, a phase of cell proliferation is followed by differentiation, which both define the total cell number and size and, thereby, the final leaf size. Ultrastructural analysis of cells in the SAM and leaf primordia has revealed, in cytokinin-deficient plants, distinct cytological changes indicative of early arrest of cell division activity and accelerated cellular differentiation. The meristematic cells of these plants showed early signs of vacuolation. Furthermore, in contrast to wild-type meristems, the proplastids in cytokinin-deficient SAM cells were already enlarged and mostly contained an internal pre-thylakoidal body and thylakoids (Fig. 1C). The presence of a dense plastidal inclusion is common in differentiating chloroplasts of tobacco leaf primordia but not in the SAM itself, except for some cells in the L1 (Brossard, 1975). A further indication of advanced differentiation in cells of the SAM in cytokinin-deficient plants is the marked decondensation of nuclear chromatin (Fig. 1A), which contrasts with the typical electron-dense heterochromatin of meristematic cells (Verdeil et al., 2007). Chromatin decondensation was also observed during cell differentiation in Crepis capillaris pith (Brossard, 1978) and the maize root cap (Barlow, 1985). Together these results suggest that cytokinin prevents early cellular differentiation in cells of the SAM. This contrasts with the promoting role of cytokinin on cell differentiation in the root meristem (Werner et al., 2003; Dello Ioio et al., 2007). It will be interesting to examine to what extent the premature differentiation of cytokinin-deficient meristematic cells is a direct consequence of reduced mitotic activity or whether the onset of the differentiation process is independent of the cell cycle.
Other ultrastructural changes observed in cytokinin-deficient meristem and leaf cells are indicative of enhanced oxidative stress. Mitochondria in cytokinin-deficient meristematic cells were swollen and showed changes in their internal organization, the inner membrane giving rise to large and long tubuli. Similar mitochondria have been described upon food deprivation in rat liver cells (Domenicali et al., 2001), oxidative stress in human cells (Lee et al., 2005), and accumulation of reactive oxygen species preceding Arabidopsis ovule abortion (Hauser et al., 2006). Comparable ultrastructural aberrations in human mitochondria carrying a diabetes-associated mutation were associated with a changed glycolytic metabolism, reduced ATP content, and higher intracellular levels of reactive oxygen species (de Andrade et al., 2006; Maassen et al., 2006). Mitochondria are the main targets for oxidative damage (Bartoli et al., 2004). Cytokinins are known to have anti-oxidative properties (Krishna Chaitanya and Naithani, 1998; Rattan, 2002; Dertinger et al., 2003; Synková et al., 2004). The altered mitochondrial structure and occasional necrotic cells in cytokinin-deficient plants indicated a stress situation, possibly caused by a reduced antioxidant capacity which might be caused or enhanced by a reduced carbohydrate status.
As well, chloroplasts in the developing cytokinin-deficient leaves showed specific structural changes. Cytokinins stimulate chloroplast maturation and grana development (Synková et al., 2006) as well as amyloplast formation (Miyazawa et al., 2002). Opposite to these effects, chloroplasts of developing cytokinin-deficient leaf cells were longer and larger with few starch grains and reduced grana stacking. Some plastids displayed swelling of thylakoids typical of early stages of senescence, but never contained plastoglobules that are usually present in senescing plastids (Ikeda and Veda, 1964; Barton, 1966; Dodge, 1970). Cytokinins are known to retard senescence (Richmond and Lang, 1957; Gan and Amasino, 1995). Thus, these observations made in the cytokinin-deficient plants are consistent with earlier results obtained upon cytokinin enhancement. However, further development of these leaves did not indicate a strong negative influence of these early changes on photosynthetic activity.
G2/M transition appears to be the rate-limiting step for cell division in cytokinin-deficient shoots
It has been shown that cytokinin influences different phases of the cell cycle (Jacqmard et al., 1994; Dewitte and Murray, 2003) and cytokinin deficiency may limit this regulatory activity, causing an eventual arrest at a particular stage. In this study, it has been shown that cytokinin deficiency in developing leaf primordia increased the fraction of cells with 4C nuclei in the G2 phase. This indicates that cells were unable to enter and/or complete the mitotic phase and thus their proliferation ceased. A similar increase in the fraction of 4C cells was observed in transgenic tobacco plants expressing a dominant-negative mutant of CDKB, a cyclin-dependent kinase involved in the control of G2/M progression (Porceddu et al., 2001). It is concluded that in developing leaves the G2/M transition is the most sensitive target of cytokinin during cell cycle progression and it becomes rate-limiting upon restricted cytokinin availability.
Zhang et al. (1996, 2005) have proposed a mechanism for the regulation of G2/M transition by cytokinin. They showed that cultured cytokinin-dependent Nicotiana plumbaginifolia cells arrest in G2 when cytokinin was depleted. Replenishment of cytokinin or expression of the fission yeast Cdc25 phosphatase could dephosphorylate plant cyclin-dependent protein kinase (CDK) and restore mitotic competence. It was suggested that such a post-translational activation of CDK through Tyr dephosphorylation is a key function of cytokinin at the transition to mitosis. Consistent with this model, the concentrations of cytokinins oscillate in cytokinin-autonomous tobacco BY-2 cells during cell cycle progression (Redig et al., 1996; Hartig and Beck, 2005), and inhibition of cytokinin synthesis during G2 causes arrest of cell division, which can be overcome by addition of zeatin or expression of yeast Cdc25 (Laureys et al., 1998; Orchard et al., 2005). However, the existence of a CDC25-like protein in higher plants, which could be the target for cytokinin, is still a matter of debate (Boudolf et al., 2006). The present results suggest that similar to the situation in isolated cell cultures the regulation of G2/M transition by cytokinin can be the rate-limiting step within the developmental context of the shoot meristem and leaf primordia. Consistent with a predominant role of G2/M regulation is the result that enhanced expression of the cytokinin-regulated CYCD3;1 gene, which operates at the G1/S transition (Riou-Khamlichi et al., 1999), did not complement the cytokinin deficiency phenotype (TW, KH, and TS, unpublished result). Furthermore, it was recently shown that triple cycd3 mutants lack morphological alterations comparable to those of cytokinin-deficient plants, despite a reduced cell number in their shoot lateral organs (Dewitte et al., 2007).
It should be kept in mind that the changes of cell cycle activity could be at least partially an indirect consequence of cytokinin deficiency and rather be directly caused by the reduced sugar availability. Various cell cycle genes are regulated by sugar availability, including CYCD2, CYCD3 (Riou-Khamlichi et al., 2000), and CYCD4 (De Veylder et al., 1999). However, there is little knowledge about the involvement of sugar signalling in controlling G2/M.
Shoot sink strength is reduced in cytokinin-deficient plants
Carbohydrates produced in photosynthetically active source leaves are transported to heterotrophic sink tissues of the plant, such as the growth zones and the storage organs. Partitioning of photoassimilates from source organs to various sinks is under strict developmental control. The present analysis clearly demonstrated that the capacity of the shoot sink to import and/or utilize carbohydrates is dramatically reduced in cytokinin-deficient plants. In vegetative apex tissues (including the SAM and youngest leaves), the contents of soluble sugars were reduced by >70% in comparison with wild type (Fig. 6). The strongest reduction was detected for Glc which was reduced to 10% of wild type. Such a drastic deprivation of sugars can be caused (i) by a reduced cell population attracting the photoassimilates, (ii) by a decreased capacity to utilize imported sucrose metabolically, or (iii) by compromised phloem unloading. The first possibility is very plausible in light of the above-described attenuated cell proliferation and accelerated differentiation. In this scenario, the phloem is under carbohydrate pressure during the vegetative growth phase and the activity of cell division in the meristematic tissues controls assimilate uptake from the phloem by sink formation (Beck, 1999). It would be interesting to investigate whether, and to what extent, locally enhanced cell proliferation can alone re-establish the sink capacity and restore normal growth in a cytokinin-deficient background.
Secondly, a strong reduction was detected in activities of invertase enzymes, which indicated that the cytokinin-deficient apex was impaired in metabolic utilization of imported Suc. Vacuolar invertase activity, which represented the dominant invertase activity in tobacco shoot apices, was reduced by 50% and activity of neutral invertases was decreased to a similar extent. This indicates that invertase activities could be causally involved in the establishment of the cytokinin deficiency phenotype and thus support the view that invertases provide an important link between phytohormone action and primary metabolism (Roitsch et al., 2003). Surprisingly, the cell wall invertase activity was not changed. Activity of cell wall invertases at the site of phloem unloading has been proposed as a major factor controlling the sink strength to attract Suc (Roitsch and González, 2004). Moreover, expression of CIN1, a cell wall invertase gene from Chenopodium, was induced by cytokinin (Ehneß and Roitsch, 1997), and extracellular invertase activity was indispensable for cytokinin-induced nutrient mobilization and delay of leaf senescence (Balibrea Lara et al., 2004). The present results, however, suggest that vacuolar invertases may play a more dominant role in Suc cleavage and sink maintenance within the symplastic continuity of vegetative SAM and developing leaves. This is in accordance with experiments showing that vacuolar invertase activity precedes that of cell wall invertases during initiation of other sink types (Andersen et al., 2002; Wächter et al., 2003). Consistently, expression of the CIN1 gene in young developing leaves of cytokinin-deficient Arabidopsis plants did not restore the reduced shoot growth (TW, KH, and TS, unpublished result).
A further indication of a disturbed carbohydrate metabolism in cytokinin-deficient shoot sink was the increased activity of enzymes of the initial phase of glycolysis, such as PFP and aldolase. This could indicate that a compensatory mechanism was activated in response to the lower sugar status. However, increased starch accumulation observed in cytokinin-deficient apices suggests that the triose phosphates and/or Glc-6-phosphates were not utilized for downstream glycolytic steps but were directed to starch synthesis. As a result, subsequent energy-conserving steps of glycolysis were presumably attenuated as indicated by the reduced activity of enolase. Conceivably, a lowered production of pyruvate and a decreased oxidative phosphorylation may explain the 50% reduction of ATP content in cytokinin-deficient shoot sink tissues. Whether cytokinin could directly control some glycolytic processes is currently little explored. Suzuki et al. (1994) have shown that cytokinin, in response to the nitrogen status, up-regulates the transcript level of a phosphoenolpyruvate carboxylase gene in maize.
Photosynthetic activity of cytokinin-deficient source leaves is not altered
Sugar deprivation in the cytokinin-deficient shoot apex could be caused by a reduced rate of carbon fixation in source leaves and/or reduced allocation of assimilates from source to sink. Chl biosynthesis in cytokinin-deficient leaves was diminished at the rate-limiting step of ALA synthesis and resulted in a 15–37% reduction in Chl content (Table 1). Interestingly, less Chl did not significantly compromise photosynthetic activity, as indicated by the unchanged maximum yield of PSII photochemistry and photosynthetic efficiency of CO2 uptake. This indicates that wild-type source leaves contained, under experimental growth conditions, excessive Chl and photosynthesized at a lower than maximal rate. A higher Fv/Fm ratio of cytokinin-deficient source leaves might indicate that there was compensation for the initially reduced maximum quantum yield. No significant change in quantum efficiency of PSII photochemistry in transgenic source leaves (ΦPSII; Table 2) suggests that the reduced ETR was primarily caused by the reduced photon absorption and apparently not associated with a reduced proportion of absorbed energy that is used in photochemistry. Hence no activation of radiationless dissipation of the absorbed energy seems to be necessary in the transgenic tissue, as is indicated by the unchanged non-photochemical quenching (qN, Fig. 5).
Starch was significantly reduced in cytokinin-deficient source leaves (Fig. 6). By contrast, of the soluble sugars, only Suc was significantly reduced in the 35S:CKX1 line. The total content of non-structural carbohydrates was reduced by 35–50% in transgenic lines, indicating that, despite unchanged photosynthetic parameters, the total carbon gain was reduced. The ATP content was decreased by 20–50% but the ATP/ADP ratio remained unchanged in comparison with wild type. A 30–50% increase was detected in the mitochondrial respiration rate in transgenic source leaves, which suggests that the photosynthetic gain could be partially lost by respiration because of the higher dark respiration rates. It has been shown that cytokinin inhibits cyanide-resistant respiration and it was suggested that cyanide-resistant respiration may be involved in some plant responses to cytokinin (Musgrave, 1994). However, whether cytokinin participates in direct control of respiration and energy production in plants remains to be studied.
Cytokinin deficiency in roots
The faster-growing roots of cytokinin-deficient plants have a strongly reduced content of soluble sugars and, at least in the case of 35S:CKX1-expressing tobacco plants, increased invertase activities. The lower sugar contents could be caused by their rapid metabolic utilization due to the increased growth rate. However, the activity of glycolytic enzymes and the ATP content were not altered. One might suspect that the faster-growing roots act as a strong sink and grow at the expense of the shoot sink. However, grafting experiments showed that growth of a wild-type scion was not negatively affected by a strongly growing cytokinin-deficient rootstock (TW and TS, unpublished results). The results of the physiological analysis of roots have not provided a clue to understanding their enhanced growth. It could be that the formation of a larger root meristem, which is caused by retarding the exit of cells from the cell division phase and their entry into the elongation phase (Werner et al., 2003; Dello Ioio et al., 2007), is more relevant for root growth than changes in primary metabolism.
Supplementary data
Figure S1. Altered ultrastructure of cells in shoot meristems of cytokinin-deficient tobacco plants.
Supplementary Material
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft to TS and BG in the frame of Sfb 429. Special thanks are given to S Brown and O Catrice (ISV, CNRS, Gif-sur-Yvette, France) for their help in flow cytometry and C Fayet (UPMC, IFR83, Paris, France) for her assistance in electron microscopy. We thank Thomas Roitsch for the CIN1 gene.
References
- Andersen MN, Asch F, Wu Y, Jensen CR, Nasted H, Mogensen VO, Koch KE. Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiology. 2002;130:591–604. doi: 10.1104/pp.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balibrea Lara ME, Gonzalez Garcia M-C, Fatima T, Ehness R, Lee TK, Proels R, Tanner W, Roitsch T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. The Plant Cell. 2004;16:1276–1287. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow PW. Nuclear chromatin structure in relation to cell differentiation and cell activation in the cap and quiescent centre of Zea mays L. Journal of Experimental Botany. 1985;36:1492–1503. [Google Scholar]
- Bartoli CG, Goméz F, Martínez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.) Journal of Experimental Botany. 2004;55:1663–1669. doi: 10.1093/jxb/erh199. [DOI] [PubMed] [Google Scholar]
- Barton R. Fine structure of mesophyll cells in senescing leaves of Phaseolus. Planta. 1966;71:314–325. doi: 10.1007/BF00396319. [DOI] [PubMed] [Google Scholar]
- Beck E. Towards an understanding of plant growth regulation: cytokinins as major signals for biomass distribution. In: Strnad M, Pec P, Beck E, editors. Advances in regulation of plant growth and development. Prague: Peres Publishers; 1999. pp. 97–110. [Google Scholar]
- Boudolf V, Inzé D, De Veylder L. What if higher plants lack a CDC25 phosphatase? Trends in Plant Science. 2006;11:474–479. doi: 10.1016/j.tplants.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner WG, Romanov GA, Köllmer I, Bürkle L, Schmülling T. Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. The Plant Journal. 2005;44:314–333. doi: 10.1111/j.1365-313X.2005.02530.x. [DOI] [PubMed] [Google Scholar]
- Brossard D. La néoformation de bourgeons végétatifs à partir de la moelle du tabac (Nicotiana tabacum L. var. Wisconsin 38) cultivée in vitro: analyse cytochimique, histoautoradiographique et cytophotométrique. Annales des Sciences Naturelles, Botanique. 1975;16:43–150. [Google Scholar]
- Brossard D. Microspectrophotometric and ultrastructural analyses of a case of cell differentiation without endopolyploidisation: the pith of Crepis capillaris (L.) Wallr. Protoplasma. 1978;93:369–380. [Google Scholar]
- de Andrade PBM, Rubi B, Frigerio F, van den Ouweland JMW, Maassen JA, Maechler P. Diabetes-associated mitochondrial DNA mutation A3243G impairs cellular metabolic pathways necessary for beta cell function. Diabetologia. 2006;49:1816–1826. doi: 10.1007/s00125-006-0301-9. [DOI] [PubMed] [Google Scholar]
- De Veylder L, de Almeida Engler J, Burssens S, Manevski A, Lescure B, Van Montagu M, Engler G, Inzé D. A new D-type cyclin of Arabidopsis thaliana expressed during lateral root primordia formation. Planta. 1999;208:453–462. doi: 10.1007/s004250050582. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Current Biology. 2007;17:678–682. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Dertinger U, Schaz U, Schulze E-D. Age-dependence of the antioxidative system in tobacco with enhanced glutathione reductase activity or senescence-induced production of cytokinins. Physiologia Plantarum. 2003;119:19–29. [Google Scholar]
- Dewitte W, Murray JAH. The plant cell cycle. Annual Review of Plant Biology. 2003;54:235–264. doi: 10.1146/annurev.arplant.54.031902.134836. [DOI] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proceedings of the National Academy of Sciences, USA. 2007;104:14537–14542. doi: 10.1073/pnas.0704166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JD. Changes in chloroplast fine structure during the autumnal senescence of Betula leaves. Annals of Botany. 1970;34:817–824. [Google Scholar]
- Domenicali M, Caraceni P, Vendemiale G, et al. Food deprivation exacerbates mitochondrial oxidative stress in rat liver exposed to ischemia-reperfusion injury. Journal of Nutrition. 2001;131:105–110. doi: 10.1093/jn/131.1.105. [DOI] [PubMed] [Google Scholar]
- Ehneß R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. The Plant Journal. 1997;11:539–548. doi: 10.1046/j.1365-313x.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- Ferreira FJ, Kieber JJ. Cytokinin signaling. Current Opinion in Plant Biology. 2005;8:518–525. doi: 10.1016/j.pbi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Flores S, Tobin EM. Cytokinin modulation of LHCP mRNA levels: the involvement of post-transcriptional regulation. Plant Molecular Biology. 1988;11:409–415. doi: 10.1007/BF00039021. [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science. 1983;220:1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Guivarc'h A, Rembur J, Goetz M, Roitsch T, Noin M, Schmülling T, Chriqui D. Local expression of the ipt gene in transgenic tobacco (Nicotiana tabacum L. cv. SR1) axillary buds establishes a role for cytokinins in tuberization and sink formation. Journal of Experimental Botany. 2002;53:621–629. doi: 10.1093/jexbot/53.369.621. [DOI] [PubMed] [Google Scholar]
- Hartig K, Beck E. Endogenous cytokinin oscillations control cell cycle progression of tobacco BY-2 cells. Plant Biology. 2005;7:33–40. doi: 10.1055/s-2004-830474. [DOI] [PubMed] [Google Scholar]
- Hauser B, Sun K, Oppenheimer D, Sage T. Changes in mitochondrial membrane potential and accumulation of reactive oxygen species precede ultrastructural changes during ovule abortion. Planta. 2006;223:492–499. doi: 10.1007/s00425-005-0107-x. [DOI] [PubMed] [Google Scholar]
- Heyl A, Werner T, Schmülling T. Metabolism and signal transduction of cytokinin. In: Hedden P, Thomas S, editors. Plant hormone signalling, Annual Plant Reviews. Oxford: Blackwell Publishing; 2006. pp. 93–123. [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences, USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Veda RR. Light and electron microscopical studies on the senescence in Elodea leaf cells. Botanical Magazine. 1964;77:336–341. [Google Scholar]
- Jacqmard A, Houssa C, Bernier G. Regulation of the cell cycle by cytokinins. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton, FL: CRC; 1994. pp. 197–215. [Google Scholar]
- Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology. 2004;7:235–246. doi: 10.1016/j.pbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Krall JP, Edwards GE. Relationship between photosystem II activity and CO2 fixation in leaves. Physiologia Plantarum. 1992;86:180–187. [Google Scholar]
- Krishna Chaitanya KS, Naithani SC. Kinetin-mediated prolongation of viability in recalcitrant sal (Shorea robusta Gaertn. f.) seeds at low temperature: role of kinetin in delaying membrane deterioration during desiccation-induced injury. Journal of Plant Growth Regulation. 1998;17:63–69. [Google Scholar]
- Kuiper D. Sink strength: established and regulated by plant growth regulators. Plant, Cell and Environment. 1993;16:1025–1026. [Google Scholar]
- Laureys F, Dewitte W, Witters E, Van Montagu M, Inze D, Van Onckelen H. Zeatin is indispensable for the G2-M transition in tobacco BY-2 cells. FEBS Letters. 1998;426:29–32. doi: 10.1016/s0014-5793(98)00297-x. [DOI] [PubMed] [Google Scholar]
- Lee C-F, Liu C-Y, Hsieh R-H, Wei Y-H. Oxidative stress-induced depolymerization of microtubules and alteration of mitochondrial mass in human cells. Annals of the New York Academy of Sciences. 2005;1042:246–254. doi: 10.1196/annals.1338.027. [DOI] [PubMed] [Google Scholar]
- Lerbs S, Lerbs W, Klyachko NL, Romanko EG, Kulaeva ON, Wollgiehn R, Parthier B. Gene expression in cytokinin- and light-mediated plastogenesis of Cucurbita cotyledons: ribulose-1,5-bisphosphate carboxylase/oxygenase. Planta. 1984;162:289–298. doi: 10.1007/BF00396739. [DOI] [PubMed] [Google Scholar]
- Maassen JA, ‘t Hart LM, Janssen GMC, Reiling E, Romijn JA, Lemkes HH. Mitochondrial diabetes and its lessons for common Type 2 diabetes. Biochemical Society Transactions. 2006;34:819–823. doi: 10.1042/BST0340819. [DOI] [PubMed] [Google Scholar]
- Miyazawa Y, Kato H, Muranaka T, Yoshida S. Amyloplast formation in cultured tobacco BY-2 cells requires a high cytokinin content. Plant and Cell Physiology. 2002;43:1534–1541. doi: 10.1093/pcp/pcf173. [DOI] [PubMed] [Google Scholar]
- Mok MC. Cytokinins and plant development: an overview. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton, FL: CRC; 1994. pp. 155–166. [Google Scholar]
- Mothes K, Engelbrecht L, Schütte HR. Über die Akkumulation von a-Aminoisobuttersäure im Blattgewebe unter dem Einfluss von Kinetin. Physiologia Plantarum. 1961;14:72–75. [Google Scholar]
- Musgrave ME. Cytokinins and oxidative processes. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton, FL: CRC; 1994. pp. 167–178. [Google Scholar]
- Mustroph A, Albrecht G. Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiologia Plantarum. 2003;117:508–520. doi: 10.1034/j.1399-3054.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Mustroph A, Boamfa E, Laarhoven L, Harren F, Albrecht G, Grimm B. Organ-specific analysis of the anaerobic primary metabolism in rice and wheat seedlings. I. Dark ethanol production is dominated by the shoots. Planta. 2006a;225:103–114. doi: 10.1007/s00425-006-0333-x. [DOI] [PubMed] [Google Scholar]
- Mustroph A, Boamfa E, Laarhoven L, Harren F, Pörs Y, Grimm B. Organ specific analysis of the anaerobic primary metabolism in rice and wheat seedlings. II. Light exposure reduces needs for fermentation and extends survival during anaerobiosis. Planta. 2006b;225:139–152. doi: 10.1007/s00425-006-0336-7. [DOI] [PubMed] [Google Scholar]
- Nagl W, Fusenig HP. Types of chromatin organization in plant nuclei. Plant Systematics and Evolution Supplement 2. 1979:221–233. [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. The Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard CB, Siciliano I, Sorrell DA, et al. Tobacco BY-2 cells expressing fission yeast cdc25 bypass a G2/M block on the cell cycle. The Plant Journal. 2005;44:290–299. doi: 10.1111/j.1365-313X.2005.02524.x. [DOI] [PubMed] [Google Scholar]
- Porceddu A, Stals H, Reichheld J-P, Segers G, De Veylder L, de Pinho Barrôco R, Casteels P, Van Montagu M, Inzé D, Mironov V. A plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. Journal of Biological Chemistry. 2001;276:36354–36360. doi: 10.1074/jbc.M011060200. [DOI] [PubMed] [Google Scholar]
- Pörs Y, Hansen U, Hoffmann P. Compensation of differences in light absorption at the levels of photosynthetic primary processes, CO2 uptake and growth of tobacco plants. Journal of Plant Physiology. 2001;158:1555–1564. [Google Scholar]
- Rattan SIS. N6-furfuryladenine (kinetin) as a potential anti-aging molecule. Journal of Anti-Aging Medicine. 2002;5:113–116. [Google Scholar]
- Redig P, Shaul O, Inze D, Van Montagu M, Van Onckelen H. Levels of endogenous cytokinins, indole-3-acetic acid and abscisic acid during the cell cycle of synchronized tobacco BY-2 cells. FEBS Letters. 1996;391:175–180. doi: 10.1016/0014-5793(96)00728-4. [DOI] [PubMed] [Google Scholar]
- Reski R. Plastid genes and chloroplast biogenesis. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton, FL: CRC; 1994. pp. 179–195. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond AE, Lang A. Effect of kinetin on protein content and survival of detached Xanthium leaves. Science. 1957;125:650–651. [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH. Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Molecular and Cellular Biology. 2000;20:4513–4521. doi: 10.1128/mcb.20.13.4513-4521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK. Extracellular invertase: key metabolic enzyme and PR protein. Journal of Experimental Botany. 2003;54:513–524. doi: 10.1093/jxb/erg050. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Bittner M, Godt DE. Induction of apoplastic invertase of Chenopodium rubrum by D-glucose and a glucose analog and tissue-specific expression suggest a role in sink-source regulation. Plant Physiology. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, González M-C. Function and regulation of plant invertases: sweet sensations. Trends in Plant Science. 2004;9:606–613. doi: 10.1016/j.tplants.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Takei K, Hirose N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends in Plant Science. 2006;11:440–448. doi: 10.1016/j.tplants.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sugiharto B, Burnell JN, Sugiyama T. Cytokinin is required to induce the nitrogen-dependent accumulation of mRNAs for phosphoenolpyruvate carboxylase and carbonic anhydrase in detached maize leaves. Plant Physiology. 1992;100:153–156. doi: 10.1104/pp.100.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Cretin C, Omata T, Sugiyama T. Transcriptional and posttranscriptional regulation of nitrogen-responding expression of phosphoenolpyruvate carboxylase gene in maize. Plant Physiology. 1994;105:1223–1229. doi: 10.1104/pp.105.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synková H, Schnablová R, Polanská L, Hušák M, Šiffel P, Vácha Fe, Malbeck J, Machácková I, Nebesárová J. Three-dimensional reconstruction of anomalous chloroplasts in transgenic ipt tobacco. Planta. 2006;223:659–671. doi: 10.1007/s00425-005-0119-6. [DOI] [PubMed] [Google Scholar]
- Synková H, Semorádová Á, Burketová L. High content of endogenous cytokinins stimulates activity of enzymes and proteins involved in stress response in Nicotiana tabacum. Plant Cell, Tissue and Organ Culture. 2004;79:169–179. [Google Scholar]
- Verdeil J-L, Alemanno L, Niemenak N, Tranbarger TJ. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends in Plant Science. 2007;12:245–252. doi: 10.1016/j.tplants.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Wächter R, Langhans M, Aloni R, et al. Vascularization, high-volume solution flow, and localized roles for enzymes of sucrose metabolism during tumorigenesis by Agrobacterium tumefaciens. Plant Physiology. 2003;133:1024–1037. doi: 10.1104/pp.103.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing PF, Khalifa MM, Treharne KJ. Rate-limiting processes in photosynthesis at saturating light intensities. Nature. 1968;220:453–457. doi: 10.1038/220453a0. [DOI] [PubMed] [Google Scholar]
- Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T. New insights into the biology of cytokinin degradation. Plant Biology. 2006;8:1–12. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yu H, Xu Y, Goh CJ. Investigation of cytokinin-deficient phenotypes in Arabidopsis by ectopic expression of orchid DSCKX1. FEBS Letters. 2003;555:291–296. doi: 10.1016/s0014-5793(03)01259-6. [DOI] [PubMed] [Google Scholar]
- Yaronskaya E, Vershilovskaya I, Poers Y, Alawady AE, Averina N, Grimm B. Cytokinin effects on tetrapyrrole biosynthesis and photosynthetic activity in barley seedlings. Planta. 2006;224:700–709. doi: 10.1007/s00425-006-0249-5. [DOI] [PubMed] [Google Scholar]
- Zhang K, Diederich L, John PCL. The cytokinin requirement for cell division in cultured Nicotiana plumbaginifolia cells can be satisfied by yeast Cdc25 protein tyrosine phosphatase: implications for mechanisms of cytokinin response and plant development. Plant Physiology. 2005;137:308–316. doi: 10.1104/pp.104.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Letham DS, John PCL. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta. 1996;200:2–12. doi: 10.1007/BF00196642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.