Abstract
To study the effects of cytokinin O-glucosylation in monocots, maize (Zea mays L.) transformants harbouring the ZOG1 gene (encoding a zeatin O-glucosyltransferase from Phaseolus lunatus L.) under the control of the constitutive ubiquitin (Ubi) promoter were generated. The roots and leaves of the transformants had greatly increased levels of zeatin-O-glucoside. The vegetative characteristics of hemizygous and homozygous Ubi:ZOG1 plants resembled those of cytokinin-deficient plants, including shorter stature, thinner stems, narrower leaves, smaller meristems, and increased root mass and branching. Transformant leaves had a higher chlorophyll content and increased levels of active cytokinins compared with those of non-transformed sibs. The Ubi:ZOG1 plants exhibited delayed senescence when grown in the spring/summer. While hemizygous transformants had reduced tassels with fewer spikelets and normal viable pollen, homozygotes had very small tassels and feminized tassel florets, resembling tasselseed phenotypes. Such modifications of the reproductive phase were unexpected and demonstrate a link between cytokinins and sex-specific floral development in monocots.
Keywords: Corn, cytokinin, plant development, tasselseed, Zea mays, zeatin O-glucosyltransferase
Introduction
Cytokinins are plant hormones that are essential for cell division and plant development (Mok and Mok, 1994). Natural cytokinins are adenine derivatives, generally with an isoprenoid side chain at the N6 position, although cytokinins with aromatic side chains are also known to occur (Strnad, 1997). Zeatin is considered to be an essential cytokinin in higher plants due to its ubiquitous nature and high activity. Other free bases with cytokinin activity, cis-zeatin, dihydrozeatin, and N6-(Δ2-isopentenyl) adenine, are also present in most plant tissues. Derivatives of these bases include the corresponding ribosides and nucleotides, as well as glucosides with the sugar moiety at the O of the side chain or at the N7, N9, or N3 of the adenine ring.
Until recently, the true activities of the various cytokinin metabolites were difficult to assess. The activity of natural as well as synthetic compounds have been traditionally determined by bioassays such as the tobacco callus, bean callus, or radish cotyledon bioassays (Murashige and Skoog, 1962; Letham, 1971; Mok et al., 1978). The results of these bioassays indicated that free bases and ribosides had high cytokinin activity. However, apparent cytokinin activities may not necessarily reflect their true activities in planta due to rapid metabolism or catabolism. For instance, while the N7 and N9 glucosides of zeatin are mostly inactive in bioassays (Letham et al., 1983), the O-glucoside is very active in bioassays, sometimes more than zeatin itself (Mok et al., 1992). The high activity of O-glucosylzeatin was assumed to result from conversion to the aglycone since the side chain far exceeds the optimal molecule size for cytokinin activity (Skoog and Armstrong, 1970).
The identification of cytokinin receptors provided an alternative evaluation of cytokinin activity. For example, studies with the CRE1/AHK4 and AHK3 receptors of Arabidopsis showed that indeed, zeatin O-glucoside is not an active cytokinin (Spíchal et al., 2004). In general, these studies indicate that the highest cytokinin activity is conferred by free bases. Relatively lower activity was displayed by ribosides, while no activity was detected with glucosides (Spíchal et al., 2004; Yonekura-Sakakibara et al., 2004). There are, however, differences between receptors in cytokinin recognition as illustrated by the binding of only the trans isomer of zeatin by the Arabidopsis CRE1/AHK4 receptor, but both the cis and trans isomers by the maize ZmHK1 receptor (Yonekura-Sakakibara et al., 2004; Mok et al., 2005).
Cytokinin O-glucosides are generally assumed to be storage products. While they lack activity per se, they can be activated through hydrolysis by β-glucosidases. Although conversion of zeatin to its O-glucoside by O-glucosyltransferases temporarily inactivates zeatin, this process also protects zeatin from cytokinin oxidases/dehydrogenases, which can degrade zeatin but not its O-glucoside (McGaw and Horgan, 1983; Armstrong, 1994). Complicating this picture is the distribution of these metabolites in the cell, resulting possibly in differential localization of substrates and enzymes. For instance, dihydrozeatin O-glucoside and the glucosides of a number of other plant growth regulators were found in the vacuoles (Garcia-Martinez et al., 1981; Schmitt and Sandermann, 1982; Bray and Zeevaart, 1985; Lehmann and Glund, 1986; Fusseder and Ziegler, 1988; Dean et al., 2003) whereas the maize β-glucosidase is targeted to plastids (Kristoffersen et al., 2000). Thus, while the high activity of O-glucosylzeatin in bioassays indicates that it can be converted to zeatin, the extent of this conversion in plants is unclear. It is possible that it is limited to certain tissues or particular stages of plant development or that it only occurs under specific conditions.
Enzymes and genes involved in zeatin glycosylation and glucoside hydrolysis have been identified. The first zeatin O-glucosyltransferase (EC 2.4.1.203) was isolated from immature P. lunatus seeds (Dixon et al., 1989) while a variant of this enzyme, a zeatin O-xylosyltransferase (EC 2.4.2.40), was obtained from those of P. vulgaris (Turner et al., 1987). Subsequently, the genes encoding these enzymes, ZOG1 and ZOX1, were cloned (Martin et al., 1999a, b). In addition, two genes encoding O-glucosyltransferases with a preference for cis-zeatin were isolated from maize (Martin et al., 2001b; Veach et al., 2003). Other zeatin O-glucosyltransferase genes as well as two cytokinin N-glucosyltransferase (EC 2.4.1.118) genes were identified in the Arabidopsis genome (Hou et al., 2004). These enzymes all belong to Family 1 of the UDP-sugar requiring glycosyltransferases (http://www.cazy.org) and contain the signature sequence for this family in the C-proximal portion.
An enzyme converting zeatin O-glucoside to zeatin was first identified in maize (Brzobohatý et al, 1993). This β-glucosidase (EC 3.2.1.21) has somewhat broader substrate specificity than the O-glucosyltransferases since it cleaves kinetin-N3-glucoside as well as some other substrates. The corresponding gene was cloned (Zm-p60.1) and found to be highly expressed in root meristems (Brzobohatý et al., 1993). A similar gene was isolated from Brassica napus (Falk and Rask, 1995).
We are interested in the effects of over-expressing as well as repressing ZOG-type genes to determine the regulatory properties of O-glucosylation on plant development. The developmental modifications of maize transformants harbouring ZOG1 driven by the constitutive ubiquitin (Ubi) promoter is reported here. Our data indicate that zeatin O-glucosylation clearly affects root formation, leaf development, chlorophyll content, senescence, and male flower differentiation. Most interesting are the effects of the ZOG1 transgene on tassel development, with the formation of tasselseed in the homozygous transformants.
Materials and methods
Generation and selection of transgenic lines
Transgenic Zea mays plants were obtained using an Agrobacterium-mediated transformation procedure of GS3×HC69 hybrid embryos as previously described (Zhao et al., 1998). The construct that was used contained the Ubi (ubiquitin) promoter (Christensen et al., 1992) upstream of the ZOG1 gene (accession no. AF101972) and a bialaphos resistance gene (Block et al., 1987) as the selectable marker. For further details on the construct see Fig. S1 in Supplementary data at JXB online. T0 plants were backcrossed four times to HC69.
Five replications of four BC4 lines (P1, P3, P6, and P7) were planted from February through April and grown in the greenhouse at 25/20 °C (day/night) under natural light. Plants were identified as bialaphos-resistant or bialaphos-sensitive by painting part of the lower leaf with 200 mg l−1 bialaphos. For each replication, data were obtained from four plants per BC4 line.
Crosses between sibs of the BC4 lines produced progeny equivalent to F2s. These progenies segregated at the expected 3:1 ratio of bialaphos-resistant to bialaphos-sensitive plants. Three repeats of two F2 lines (P3 and P7) with 30 plants per line were planted in early October to study the F2 generation. The photoperiod was gradually extended by using high-pressure sodium lamps (Hortilux LU1000B/Ht1/En) to simulate daylength conditions in spring and summer (April planting). Two additional 30 plant replications of P3 and P7 were planted in the spring and grown under natural light.
Distribution of materials
Novel materials described in this publication may be available for non-commercial research purposes upon acceptance and signing of a material transfer agreement. In some cases such materials may contain or be derived from materials obtained from a third party. In such cases, distribution of material will be subject to the requisite permission from any third-party owners, licensors or controllers of all or parts of the material. Obtaining any permission will be the sole responsibility of the requestor. Plant germplasm and transgenic material will not be made available except at the discretion of the owner and then only in accordance with all applicable governmental regulations.
PCR
DNA was isolated from 100 mg tissue samples by sequential treatment with 500 μl extraction buffer (200 mM TRIS-HCl, 250 mM NaCl, 25 mM EDTA, 0.5% SDS), 500 μl saturated phenol, and 500 μl chloroform, followed by precipitation with 400 μl isopropanol. Internal primers for ZOG1, ZOG411F (5′-CATCTCAAATGTTGAA-AACTAC-3′) and ZOG930B (5′-CTTCACTTCCGGCAAAGATGTC-3′), were used for PCR. An initial denaturation cycle at 95 °C for 4 min was followed by 30 cycles of 95 °C for 1 min, 52 °C for 1 min, and 72 °C for 1 min. After an additional cycle at 72 °C for 10 min, samples were stored at 4 °C.
RT-PCR
Total RNA was isolated from leaves, roots, and tassels at various stages using TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Polysaccharide-rich samples were treated with 20% polyvinylpyrrolidine (PVP) and 8 M lithium chloride and incubated overnight at –20 °C. Synthesis of cDNA was achieved using the SuperScript™ II reverse transcriptase kit (Invitrogen, Carlsbad, CA, USA). The resulting cDNA product was subjected to semi-quantitative PCR using actin to normalize samples. Primers for actin PCR were mzACTIN-32F (5′-GTGACAATGGCACTGGAATG-3′) and mzACTIN-741B (5′-GACCTGACCATCAGGCATCTC-3′). The internal primers ZOG411F and ZOG930B were used to amplify ZOG1. The following conditions were used for PCR: initial denaturation at 94 °C for 4 min followed by 30 cycles of 94 °C for 1 min, 57 °C for 45 s, and 72 °C for 1 min. The last cycle was followed by incubation at 72 °C for 10 min.
Western analyses
Protein was extracted from leaves with buffer containing 55 mM TRIS, 50 μM EDTA, 5 mM DTT, pH 7.4 (800 μl extraction buffer for 200 mg leaf tissue) followed by 30–75% NH4SO4 fractionation. Samples were desalted and then purified by Blue Sepharose 6B affinity chromatography (Dixon et al., 1989). A fraction equivalent to 7 mg fresh leaf tissue was separated on a 12% SDS polyacrylamide gel and blotted to Immobilon-P transfer membrane (Millipore, Billerica, MA, USA). Western blots were developed as described in Martin et al. (1990).
Cytokinin analyses
Cytokinin levels were determined in roots and leaves of 28-d-old and in leaves of 110-d-old non-transformed and hemizygous plants. For extraction and purification of cytokinins as well as determination of cytokinin levels, the procedures described by Veach et al. (2003) were followed. The HPLC/MS system consisted of a HTS-Pal auto-sampler with a cooled sample stack (CTC Analytics, Zwingen, Switzerland), quaternary HPLC pump Rheos 2200 (Flux Instruments, Basel, Switzerland), Delta Chrom CTC 100 Column oven (Watrex, Prague, Czech Republic) and TSQ Quantum Ultra AM triple-quad high resolution mass spectrometer (Thermo Electron, San Jose, USA). For HPLC a Synergi Hydro column (Phenomenex, Torrance, USA) was used. Data were obtained from three samples per genotype, with two HPLC injections and analyses per sample.
Phenotypic characterizations
Phenotypic characterization of transformed lines included measurements on vegetative growth rate, plant height, root length, chlorophyll content, leaf width, stomatal distribution, meristem structure, development of reproductive organs (time of appearance, size), pollen viability, and kernel development.
Foliar data presented were taken from the 10th leaf, which was found to be representative of the whole plant by measuring all leaves from fewer plants (data not shown). Foliar chlorophyll levels were monitored with a chlorophyll content meter CCM-200 (Optisciences Inc., Tyngsboro, MA, USA) over a 175-day period. Four measurement points were taken from each leaf. To convert these measurements to chlorophyll units per leaf fresh weight, chlorophyll was extracted by incubating leaf tissues in N,N′-dimethylformamide (7% leaf weight/solvent volume) at 4 °C for 48 h, as described in Inskeep and Bloom (1985). Sample absorbances were measured at 647 nm and 664.5 nm (maximum for chlorophyll b and a, respectively) and absolute chlorophyll contents calculated according to the equation:
Optimeter CCI units were then transformed into μg of chlorophyll per mg fresh weight with restriction curves. Random leaf samples were examined with a light microscope to determine stomatal cell distribution patterns.
Apical meristem samples were collected from 30-d-old plants, fixed in FAA (50% ethanol, 10% acetic acid, 5% formalin, by vol.), dehydrated in a graded ethanol series, and embedded in Technovit 7100 plastic (Heraeus Kulzer, Wehrheim, Germany). Sections were cut on an AO 820 rotary microtome to obtain 46-μm-thick serial sections. Samples were stained with Toluidine Blue-O, and analysed with Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
Pollen viability was tested prior to pollination assays. Fresh pollen grains were collected at 09.00 h and kept for 2 h on liquid germination media containing (l−1) 236.2 mg Ca(NO3)2, 24.8 mg H3BO3, and 30% (w/v) of polyethyleneglycol (PEG). Silk filaments from the female flower were added to enhance pollen tube growth. A pollen cryo-preservation protocol was optimized to ensure a constant supply of viable pollen during pollination assays. Pollen grains were collected, dried at 20 °C and 20–40% humidity for 2 h and shock-frozen by immersion in liquid nitrogen as described by Barnabas (1994). A saturated MgCl2 solution was used to stabilize the humidity in the desiccator. Prior to use, pollen was thawed in a water bath at 40 °C for 2 min. The combination of fresh and cryopreserved pollen allowed uniform pollination coverage during the receptive period of the maize ear.
The experimental design for all phenotypic traits was fully randomized. Data were analysed statistically by multivariate analysis of variance (MANOVA) with repeat, line, and type as the main sources of variation, followed by a Tukey test at 5% significance level. The analyses aimed to identify the interactions among the independent variables as well as their effect on the response variables. For those traits where no relevant variable interactions were observed, two-way and one-way ANOVA were performed.
Results
Transgenic Ubi:ZOG1 maize lines were generated using Agrobacterium-mediated transformation. Bialaphos resistance was the selectable marker and initial transformants were backcrossed to the parental inbred HC69 for four generations to generate transformants with genetic backgrounds similar to this inbred. Four BC4 lines (P1, P3, P6, and P7), derived from independent transformation events and containing a single insert each, were selected for further study. The BC4 lines segregated into bialaphos-resistant hemizygous (P+) and bialaphos-sensitive non-transformed (NT) plants (1:1). To obtain homozygous transformants (P++), hemizygous BC4 plants were selfed. For simplicity, the progeny obtained after selfing the hemizygous BC4 is referred to as F2.
Bialaphos-resistant plants transcribe and translate ZOG1
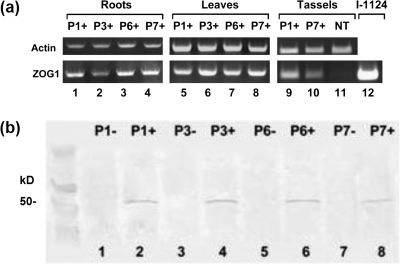
RT-PCR (Fig. 1a) and western (Fig. 1b) blots of BC4 plants indicated that transcription and translation of Ubi:ZOG1 occurs in transgenic maize tissues. Tissue samples from bialaphos-resistant plants of all four lines produced clear RT-PCR and protein bands, but those from bialaphos-sensitive plants did not.
Fig. 1.
Ubi::ZOG1 expression. (a) RT-PCR products with ZOG1-specific primers and actin as a control showing expression of the transgene in roots, leaves, and tassels. P1+, P3+, P6+, and P7+ are independent hemizygous lines. NT is a non-transformed control. Plasmid I-1124 contains the Ubi::ZOG1 insert. (b) Western blot of ZOG1 product in leaves (4 mg equivalent) from hemizygous transformants and non-transformed sibs, developed with monoclonal antibodies specific to ZOG1.
Over-expression of ZOG1 leads to large increases in zeatin O-glucoside
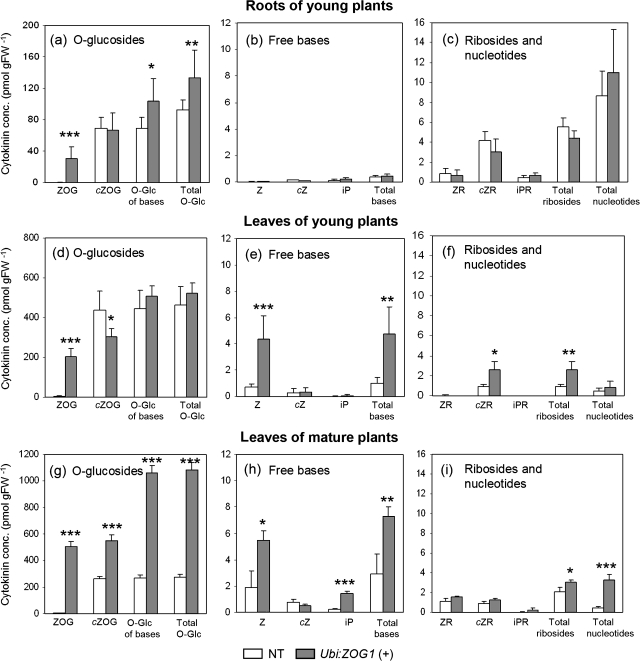
Cytokinins were analysed in roots and leaves of young plants and in leaves of mature plants (Fig. 2). As expected, the zeatin O-glucoside levels were increased significantly in the hemizygous transformants: 100-fold in roots, and 38-fold and 88-fold higher in leaves of young and older plants, respectively (Fig. 2a, d, g). By contrast, the cis-zeatin O-glucoside levels differed only up to 2-fold and glucosylation of ribosides was low. These levels are both in agreement with the substrate preference of the ZOG1 enzyme (Martin et al., 1999a). Overall, O-glucosides constituted the main pool of cytokinin metabolites, even in the non-transformed plants. The levels of N-glucosides were negligible compared with those of O-glucosides (data not shown).
Fig. 2.
Cytokinin concentrations in roots and leaves. (a, b, c) Cytokinin concentrations in roots of 28-d-old non-transformed (NT) and hemizygous Ubi::ZOG1 (+) maize plants. (d, e, f) Cytokinin concentrations in leaves of 28-d-old maize plants. (g, h, i) Cytokinin concentrations in leaves of 95-d-old maize plants. (a, d, g) O-glucosides; (b, e, h) free bases; (c, f, i) ribosides and nucleotides. Z, trans-zeatin; cZ, cis-zeatin; iP, N6-(Δ2-ispentenyl)adenine; ZR, trans-zeatin riboside; cZR, cis-zeatin riboside; iPR, N6-(Δ2-ispentenyl)adenosine; ZOG, trans-zeatin O-glucoside; cZOG, cis-zeatin O-glucoside. Values are means (±SE) of three samples with two HPLC analyses for each. Significant differences between transformants and controls are indicated by *P <0.05, **P <0.01, or ***P <0.001.
In roots, levels of free bases were extremely low, both in transformed and control plants (Fig. 2b). Root riboside levels were higher than free bases but did not differ between transformants and controls (Fig. 2c). In leaves of both young and old plants, the levels of zeatin were higher in transformants than in controls (Fig. 2e, h). Although this increase in zeatin could be accounted for by hydrolysis of only 1% of the zeatin O-glucosides in transformants during storage and extraction, the ribosides and nucleotides were also elevated in transformants (Fig. 2f, i), which could not stem from glycoside hydrolysis. The levels of dihydrozeatin and its derivatives were generally below detection levels. Altogether, the data indicate that the levels of active cytokinins (free bases and ribosides) are slightly lower in transformed roots but higher in transformed leaves.
Increased cytokinin conjugation leads to pronounced changes in maize plant architecture and leaf width
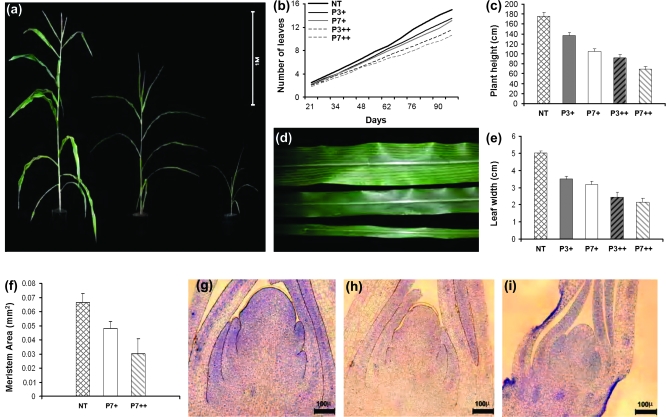
The hemizygous Ubi:ZOG1 BC4 plants were morphologically distinguishable from non-transformed sibs. They grew slower, were shorter, and had narrower leaves. There were three types of F2 plants: bialaphos-sensitive non-transformed plants, plants resembling the hemizygous BC4 transformants, and a class of extremely small plants (Fig. 3a). The segregation ratios were 16:36:10 and 15:27:22 in the P3 and P7 lines, respectively, fitting a 1:2:1 ratio and thus supporting the hypothesis that the very small plants were homozygous for the transgene. There were significantly fewer leaves on the hemizygous transformants than on the non-transformed plants and even fewer leaves on the homozygous transformants (Fig. 3b). At the time of tassel emergence, plant height was reduced by 22–41% in the hemizygous and by 48–60% in the homozygous Ubi:ZOG1 plants compared with non-transformed plants (Fig. 3c). There was a greater reduction in plant height than in the number of leaves, indicating that internodes were shortened in response to transgene activity. Stems were slender in hemizygous and very slender in homozygous Ubi:ZOG1 plants. The leaves of hemizygous plants were significantly narrower than those of non-transformed sibs (Fig. 3d, e), but their length was the same (data not shown). The leaves of homozygous transformants were extremely narrow, even at plant maturity (Fig. 3e), and also shorter than control and hemizygous plants. The thinner stems and leaves of transformants suggested smaller meristematic regions and microtome sections confirmed that hemizygous and homozygous meristems were indeed significantly smaller (Fig. 3f-i). These characteristics were not influenced by environmental fluctuations (light intensity or day length).
Fig. 3.
Vegetative development. (a) Non-transformed (left), hemizygous (centre) and homozygous (right) 97-d-old plants. (b) Number of leaves over time. P+ and P++ represent hemizygous and homozygous lines, respectively. (c) Plant height at tassel emergence. (d) Non-transformed (top), hemizygous (centre) and homozygous (bottom) leaves. (e) Average width of the tenth leaf. (f) Shoot meristem size of 40-d-old plants. (g) Non-transformed meristem. (h) Hemizygous meristem. (i) Homozygous meristem. Values are means (±SE), obtained from two experiments with a total of 60 plants each. Values for (f) are means (±SD) of 10 meristems.
Chlorophyll formation and distribution of stomates are altered in transformants
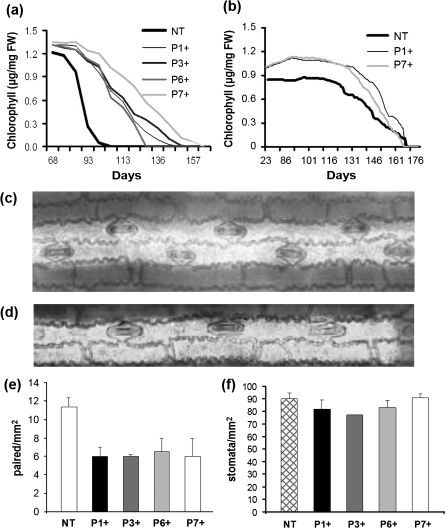
Differences in foliar chlorophyll content were noticeable as early as 23 d after germination. The transformed plants were darker green than the non-transformed ones. Chlorophyll levels in transformants were elevated throughout their life span and ultimately resulted in the delay of senescence which kept transformed plants green weeks after control sibs had senesced (Fig. 4a). This is interesting since increased chlorophyll formation and delay of senescence are traits usually associated with an increase in cytokinins (Richmond and Lang, 1957; Gan and Amasino, 1995) whereas the plant architecture of Ubi::ZOG1 transformants is generally indicative of a decrease in active cytokinins (see Discussion). However, these findings are supported by the cytokinin analyses, which showed increases in active cytokinins in transformant leaves (Fig. 2). The delay of senescence was influenced by the environment since it occurred in the summer but not in the winter under artificial light (Fig. 4b), even though the daylength was adjusted to mimic summer conditions. It is most likely that the difference in light intensity and/or quality was a major factor in this seasonal effect.
Fig. 4.
Leaf characteristics. (a, b) Chlorophyll levels in the 10th leaf through senescence. Plants grown in the spring/summer under natural long-day conditions (a) and in the autumn under artificial lighting (b). (c) Paired chain of stomata from a non-transformed leaf. (d) Single chain of stomata from Ubi::ZOG1 leaf. (e) Number of paired stomata mm−2 (f) Total number of stomata mm−2. Values for (a) and (b) are means (±SE) of two experiments with four plants per line and six measurements per leaf. Values for (e) and (f) are means (±SD) of cross-sections of 20 random mm2 fields.
Closer examination of the leaves revealed that the number of major veins was the same in non-transformed plants and hemizygous transformants but that the distance between the veins was smaller in the latter. On normal maize leaves, the stomata usually occur in rows, both in double files with stomata at alternate positions (Fig. 4c; Hernandez et al., 1999) and single files (Fig. 4d). Transformants had fewer double file stomata (Fig. 4e) but more single files. The overall density of the stomata in the leaves did not differ between the two groups (Fig. 4f).
Root mass and branching is increased in Ubi:ZOG1 transformants
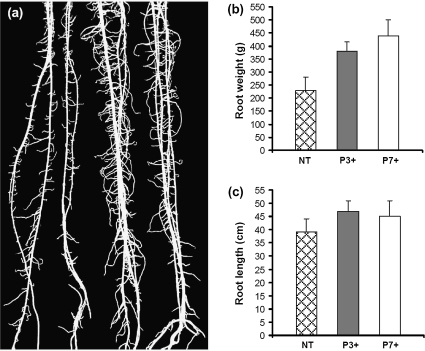
Root morphology differed between non-transformed plants and hemizygous transformants. Ubi:ZOG1 roots were thicker, more branched (Fig. 5a) and longer (Fig. 5b). As a result, the total root weight was much greater in the transformants (Fig. 5c).
Fig. 5.
Root development. (a) Non-transformed (two at left) and hemizygous (two at right) roots 3 weeks after planting. (b) Root fresh weight. (c) Root length. Values for (b) and (c) are means (±SD) of 10 plants.
Ubi:ZOG1 causes reduction of tassel size and feminization of florets
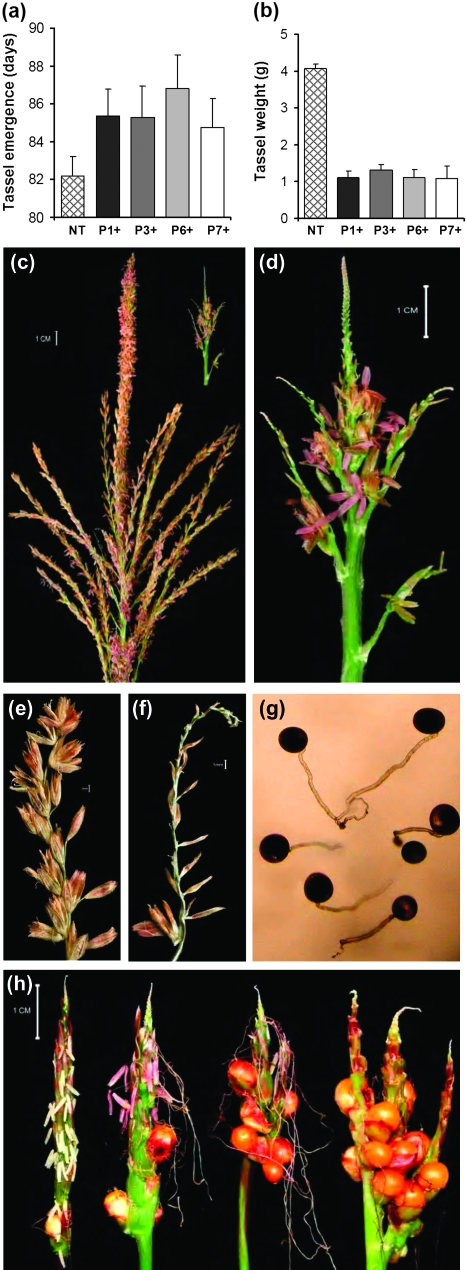
Hemizygous Ubi:ZOG1 plants showed delayed tassel initiation (Fig. 6a) and, more strikingly, a drastic reduction in tassel size, branching, and spikelet production compared with the non-transformed control (Fig. 6b, c, d). The weight of transformed tassels was about 75% less than that of non-transformed tassels (Fig. 6b), due to the decreased number and smaller size of branches. Moreover, the tassels were abnormal. Normal maize tassels have many florets up to the tip of each branch (Fig. 6e), but the hemizygous Ubi:ZOG1 tassels had functional spikelets only at the lower end of the tassels while the tips of the branches were devoid of most floral structures (Fig. 6f). Sterile spikelets consisted of two external glumes with no florets, lemma or stamens. The normal florets underwent anthesis and produced functional pollen grains as demonstrated by the ability of the pollen to germinate (Fig. 6g). Pollen viability was further supported by the roughly equal numbers of transformed and untransformed kernels in the BC4 populations, which were generated using BC3 transformants as male parents.
Fig. 6.
Tassel development. (a) Age of plant at tassel emergence. (b) Tassel fresh weight at maturity. (c) Non-transformed tassel. (d) Hemizygous Ubi::ZOG1 tassel. (e) Control lateral rachis with fully developed fertile spikelets. (f) Hemizygous lateral rachis with empty spikelets. (g) Viable Ubi::ZOG1 pollen in liquid germination medium. (h) Homozygous tassels with various degrees of floret feminization. Values for (a) and (b) are means (±SE) of five experiments with four plants each.
Homozygous F2 tassels were phenotypically even more extreme, showing a dosage effect of the ZOG1 gene. They were very small, had no or very few branches, and most interestingly, showed various degrees of floret feminization (Fig. 6h). Fertilization of these female florets resulted in formation of complete kernels (Fig. 6h). The feminization varied in the homozygous population, from a few female florets and seeds to almost complete silking of the tassel (Fig. 6h, left to right). Normal tassel floret development is initially bisexual, after which florets become unisexual through gynoecium abortion (Cheng and Pareddy, 1994). This programmed gynoecium abortion was inhibited by ZOG1 over-expression in some of the lower florets since fertile ovules and elongated silk were formed. The few male florets on the apical portion of the homozygous tassels yielded a small amount of viable pollen. The phenotype varied depending on the light source during plant growth. Plants grown in the winter under artificial light always showed some degree of feminization while plants grown in the spring and summer under ambient light conditions had only a few tasselseeds.
Ubi:ZOG1 reduces seed weight
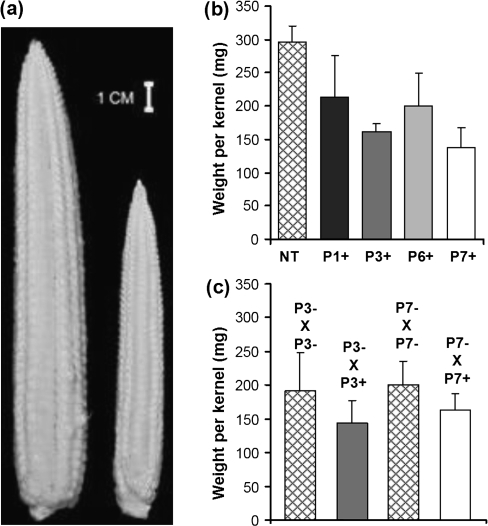
Ear development on hemizygous Ubi:ZOG1 plants lagged behind that of non-transformed plants but the ears looked normal (Fig. 7a). Hemizygous ears had only partial seed set, in contrast to fully filled non-transformed ears under the same greenhouse conditions; however, when the ears were artificially pollinated, the hemizygous ears were also completely filled, indicating that pollen availability (not viability) was the limiting factor. When the Ubi:ZOG1 plants were used as the male parent, plants produced kernels with a lower average weight than those pollinated with non-transformed pollen (Fig. 7b). To confirm this initial observation, hemizygous plants were used to pollinate non-transformed plants and the resulting kernels were weighed and classified as transgene positive or negative (using PCR with ZOG1-specific primers). The data indicate that kernels on the same ear resulting from pollination with Ubi:ZOG1 pollen grains were significantly smaller than those resulting from pollination with non-transformed pollen (Fig. 7c).
Fig. 7.
Ear and kernel development. (a) Non-transformed (left) and hemizygous (right) ears from plants of the same age before pollination. (b) Weight of kernels from non-transformed ears pollinated with pollen from non-transformed (NT) or hemizygous Ubi::ZOG1 (P+) plants. (c) Weight of kernels from non-transformed ears pollinated with pollen from hemizygous transformants. Kernels were taken from the same ear and classified as P–×P– or P–×P+ by PCR with ZOG1-specific primers. Values for (b) are means (±SE) of all kernels of four ears. Values for (c) are means (±SD) of 15±2 kernels.
Discussion
Relevance of cytokinin determinations
Constitutive over-expression of ZOG1 resulted in elevated levels of O-glucosides, as expected. There was, however, no concomitant decrease in active cytokinins (bases and ribosides) in roots and an increase in overall active cytokinins in leaves. This indicates that the initial decrease in zeatin led to an increase in cytokinin biosynthesis. It should be noted though that cytokinin analyses can only determine the cytokinin composition in a large amount of tissue, whereas the distribution of cytokinins within specific tissues and cells may be most critical. As a consequence of ZOG1 over-expression, there are probably changes in the inter- and intracellular distribution of various active cytokinins and their metabolites due to differential expression of cytokinin biosynthetic genes. Thus it is possible that cytokinins are elevated at or near biosynthetic sites but not at cytokinin receptor sites.
Root development and plant architecture of Ubi:ZOG1 transformants are characteristic of cytokinin deficiency
Root development was enhanced in the Ubi:ZOG1 maize plants similar to that observed in Arabidopsis and Lotus japonicus transformed with maize cytokinin oxidase/dehydrogenase genes (Werner et al., 2001, 2003; Lohar et al., 2004; Kopečný et al., 2006). This suggests that the decrease in physiologically active cytokinins caused by increased glucosylation stimulated initiation and growth of root tips. Both the increased number of root tips and possible increased cytokinin biosynthesis may have led to the adjustment in free cytokinins to levels of non-transformed maize.
While root development was stimulated by the presence of the transgene, the opposite was the case with shoot development. The hemizygous Ubi:ZOG1 transformants were shorter and less robust than the controls and this size reduction was more severe in the homozygous plants, indicating dosage effects of the transgene. The phenotypes were similar to those of maize plants having a CKX transgene (N Brugière, unpublished results). Slower growth rates and reduced plant stature were also observed in dicots having decreased cytokinin levels or reduced cytokinin sensitivity due to increased expression of oxidases/dehydrogenases (Werner et al., 2003; Kopécný et al., 2006), deficiencies in biosynthesis (Miyawaki et al., 2006), or mutations in receptor genes (Higuchi et al., 2004; Nishimura et al., 2004; Riefler et al., 2006).
Observations regarding cytokinin effects on monocot leaf development are limited. Classical experiments involving exogenous cytokinin applications conducted with dicots demonstrated that cytokinins stimulated leaf expansion (Kuraishi and Okumura, 1956). However, transformants and mutants of Arabidopsis with either reduced cytokinin levels or deficient in cytokinin perception have smaller leaves, but the ratio between the length and width remains constant (Riefler et al., 2006; Werner et al., 2001, 2003). The leaf width of Ubi:ZOG1 hemizygous maize plants was significantly reduced by over-expression of ZOG1, while both the leaf width and length were decreased in Ubi:ZOG1 homozygous plants. Maize genetic mutants with smaller leaves usually have smaller meristems (Scanlon et al., 1996). This is also the case with the Ubi:ZOG1 transformants. The meristems of the homozygous Ubi:ZOG1 plants were slightly smaller than those of the hemizygous Ubi:ZOG1 plants which were smaller than those of the non-transformed control plants. The meristems of Arabidopsis plants over-expressing cytokinin oxidase/dehydrogenase genes were also smaller those of control plants (Werner et al., 2003; Kopečný et al., 2006). Another notable difference between the Ubi:ZOG1 transformants and control plants was the arrangement of leaf stomata. There were more single file stomata and fewer double file stomata in the transformants, which may be related to the reduced number of cell files between veins.
Chlorophyll formation and leaf senescence reflect higher levels of active cytokinins
Chlorophyll levels were increased and senescence was delayed in Ubi:ZOG1 transformant leaves. These characteristics are indicative of an increase in active cytokinins since exogenous cytokinins and up-regulation of cytokinin biosynthesis produce similar phenotypic changes (Richmond and Lang, 1957; Smart et al., 1991; Li et al., 1992; Gan and Amasino, 1995; Robson et al., 2004). The cytokinin analyses confirmed that active cytokinins (free bases and ribosides) are increased in leaves. Although it is possible that accumulation of extremely high amounts of O-glucosides can eventually lead to increased free bases due to the action of β-glucosidases, higher chlorophyll and delayed senescence were also reported for tobacco over-expressing the AtCKX2 gene of Arabidopsis (Mýtnová et al., 2006). A factor common to both the transgenic maize and tobacco is the generally slower development due to cytokinin O-glucosylation and degradation, respectively, thus prolonging the growth period. However, if this slower development is delaying maturation to a significant extent, time of flowering would also be greatly delayed. This was not the case since tassels emerged only about 2 d later in transformants than in untransformed controls, whereas leaf senescence showed a much longer delay (Fig. 4a). Both the ZOG1 and AtCKX2 leaves may respond to the resulting cytokinin deficiency by increasing cytokinin biosynthesis. Since biosynthesis takes place in the plastids (Kasahara et al., 2004; Sakakibara, 2006), there may be a localized increase of cytokinins in plastids. Whether higher cytokinin levels in chloroplasts could bring about an increase in chlorophyll is an interesting question. It would assume direct action of cytokinins in chloroplasts, which has never been demonstrated.
Indirect effects contributing to the higher chlorophyll in the transformed maize leaves can not be excluded. Non-transformed maize leaves expand very rapidly under natural summer conditions while those of the transformants remain much narrower, which could result in differential chlorophyll accumulation. Furthermore, the ratio between root and shoot growth is much higher in the transformants, possibly leading to higher accumulation of nutrients (including N) in the leaves. If this is the case, the much higher cytokinin levels in the mature transformant leaves may not be the cause, but rather a consequence, of the delayed senescence.
Increased cytokinin conjugation leads to defective tassel development and feminization of the lower florets
The drastic reduction in tassel size of the Ubi:ZOG1 transformants points to the importance of cytokinins in maize reproductive development. Normal tassels have 10–50 lateral branches which contain paired spikelets (Cheng and Pereddy, 1994). By contrast, the hemizygous Ubi:ZOG1 transformants had fewer and shorter lateral branches. Terminal florets were often missing. Maize plants transformed with the CKX1 gene driven by a pollen-specific promoter also had smaller tassels even though CKX1 expression was targeted to the pollen (Huang et al., 2003). A rice cultivar with reduced expression of a cytokinin oxidase/dehydrogenase gene and increased cytokinins had more reproductive organs (Ashikari et al., 2005). Although reproductive programmes of maize and rice are different in a number of aspects, these studies suggest that cytokinins have a positive effect on terminal flower development, most probably through its effects on meristem size.
Most intriguing was the occurrence of female florets at the lower end of the homozygous Ubi:ZOG1 tassels which formed seed when pollinated. Maize florets begin as complete bisexual flowers containing pistil and anther initials, but later the pistils abort (Cheng and Pareddy, 1994). The process of pistil abortion must have been inhibited in the homozygous Ubi:ZOG1 plants. Tasselseeds were found on all four transformed lines examined indicating that they were not a consequence of accidental insertion in one of the tasselseed (TS) loci but rather as the result of altered cytokinin levels or composition. Tasselseed in maize is a known phenomenon and several genetic mutations causing feminization of the tassel have been described (Irish and Nelson, 1989; Dellaporta and Calderon-Urrea, 1994). The most prominent phenotypes occur in recessive mutants ts1/ts1 and ts2/ts2, which display complete reversion from male to female inflorescences, with the failure of pistil abortion and the induction of stamen abortion (Emerson, 1920; Irish et al., 1994; DeLong et al., 1993). Somewhat less extreme is the dominant Ts5 mutant, which shows positional effects, with female florets occurring at the basal portion of the tassel (Nickerson and Dale, 1955), similar to the Ubi:ZOG1 transformants. The ts4/ts4 and ts6/ts6 mutants also show partial reversions (Dellaporta and Calderon-Urea, 1994). The TS2 gene was cloned and found to have homology to short chain alcohol dehydrogenases (DeLong et al., 1993). The maize transformants described here establish the first link between tasselseed and cytokinins.
Previously, cytokinins have been implicated in sex expression of a number of plants (Durand and Durand, 1994), but to our knowledge they have not been researched in connection with sex expression in maize tassels. The occurrence of tasselseed on the homozygous Ubi:ZOG1 transformants indicates that increased zeatin O-glucosylation and the associated disturbance in cytokinin homeostasis result in feminization. Whether this is due to a decrease in active cytokinins (as shown by the retarded shoot development and smaller meristem size) is difficult to assess. However, tasselseed formation was also observed on maize transformants over-expressing a cytokinin oxidase/dehydrogenase gene under the control of the Ubi promoter (N Brugière, unpublished results). In most plant species (although primarily dicots), cytokinins are feminizing, but exceptions are known (Durand and Durand, 1994). Interestingly, only the lower inflorescences showed this feminization whereas the more apical florets had the usual male characteristics. Thus there may be a gradient of active cytokinins or some polarity in signal distribution, causing female flowers to develop at the basal end of the tassel.
A possible alternative explanation for the tasselseed characteristic could reside in the changed composition of cytokinins. Not much is known about the influence of particular cytokinin metabolites on sex expression. In the most extensively studied system, Mercurialis, where a number of genes control sex expression, specific cytokinins have been linked with sex differentiation and male sterility. For instance, occurrence of trans-zeatin in apices was correlated with femaleness, while its riboside and nucleotide were more abundant in males (Durand and Durand, 1994). The presence of cis-zeatin and its riboside were associated with male sterility. Thus the changes in the ratios between cis and trans isomers in the Ubi::ZOG1 homozygotes, as a result of the preference of ZOG1 for trans-zeatin, may also be contributing to the abnormal tassel phenotypes. However, this is difficult to assess since any changes in cytokinin levels and composition could be the cause, but also merely the consequence, of altered development.
The influence of light intensity or quality on the tasselseed trait in Ubi:ZOG1 transformants indicates an interaction between the cytokinin and light signalling pathways. Such interactions have been previously observed. For instance, exogenous cytokinin caused de-etiolation of dark-grown Arabidopsis seedlings (Chory et al., 1994). Furthermore, Arabidopsis transformants and mutants with altered expression of the cytokinin type A response regulator ARR4 showed altered red light sensitivity (Sweere et al., 2001; To et al., 2004).
Pollen grains of hemizygous Ubi:ZOG1 plants appeared normal and germinated on artificial medium. Even the few anthers on homozygous transformants contained normal pollen. Also Arabidopsis transformants with constitutive over-expression of CKX genes produced fewer but still functional pollen grains (Werner et al., 2003); however, maize plants over-expressing CKX1 via a pollen-specific promoter (pZtap) showed pollen sterility (Huang et al., 2003).
Female inflorescence development is normal but seeds are smaller in Ubi:ZOG1 plants
The female inflorescences are not altered in Ubi:ZOG1 maize plants indicating that the changes in cytokinin levels have stronger effects on male flower development. Also, no obvious abnormalities in the flowers were observed in transgenic tobacco over-expressing ZOG1 (Martin et al., 2001a) even though tobacco flowers are derived from the terminal bud. Flower abnormalities were also absent from Arabidopsis plants constitutively over-expressing CKX genes (Werner et al., 2003). However, when cytokinin levels were increased through senescence-induced expression of the Agrobacterium IPT gene, the pistil was retained in the lower floret and fused kernels were formed (Young et al., 2004).
Until recently, very few studies have addressed the effects of cytokinin on seed size. Transgenic Arabidopsis with decreased cytokinin levels or sensitivity produced fewer but larger seeds (Werner et al., 2003; Kopečný et al., 2006; Riefler et al., 2006). However, the maize seeds with increased cytokinin conjugation were smaller than non-transformed seeds from the same ears when control plants were pollinated with pollen from hemizygous Ubi:ZOG1 transformants. It should be noted though that the increased seed size in Arabidopsis was associated with a decrease in the number of seeds, while seed fill of the maize cobs was complete. Moreover, maize and Arabidopsis seed development can not be compared due to the fact that maize kernels are largely endosperm while Arabidopsis seeds consist mainly of embryos.
Implications of high levels of cis isomers
In the present study, the predominant cytokinin in non-transformed maize leaves was the O-glucoside of cis-zeatin. In a previous study, cis-zeatin and its derivatives were found to be the major components in maize roots and stems (Veach et al., 2003). Levels of cis isomers were also very high in kernels although lower than the trans counterparts (Veach et al., 2003). Maize has a cytokinin glucosyltransferase with a preference for cis-zeatin over trans-zeatin (Martin et al., 2001b). In addition, a maize cytokinin receptor, ZmHK1, with high affinity for cis-zeatin and trans-zeatin has been identified (Yonekura-Sakakibara et al., 2004). These findings suggest that cis-zeatin is an active cytokinin in tissues where ZmHK1 is expressed.
A related issue is the possible origin of cis-zeatin. The presence of a hydroxylase for the synthesis of cis-zeatin, similar to the hydroxylase found in Arabidopsis for the formation of trans-zeatin (Takei et al., 2004), is a distinct possibility. Recent findings in Arabidopsis (Miyawaki et al., 2006) favoured tRNA degradation rather than de novo synthesis as the source of cis-zeatin. Maize may be different from Arabidopsis in that the cis-zeatin level is too high to be accounted for solely by tRNA breakdown. In addition, the presence in maize of a receptor responsive to cis-zeatin and a cis-specific glucosyltransferase indicates species-specific pathways, supporting direct synthesis of cis-zeatin in monocots.
Conclusions
The changes in plant architecture associated with ZOG1 over-expression are consistent with the reduction of active cytokinins, but chlorophyll levels and retention seem to reflect increases in cytokinins, as supported also by the cytokinin analyses. The most interesting effect of ZOG1 over-expression was the feminization of tassel floret development. This novel observation provides a link between cytokinins and sex-specific floral development in maize.
Supplementary data
A supplementary figure with specifics of the construct used for transformation is available at JXB online.
Supplementary Material
Acknowledgments
We thank K Cook for her assistance with microtome sectioning. We thank M Rossman and C Pereira for their help with the statistical analyses. This work was supported by the National Science Foundation under Grant No. 0514024 and by the Ministry of Education, Youth and Sports of the CR under project no. ME868.
References
- Armstrong DJ. Cytokinin oxidase and the regulation of cytokinin degradation. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton: CRC Press; 1994. pp. 139–154. [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- Barnabas B. Preservation of maize pollen. In: Bajaj YPS, editor. Biotechnology in agriculture and forestry, Vol. 25. Maize. Berlin: Springer-Verlag; 1994. pp. 607–618. [Google Scholar]
- Block M, Botterman J, Vandewiele M, Dockx J, Thoen C, Gossele V, Movva N, Thompson C, Montagu M, Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO Journal. 1987;6:2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA, Zeevaart JAD. The compartmentation of abscisic acid and β-D-glucopyranosyl abscisate in mesophyll cells. Plant Physiology. 1985;79:719–722. doi: 10.1104/pp.79.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzobohatý B, Moore I, Kristoffersen P, Bakó L, Campos N, Schell J, Palme K. Release of active cytokinin by a β-glucosidase localized to the maize root meristem. Science. 1993;262:1051–1054. doi: 10.1126/science.8235622. [DOI] [PubMed] [Google Scholar]
- Cheng PC, Pareddy DR. Morphology and development of the tassel and ear. In: Freeling M, Walbot V, editors. The maize handbook. New York: Springer-Verlag; 1994. pp. 37–47. [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M. A role for cytokinins in de-etiolation in Arabidopsis. Plant Physiology. 1994;104:339–347. doi: 10.1104/pp.104.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Molecular Biology. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Dean JV, Shah RP, Mohammed LA. Formation and vacuolar localization of salicylic acid glucose conjugates in soybean cell suspension cultures. Physiologia Plantarum. 2003;118:328–336. [Google Scholar]
- Dellaporta SL, Calderon-Urrea A. The sex determination process in maize. Science. 1994;266:1501–1505. doi: 10.1126/science.7985019. [DOI] [PubMed] [Google Scholar]
- DeLong A, Calderon-Urrea A, Dellaporta SL. Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage-specific floral organ abortion. Cell. 1993;74:757–768. doi: 10.1016/0092-8674(93)90522-r. [DOI] [PubMed] [Google Scholar]
- Dixon SC, Martin RC, Mok MC, Shaw G, Mok DWS. Zeatin glycosylation enzymes in Phaseolus: isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris. Plant Physiology. 1989;90:1316–1321. doi: 10.1104/pp.90.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand R, Durand B. Cytokinins and reproductive organogenesis in Mercurialis. In: Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Raton: CRC Press; 1994. pp. 295–304. [Google Scholar]
- Emerson RA. Heritable characters in maize. II. Pistillate flowered maize plants. Journal of Heredity. 1920;11:65–76. [Google Scholar]
- Falk A, Rask L. Expression of a zeatin-O-glucoside-degrading β-glucosidase in Brassica napus. Plant Physiology. 1995;108:1369–1377. doi: 10.1104/pp.108.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusseder A, Ziegler P. Metabolism and compartmentation of dihydrozeatin exogenously supplied to photoautotrophic suspension cultures of Chenopodium rubrum. Planta. 1988;173:104–109. doi: 10.1007/BF00394494. [DOI] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez JL, Ohlrogge JB, Rappaport L. Differential compartmentation of gibberellin A1 and its metabolites in vacuoles of cowpea and barley leaves. Plant Physiology. 1981;68:865–867. doi: 10.1104/pp.68.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ML, Passas HJ, Smith LG. Clonal analysis of epidermal patterning during maize leaf development. Developmental Biology. 1999;216:646–658. doi: 10.1006/dbio.1999.9429. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Pischke M, Mähönen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences, USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Lim E-K, Higgins GS, Bowles DJ. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. Journal of Biological Chemistry. 2004;279:47822–47832. doi: 10.1074/jbc.M409569200. [DOI] [PubMed] [Google Scholar]
- Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, Malloy KP, Ness LA. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiology. 2003;131:1270–1282. doi: 10.1104/pp.102.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients. Plant Physiology. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish EE, Langdale TM, Nelson T. Interactions between tassel seed genes and other sex determining genes in maize. Developmental Genetics. 1994;15:155–171. [Google Scholar]
- Irish EE, Nelson T. Sex determination in monoecious and dioecious plants. The Plant Cell. 1989;1:737–744. doi: 10.1105/tpc.1.8.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H. Distinct isoprenoid origins of cis- and trans-zeatin biosynthesis in Arabidopsis. Journal of Biological Chemistry. 2004;279:14049–14054. doi: 10.1074/jbc.M314195200. [DOI] [PubMed] [Google Scholar]
- Kopečný D, Tarkowski P, Majira A, Bouchez-Mahiout I, Nogué F, Laurière M, Sandberg G, Laloue M, Houba-Hérin N. Probing cytokinin homeostasis in Arabidopsis thaliana by constitutively overexpressing two forms of the maize cytokinin oxidase/dehydrogenase 1 gene. Plant Science. 2006;171:114–122. [Google Scholar]
- Kristoffersen P, Brzobohatý B, Höhfeld I, Bako L, Melkonian M, Palme K. Developmental regulation of the maize Zm-p60.1 gene encoding a β-glucosidase located to plastids. Planta. 2000;210:407–415. doi: 10.1007/pl00008149. [DOI] [PubMed] [Google Scholar]
- Kuraishi S, Okumara FS. The effect of kinetin on leaf growth. Botanical Magazine. 1956;69:300–306. [Google Scholar]
- Lehmann H, Glund K. Abscisic acid metabolism: vacuolar/extravacuolar distribution of metabolites. Planta. 1986;168:559–562. doi: 10.1007/BF00392276. [DOI] [PubMed] [Google Scholar]
- Letham DS. Regulators of cell division in plant tissues. XII. A cytokinin bioassay using excised radish cotyledons. Physiologia Plantarum. 1971;25:391–396. [Google Scholar]
- Letham DS, Palni LMS, Tao G-Q, Gollnow BI, Bates CM. Regulators of cell division in plant tissues. XXIX. The activities of cytokinin glucosides and alanine conjugates in cytokinin bioassays. Plant Growth Regulation. 1983;2:103–115. [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Developmental Biology. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DMcK. Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbiosis. The Plant Journal. 2004;38:203–214. doi: 10.1111/j.1365-313X.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- Martin RC, Martin RR, Mok MC, Mok DWS. A monoclonal antibody specific to zeatin O-glycosyltransferases of Phaseolus. Plant Physiology. 1990;94:1290–1294. doi: 10.1104/pp.94.3.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok DWS, Mok MC. Development of transgenic tobacco harboring a zeatin O-glucosyltransferase gene from Phaseolus. In Vitro Cellular and Developmental Biology–Plant. 2001a;37:354–360. [Google Scholar]
- Martin RC, Mok MC, Habben JE, Mok DWS. A cytokinin gene from maize encoding an O-glucosyltransferase specific to cis-zeatin. Proceedings of the National Academy of Sciences, USA. 2001b;98:5922–5926. doi: 10.1073/pnas.101128798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DWS. Isolation of a cytokinin gene, ZOG1, encoding zeatin O-glucosyltransferase of Phaseolus lunatus. Proceedings of the National Academy of Sciences, USA. 1999a;96:284–289. doi: 10.1073/pnas.96.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DWS. A gene encoding the cytokinin enzyme zeatin O-xylosyltransferase of Phaseolus vulgaris. Plant Physiology. 1999b;120:553–557. doi: 10.1104/pp.120.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw BA, Horgan R. Cytokinin oxidase from Zea mays kernels and Vinca rosea crown-gall tissue. Planta. 1983;159:30–37. doi: 10.1007/BF00998811. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Mok MC, editors. Cytokinins: chemistry, activity, and function. Boca Rotan, FL: CRC Press; 1994. [Google Scholar]
- Mok MC, Martin RC, Mok DWS, Shaw G. Cytokinin activity, metabolism and function in Phaseolus. In: Kaminek M, Mok DWS, Zazimalova E, editors. Physiology and biochemistry of cytokinins in plants. The Hague: SPB Academic Publishers; 1992. pp. 41–46. [Google Scholar]
- Mok MC, Martin RC, Dobrev PI, Vaňková R, Ho PS, Yonekura-Sakakibara K, Sakakibara H, Mok DWS. Topolins and hydroxylated thidiazuron derivatives are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition. Plant Physiology. 2005;137:1057–1066. doi: 10.1104/pp.104.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok MC, Mok DWS, Armstrong DJ. Differential structure–activity relationships in Phaseolus. Plant Physiology. 1978;61:72–75. doi: 10.1104/pp.61.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Planarum. 1962;15:473–497. [Google Scholar]
- Mýtnová Z, Haisel D, Wilhelmová N. Photosynthesis and protective mechanisms during ageing in transgenic tobacco leaves with over-expressed cytokinin oxidase/dehydrogenase and thus lowered cytokinin content. Photosynthetica. 2006;44:599–605. [Google Scholar]
- Nickerson NH, Dale EE. Tassel modifications in Zea mays. Annals of the Missouri Botanical Garden. 1955;42:195–212. [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. The Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond AE, Lang A. Effect of kinetin on protein content and survival of detached Xanthium leaves. Science. 1957;125:650–651. [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Donnison IS, Wang K, Frame B, Pegg SE, Thomas A, Thomas H. Leaf senescence is delayed in maize expressing the Agrobacterium IPT gene under the control of a novel maize senescence-enhanced promoter. Plant Biotechnology Journal. 2004;2:101–112. doi: 10.1046/j.1467-7652.2004.00054.x. [DOI] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Physiology and Plant Molecular Biology. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Schneeberger RG, Freeling M. The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development. 1996;122:1683–1691. doi: 10.1242/dev.122.6.1683. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Sandermann H., Jr Specific localization of β-D-glucoside conjugates of 2,4-dichlorophenoxyacetic acid in soybean vacuoles. Zeitschrift für Naturforschung. 1982;37:772–777. [Google Scholar]
- Skoog F, Armstrong DJ. Cytokinins. Annual Review of Plant Physiology. 1970;21:359–384. [Google Scholar]
- Smart CM, Scofield SR, Bevan MW, Dyer TA. Delayed leaf senescence in tobacco plants transformed with tmr, a gene for cytokinin production in Agrobacterium. The Plant Cell. 1991;3:647–656. doi: 10.1105/tpc.3.7.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T. Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant and Cell Physiology. 2004;45:1299–1305. doi: 10.1093/pcp/pch132. [DOI] [PubMed] [Google Scholar]
- Strnad M. The aromatic cytokinins. Physiologia Plantarum. 1997;101:674–688. [Google Scholar]
- Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Baurle I, Kudla J, Nagy F, Schafer E, Harter K. Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science. 2001;294:1108–1111. doi: 10.1126/science.1065022. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sugiyama T. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. Journal of Biological Chemistry. 2004;279:41866–41872. doi: 10.1074/jbc.M406337200. [DOI] [PubMed] [Google Scholar]
- To JP, Haberer G, Ferreira FJ, Deruere J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. The Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Mok DWS, Mok MC, Shaw G. Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proceedings of the National Academy of Sciences, USA. 1987;84:3714–3717. doi: 10.1073/pnas.84.11.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veach YK, Martin RC, Mok DWS, Malbeck J, Vankova R, Mok MC. O-Glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiology. 2003;131:1374–1380. doi: 10.1104/pp.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H. Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiology. 2004;134:1654–1661. doi: 10.1104/pp.103.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Giesler-Lee J, Gallie DR. Senescence-induced expression of cytokinin reverses pistil abortion during maize flower development. The Plant Journal. 2004;38:910–922. doi: 10.1111/j.1365-313X.2004.02093.x. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Gu W, Cai T, Tagliani LA, Hondred D, Bond D, Krell S, Rudert ML, Bruce WB, Pierce DA. Molecular analysis of T0 plants transformed by Agrobacterium and comparison of Agrobacterium-mediated transformation with bombardment transformation in maize. Maize Genetics Cooperative Newsletter. 1998;72:34–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.