Abstract
Protein bodies (PB) are stable polymers naturally formed by certain seed storage proteins within the endoplasmic reticulum (ER). The human immunodeficiency virus negative factor (Nef) protein, a potential antigen for the development of an anti-viral vaccine, is highly unstable when introduced into the plant secretory pathway, probably because of folding defects in the ER environment. The aim of this study was to promote the formation of Nef-containing PB in tobacco (Nicotiana tabacum) leaves by fusing the Nef sequence to the N-terminal domains of the maize storage protein γ-zein or to the chimeric protein zeolin (which efficiently forms PB and is composed of the vacuolar storage protein phaseolin fused to the N-terminal domains of γ-zein). Protein blots and pulse–chase indicate that fusions between Nef and the same γ-zein domains present in zeolin are degraded by ER quality control. Consistently, a mutated zeolin, in which wild-type phaseolin was substituted with a defective version known to be degraded by ER quality control, is unstable in plant cells. Fusion of Nef to the entire zeolin sequence instead allows the formation of PB detectable by electron microscopy and subcellular fractionation, leading to zeolin–Nef accumulation higher than 1% of total soluble protein, consistently reproduced in independent transgenic plants. It is concluded that zeolin, but not its γ-zein portion, has a positive dominant effect over ER quality control degradation. These results provide insights into the requirements for PB formation and avoidance of quality-control degradation, and indicate a strategy for enhancing foreign protein accumulation in plants.
Keywords: Endoplasmic reticulum, protein accumulation, protein bodies, plant factories, zein
Introduction
Accumulation of foreign proteins in transgenic plants is markedly affected by the subcellular compartment where they are targeted (for recent reviews, see Fischer et al., 2004; Doran, 2006; Streatfield, 2007). When the aim is high protein yield, the natural compartment of residence is not always the best or the possible choice for several reasons. The natural location may be simply impossible to reproduce because it does not exist in plants (examples are lysosomes, secretory granules, and the blood serum extracellular environment). On other occasions, a change in the tissue of accumulation results in lower stability, because of tissue- and development-specific features of certain compartments, vacuoles being the best known example (Wandelt et al., 1992; Tabe et al., 1995; Frigerio et al., 1998). Finally, there is high variability in yield of different proteins with the same natural or expected location, suggesting, for a given location, protein-specific half-lives that can sometimes be very short. For example, accumulation in tobacco leaves of the secreted proteins α-lactalbumin and phytase, both expressed under the control of the 35S promoter, was about 0.2% and 14% of total soluble protein, respectively, strongly suggesting highly different stability in the apoplast (Verwoerd et al., 1995; Takase and Hagiwara, 1998). The search for the best compartment has thus emerged as a major issue in the plant production of foreign proteins (Fischer et al., 2004; Doran, 2006; Streatfield, 2007). Accumulation in the lumen of the endoplasmic reticulum (ER) has often resulted in improved production (Wandelt et al., 1992; Tabe et al., 1995; Stöger et al. 2000; Ramírez et al., 2002; Vaquero et al., 2002). Through a biosynthetic pathway termed the secretory pathway, the ER is functionally linked to the Golgi complex, the different vacuoles (lysosomes in animal cells), the plasma membrane, and the extracellular environment. The vast majority of proteins destined to any of the above locations (collectively termed secretory proteins) are first co-translationally inserted into the ER and then reach the final destination by vesicular traffic or direct connections, the major route being from the ER to the Golgi complex and from here to vacuoles or secretion (Vitale and Denecke, 1999; Jürgens, 2004). Secretory proteins not anchored to membranes are secreted if they do not have additional signals besides the transient signal peptide for translocation into the ER lumen (Vitale and Denecke, 1999). The other destinations, including the ER itself as a compartment of final residence, need sorting signals. Soluble ER residents have a C-terminal tetrapeptide, in most cases KDEL or HDEL, which allows recycling from the Golgi complex back into the ER, through interactions with a Golgi-located receptor (Vitale and Denecke, 1999). Addition of the tetrapeptide to recombinant proteins has allowed efficient ER retention in many cases and, as mentioned above, relatively high accumulation of foreign proteins, often one or two orders higher than that obtained by expressing the wild-type forms (Wandelt et al., 1992; Tabe et al., 1995; Stöger et al., 2000; Ramírez et al., 2002; Vaquero et al., 2002). This indicates that the ER has low hydrolytic activity, which in a way is an expected feature because of its role in the folding and assembly of newly synthesized proteins. It should, however, be noticed that the ER also recognizes, and delivers for degradation, defective, misfolded newly synthesized secretory proteins, through a finely regulated mechanism termed quality control (Sitia and Braakman, 2003; Vitale and Ceriotti, 2004); this is probably the reason why addition of KDEL does not always lead to high accumulation (Frigerio et al., 2001; Patel et al., 2007; Yang et al., 2007).
The K/HDEL system is common to all eukaryotes but a number of seed storage proteins of the prolamin class use a different, not yet fully clarified, ER localization mechanism. These proteins form large insoluble polymers, termed protein bodies (PB), which stably accumulate within the ER (Shewry et al., 1995; Vitale and Ceriotti, 2004). Prolamins such as maize γ-zein also form stable PB when expressed in vegetative tissues of transgenic plants, indicating that PB formation does not require cereal seed-specific molecules besides the prolamin themselves (Geli et al., 1994); furthermore, the N-terminal domains of γ-zein can confer the ability to form PB when fused to the bean vacuolar storage phaseolin in the chimeric protein zeolin (Mainieri et al., 2004). Inter-chain disulphide bonds play an important role in PB formation and ER retention of γ-zein and zeolin (Geli et al., 1994; Pompa and Vitale, 2006). PB formation can lead to higher accumulation within the ER when compared with the addition of K/HDEL, possibly because of the exclusion from the normal turnover of ER residents (Mainieri et al., 2004). Therefore, the system is of biotechnological interest (Ludevid et al., 2005; Vitale and Pedrazzini, 2005).
In this study, an attempt was made to exploit PB formation to increase the plant accumulation of the human immunodeficiency virus negative factor (Nef) protein, a potential antigen for the development of an anti-viral vaccine (Titti et al., 2007). Nef is one of the four accessory proteins of the human immunodeficiency virus and is essential for viral replication (Das and Jameel, 2005). The major proportion of Nef is soluble in the cytosol, although about 20–30% of the protein is associated with the cytosolic side of membranes because of N-myristoylation and protein–protein interactions (Kaminchik et al., 1991; Bentham et al., 2006; Giese et al., 2006). A first effort to express Nef in the plant secretory pathway of tobacco leaf cells, by engineering an N-terminal signal peptide, revealed that the protein was translocated into the ER lumen but was less stable than the cytosolic form, possibly due to ER quality control (Marusic et al., 2007). It is shown here that the same γ-zein domains used to produce zeolin are unable to rescue Nef or a structurally defective form of phaseolin from degradation, whereas a fusion between Nef and the whole zeolin sequence successfully forms PB. These results indicate that the zein N-terminal domains are not sufficient to avoid quality control degradation, pointing to additional structural requirements for efficient PB formation, and show that zeolin fusions can be used to enhance the accumulation of foreign proteins that are unstable in the plant secretory pathway.
Materials and methods
The material used and described in this work is freely available for non-commercial purposes.
Recombinant DNA and tobacco transformation
The construct zein–Nef was produced as follows. The sequence encoding the signal peptide of γ-zein and the first 93 amino acids of the mature protein was amplified from the plasmid pBSKS.G1L (Bellucci et al., 1997) using primers 5′-GAGCTTGGATCCATGAGGGTGTTGCTCGTTGC-3′, containing a BamHI restriction site, and 5′-CGATTCGTCGACGGAACCTCCTCCACCGGAACCTCCTCCACCGGAACCTCCACCTCCCTGGCACGGGCTTGGATG-3′, containing a SalI restriction site and the sequence coding for the (GGGGS)3 flexible linker. The PCR product was inserted into BamHI/SalI-linearized pDHA vector (Tabe et al., 1995) within the expression cassette, under the control of 35S promoter, to generate the intermediate plasmid A. Nef cDNA (HIV-1 BH10 strain) was amplified from the second amino acid of the mature protein using as template the plasmid pSCNef 51(ARP#2015NIBSC-CFAR MRC) and primers 5′-GAGCTTGTCGACCTAGTACCAAGAGGTGGTGGCAAGTGGTCAAAAAGTAGTGT-3′, containing the sequence coding for the thrombin cleavage site (LVPRG), and 5′-CGATTCGCATGCTCATTATCAGCAGTTCTTGAAGTACTCCGGATGC-3′, containing a SphI restriction site downstream from three stop codons. The amplified DNA was inserted into the SphI/SalI-restricted intermediate plasmid A, to generate pDHAzein–Nef. For the production of transgenic plants, the fragment excised by EcoRI digestion of pDHAzein–Nef, including the 35S promoter, the sequence coding for the chimeric zein–Nef protein and 35S terminator, was introduced into the EcoRI site of the binary vector PBI121.1. Strain LBA4404 of Agrobacterium tumefaciens was transformed by electroporation and used to produce transgenic tobacco (Nicotiana tabacum) cv. Petit Havana SR1 as described (Pedrazzini et al., 1997). Briefly, leaf discs from Nicotiana tabacum cv. Petit Havana SR1 were transformed by co-cultivation with A. tumefaciens harbouring PBI121.1–zein–Nef. After co-cultivation, the leaf discs were grown at 25 °C under full light on the regeneration medium containing 250 mg l−1 cefotaxime and 100 mg l−1 kanamycin. Regenerated shoots of each explant were kept separated in order to guarantee regeneration of independent transformants. After 5 weeks, shoots were plated on half-strength Murashige and Skoog salts, 100 mg l−1 kanamycin, and 250 mg l−1 cefotaxime until the new plants developed. Transformed plants were grown at 25 °C in 16 h of light in axenic culture in Magenta GA-7 vessels (Sigma) without antibiotics and propagated every 5–6 weeks. Twenty-five transgenic plants (labelled with numbers from 1 to 25) were selected for analysis.
The Nef–zein fusion was assembled through amplification of the portion of γ-zein present in zeolin (Mainieri et al., 2004) using primers 5′-TGTGGGGGATCCGGAGGGGGCGGTTCA-3′, containing a BamHI restriction site, and 5′-TGTGCAGTCGACCTACTGGCACGGGCTTGGATGCGG-3′, containing a SalI restriction site and part of the flexible linker present also in the zein–Nef construct described above. The PCR product was inserted into BamHI/SalI-restricted pDHA, to generate intermediate plasmid B. The Nef coding sequence endowed with the N-terminal signal peptide of the tobacco PR1 protein was amplified using as template the construct pDAP27E (Marusic et al., 2007) with the primers 5′-TGTGCAGGATCCATGGGATTTTTTCTC-3′, containing a BamHI site, and 5′-TGTGCAGGATCCCCCACCTCCCTTGTCGTCGTCGTCCTTGTAGTC-3′ containing a BamHI site and the coding sequences of the remaining portion of the flexible linker and of the Flag epitope DYKDDDDK. The BamHI-restricted PCR product was inserted into the BamHI-linearized intermediate plasmid B, to generate pDHANef–zein. To produce transgenic plants, the fragment excised by EcoRI digestion of pDHANef–zein was inserted into the binary vector pGreenII. Strain GV3101 of Agrobacterium tumefaciens was transformed by electroporation and used to produce transgenic tobacco (Nicotiana tabacum) cv. Petit Havana SR1 as described above for plasmid PBI121.1–zein–Nef. Twenty transgenic plants (labelled with numbers from 1 to 20) were selected for further analysis.
The zeolin–Nef construct was obtained as follows. The zeolin coding sequence was amplified from plasmid pGA470zeolin (Mainieri et al., 2004) with primers 5′-GAGCTACCCGGGATGTGAGAGCAAGGGTTCCA-3′, containing a SmaI site, and 5′-GGCTCTGTCGACCTGGCACGGGCTTGGATGCGG-3′, containing a SalI site. The SmaI/SalI-restricted amplification product was inserted into the pDHA vector that had first been BamHI-linearized, blunt ended by Klenow polymerase, and SalI-restricted; the resulting construct was intermediate plasmid C. The Nef sequence was amplified from pDHA–zein–Nef with primers 5′-GGCTCAGTCGACGGAGGTGGAGGTTCCGGTGGAGGAGGTTCCGGTGGAGGAGGTTCCGTGGACCTAGTACCAAGA-3′, containing a SalI site and the coding sequence for the linker (GGGGS)3, and 5′-CGATTCGCATGCTCACTTATCGTCGTCATCCTTGTAATCGCAGTTCTTGAAGTACTCCGGATG-3′, containing a SphI site and the coding sequence for the Flag epitope. The amplification product was inserted into the SalI/SphI intermediate plasmid C and termed pDHAzeolin–Nef. For stable transformation of tobacco plants, the expression cassette containing the chimeric protein under the control of the 35S promoter was excised from pDHAzeolin–Nef by NcoI digestion, blunt-ended by Klenow polymerase, digested with HindIII and inserted into the SmaI/HindIII linearized binary vector pGreenII. The resulting plasmid was used to transform Agrobacterium and produce transgenic tobacco as described above for plasmids PBI12.1–zein–Nef and pGreenII–Nef–zein. Twenty transgenic plants (labelled with letters) were selected for further analysis.
To produce zeolinΔ364, the phaseolin portion was amplified from plasmid pDHAT343F (Pedrazzini et al., 1997) with primers 5′-TGTGGGGGGATCCATGATGAGAGCAAGGGTTCCAC-3′, containing a BamHI site and 5′-TCTCCCCGGATCCCCCACCTCCGACATTGTCCGTCTTACCTG-3′, containing part of the sequence coding for the (GGGGS)3 flexible linker and a BamHI site. The amplified sequence was digested with BamHI and inserted into plasmid pDHAzeolin (Mainieri et al., 2004) from which the original BamHI insert had been removed. The new plasmid was named pDHAzeolinΔ364 and was used for transient expression in protoplasts. All constructs were sequenced to confirm correct amplification and ligation.
mRNA analysis
Total leaf RNA was extracted with Nucleo Spin_RNA Plant Kit (Macherey-Nagel, Düren, Germany). RNA (7 μg) was electrophoretically fractionated in 1.4% formaldehyde agarose gels and transferred to Hybond-N nylon membranes (GE Healthcare, Chalfont St Giles, Bucks, UK) according to the manufacturer's instructions. The sequence encoding the γ-zein N-terminal fragment of zeolin was used as a probe. Hybridization was performed as indicated by the membrane supplier with 32P-labelled probes and the Ready-To-Go™ kit (GE Healthcare).
Leaf protein extraction, subcellular fractionation, and protein gel blot analysis
The following procedures were as described in Mainieri et al. (2004): extraction of total proteins from young (4–7 cm long) leaves in reducing conditions in the presence of non-ionic detergent; leaf homogenation in the absence of detergent and presence of sucrose followed by isopycnic sucrose gradient ultracentrifugation to separate subcellular compartments.
For protein blot, after SDS–PAGE proteins were electro-transferred on Hybond-P membrane (Amersham Bioscience, Little Chalfont, Bucks, UK, or GE Healthcare) and probed with anti γ-zein (Bellucci et al., 2000; 1:1000 dilution) or anti-phaseolin (Pedrazzini et al., 1997; 1:1500 dilution) rabbit polyclonal antisera, anti-FLAG rabbit polyclonal antibodies (Sigma; 1:1200 dilution), or anti-Nef monoclonal antibody EVA3067.4 (National Institute for Biological Standards and Control – Centralized Facility for Aids Reagents, UK Medical Research Council; 1:1000 dilution). Detection was performed using the Super-Signal West Pico Chemioluminescent Substrate (Pierce Chemical, Rockford, IL, USA), following the manufacturer's instructions. Protein molecular weight markers (Fermentas, Vilnius, Lithuania) or prestained protein marker, broad range (New England Biolabs) were used as molecular mass markers. Quantitation of zeolin–Nef expressed in leaves was repeated three times and was carried out by SDS–PAGE using dilutions of leaf extracts prepared in reducing conditions (from 0.75 μg to 5 μg of total protein) and dilutions of carboxy-terminal FLAG-BAP™ fusion protein (Sigma; from 8 ng to 32 ng), followed by protein blot with anti-FLAG antibody (Sigma; 1:1000 dilution). Protein band intensities (arbitrary units) were measured with the public-domain ImageJ software (Rasband WS, Image J; US National Institute of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/, 1997–2005).
Protoplast preparation, pulse–chase labelling, and immunoprecipitation
Protoplasts were prepared from young leaves of transgenic plants and subjected to pulse–chase labelling with Pro-Mix (a mixture of [35S]Met and [35S]Cys; Amersham Biosciences), in the absence or presence of brefeldin A (BFA; Roche) as described by Pedrazzini et al. (1997). For transient protein expression, protoplasts were isolated from small leaves of wild-type tobacco SR1 plants grown in axenic conditions and subjected to polyethylene glycol-mediated transfection as described (Pedrazzini et al., 1997) using 40 μg of plasmid. After overnight recovery, protoplasts were subjected to pulse–chase labelling as described above.
Immunoprecipitation of radioactive proteins from protoplast homogenates in the presence of 4% (v/v) 2-mercaptoethanol was performed as described (Pompa and Vitale, 2006) using the anti-phaseolin, anti γ-zein, or anti-FLAG antisera and antibodies mentioned above or the anti-Nef monoclonal antibody ARP3108 (National Institute for Biological Standards and Control – Centralized Facility for Aids Reagents, UK Medical Research Council). Protein A–Sepharose (for polyclonal antisera raised in rabbits) or protein G–Sepharose (for the anti-Nef monoclonal antibody) were used in the immunoprecipitation protocols. Immunoprecipitation of insoluble material of protoplast homogenates was performed as follows. After the first centrifugation of the immunoprecipitation protocol (performed to eliminate insoluble material before addition of antibodies) the insoluble precipitate was resuspended in 2% (w/v) SDS, 4% 2-mercaptoethanol, 150 mM TRIS-HCl pH 7.5, and heated for 5 min at 95 °C. Unbound SDS was then sequestred upon addition of Triton X-100 to 2% and bovine serum albumin (Sigma) to 0.4%; TRIS-HCl pH 7.5, NaCl, EDTA, and gelatin were then adjusted to a final concentration of 125 mM, 150 mM, 0.83 mM, 0.2%, respectively, to restore the immunoprecipitation conditions before adding the antibodies. As a control for the efficiency of immunoprecipitation after the denaturation procedure, an aliquot of pulse-labelled protoplasts was directly subjected to the denaturation protocol.
The immunoprecipitates were analysed by SDS-PAGE. Rainbow 14C-methylated proteins (Sigma-Aldrich) were used as molecular mass markers. After electrophoresis, gels were treated with 2,5-diphenyloxazole dissolved in dimethyl sulphoxide, dried, and exposed for fluorography.
Endoglycosidase H digestion
For digestion of total leaf extracts with endoglycosidase H (Endo Hf, New England Biolabs, Beverly, MA, USA), proteins were extracted from small leaves in the presence of 2% 2-mercaptoethanol. After addition of glycoprotein denaturing buffer (New England Biolabs), the solution was heated for 5 min at 95 °C and then adjusted to 1× G5 reaction buffer (New England Biolabs) and split into two equal aliquots. Four thousand units (4 μl) of Endo H or 4 μl of water (control) were added and digestion was performed for 1 h at 37 °C before analysis by SDS-PAGE and protein blot. For endoglycosidase H digestion of immunoprecipitated proteins after pulse–chase, the resin with bound antigen–antibody complex was washed twice with ice-cold water, resuspended in 50 μl of glycoprotein denaturing buffer, and heated 10 min at 95 °C. After centrifugation for 1 min at 13 700 g, 20 °C, the supernatant (45 μl) was adjusted to 1× G5 reaction buffer, split into two equal aliquots, and treated with endoglycosidase H as described above, but using 2000 units of enzyme or 2 μl of water. Proteins were then adjusted for SDS-PAGE and fluorography.
Electron microscopy
Fixation and immunoelectron microscopy of young tobacco leaves were performed as described in Mainieri et al. (2004). Grids were blocked and then incubated with anti-phaseolin (1:1000 dilution), anti-γ-zein (Bellucci et al., 2000; 1:400 dilution), anti-FLAG (1:1000 dilution), or preimmune (1:400 dilution) antiserum for 1 h at room temperature. After washing, the sections were incubated for 45 min with goat anti-rabbit secondary antibody (1:25 dilution) conjugated with 15 nm gold particles (BBInternational, Cardiff, UK). The grids were washed and examined with an electron microscope (EM 400 T; Philips, Eindhoven, The Netherlands). Untransformed tobacco leaves immunolabelled with the antisera showed no labelling.
Results
A fusion between the N-terminal portion of γ-zein and Nef is unstable
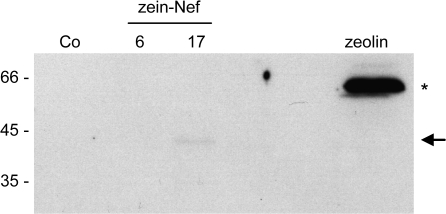
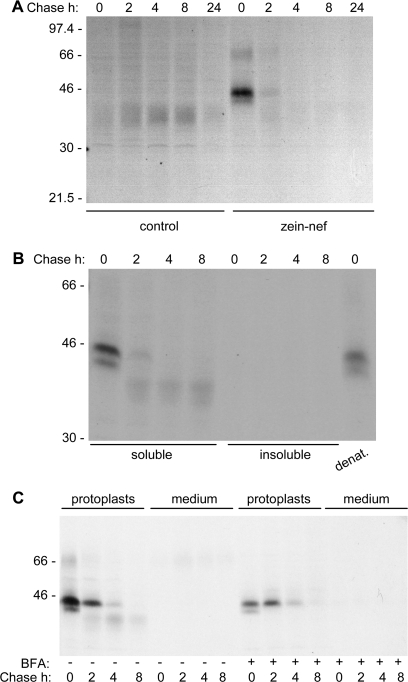
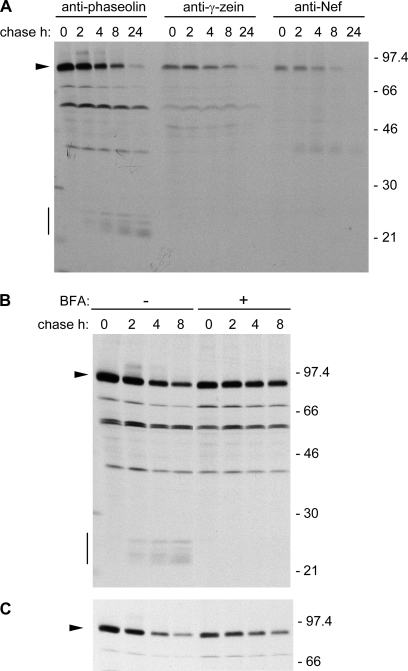
Zeolin is composed of the whole sequence of phaseolin T343F, including its signal peptide for ER translocation, followed by the (GGGGS)3 unstructured linker and 89 amino acids of γ-zein starting from the fifth residue after its signal peptide cleavage site (Mainieri et al., 2004). The zein portion includes the proline repeat domain and a shorter proline-rich non-repeated domain; overall, it contains six of the total 15 Cys residues of γ-zein. It was tested whether this zein portion promotes PB formation when fused to Nef. The ER oxidizing environment is necessary for the formation of disulphide bonds and for the possible membrane interactions of PB but, unlike phaseolin, the Nef precursor does not contain a signal peptide for translocation into the ER. The first 112 amino acids of the γ-zein precursor, which include the signal peptide and end at the same residue that constitutes the C-terminus of zeolin, were therefore used as the N-terminal portion of the new chimeric protein, followed by the (GGGGS)3 linker, the dipeptide VD (encoded by a SalI restriction site introduced for cloning strategies), the LVPRG thrombin cleavage site and the sequence of Nef without its initiator methionine (Fig. 1; zein–Nef). The thrombin cleavage site was introduced to allow in vitro removal of the extra sequences from Nef in downstream processing. Zein–Nef was expressed (like the other Nef constructs investigated in this work) in transgenic tobacco under the constitutive cauliflower mosaic virus 35S promoter. Twenty-five plants that were positive for the presence of variable levels of zein–Nef mRNA were obtained (not shown). The presence of the recombinant protein was first tested by protein blot using extracts from young leaves (4–7 cm long) of two positive plants and a control wild-type plant. As a reference for recombinant protein accumulation, an extract of transgenic leaves expressing zeolin was analysed as well. A polypeptide migrating slightly faster than the 45 kDa molecular mass marker was recognized by anti-γ-zein antiserum in zein–Nef plant 17 and was absent in control leaves (Fig. 2, arrow); its amount was, however, very low when compared with that of zeolin (Fig. 2, asterisk), suggesting that zein–Nef is unstable. This was investigated by pulse–chase labelling of leaf protoplasts followed by immunoprecipitation with anti-Nef antibody. The protocol for immunoprecipitation involves a first centrifugation step, to discard insoluble contaminants before adding antibodies, but analysis of zeolin revealed that the inter-chain disulphide bonds that lead to assembly into PB make this protein progressively insoluble with time; as a result, zeolin is in large part or totally lost during the first centrifugation unless reduced (Mainieri et al., 2004; Pompa and Vitale, 2006). To avoid misinterpretation of the results, protoplast homogenization and immunoprecipitation were therefore performed in the presence of the reducing agent 2-mercaptoethanol. A major radioactive polypeptide around 45 kDa and a minor one with slightly lower molecular mass were specifically immunoprecipitated after a 1 h pulse from protoplasts prepared from leaves of plants expressing zein–Nef but not from those of a control plant transformed with the empty vector (Fig. 3A, 0 h chase; compare zein–Nef and control). A less-defined component of around 70 kDa could represent a small proportion of undenatured dimers, whereas a similarly less-defined component around 40 kDa, detected during the chase but almost undetectable at the end of the pulse, was also immunoprecipitated from control protoplasts and is therefore unrelated to Nef. Newly synthesized zein–Nef markedly decreased in amount after 2 h chase and was below the limit of detection after 4 h and at later chase-points (Fig. 3A). Immunoprecipitation of proteins present in the protoplast incubation medium indicated that the protein was not secreted (Fig. 3C, first eight lanes). Post-translational stable association with unknown molecules that mask zein–Nef antigenic sites, independently of disulphide-bond formation, could also lead to progressive decreased detection. To rule this out, the insoluble material precipitated upon the usual centrifugation that precedes antiserum addition was resuspended in the presence of SDS and denatured at 95 °C. After sequestration of the remaining free SDS, immunoprecipitation was performed with anti-Nef antibodies; no radioactive polypeptides were detected (Fig. 3B, insoluble). A similar SDS treatment of an aliquot of 0 h chase protoplast homogenate indicated that the procedure did not markedly affect the recognition by the anti-Nef antibody (Fig. 3B, denat.). Therefore, newly synthesized zein–Nef is rapidly degraded.
Fig. 1.
Amino acid sequences of the Nef constructs used in this work. Amino acids are shown using the single-letter code. The sequence of Nef, without the initiator methionine, is on light-grey background. The (GGGGS)3 flexible linker is on dark-grey background and the DYKDDDDK Flag epitope is in white on black background. In zein–Nef and zeolin–Nef, the LVPRG thrombin cleavage site is on dark-grey background. In zeolin–Nef, the zeolin sequence is underlined.
Fig. 2.
Zein–Nef accumulates to very low amounts in transgenic tobacco leaves. Proteins were extracted with reducing buffer from young leaves of plants expressing zein–Nef (lines 6 and 17, two independent transformants), zeolin, or wild-type tobacco (Co) and were analysed by SDS-PAGE followed by protein blot using anti-γ-zein antiserum. Extract containing 50 μg (wild-type and zein–Nef plants) or 10 μg (zeolin plant) of total protein was loaded in each lane. The positions of zein–Nef (arrow) and zeolin (asterisk) are marked on the right. Numbers on the left indicate the positions of molecular mass markers, in kilodaltons.
Fig. 3.
Zein–Nef is rapidly degraded. Protoplasts isolated from young leaves of transgenic tobacco expressing zein–Nef (B, C, and zein–Nef in A) or transformed with the empty vector pGA470 (control in A) were subjected to pulse-labelling with [35S]Met and [35S]Cys for 1 h followed by chase for the indicated times. Proteins were immunoprecipitated with anti-Nef antibodies from homogenates prepared as described below. (A) Protoplasts were homogenated with reducing buffer. (B) Protoplasts were homogenated with reducing buffer (soluble). The insoluble material of the first homogenation was solubilized with denaturating buffer (insoluble). As a control, protoplasts were also directly homogenized with denaturing buffer (denat.). (C) Pulse–chase was performed using protoplasts treated (+) or untreated (–) with brefeldin A (BFA). Both protoplasts and their incubation media were collected and homogenated with reducing buffer. Analysis was by SDS–PAGE and fluorography. In each panel, numbers on the left indicate the positions of molecular mass markers, in kilodaltons.
Degradation could be due to ER quality control (Sitia and Braakman, 2003). This operates thanks to a number of ER-resident folding helpers that also contribute to the retention of newly synthesized polypeptides in the ER until the correct conformation is acquired. Permanently defective polypeptides are eventually translocated back into the cytosol for degradation or possibly targeted to the vacuole for the same purpose. ER quality control is not inhibited by BFA, an inhibitor of normal protein traffic along the secretory pathway (for plant cells, see Pedrazzini et al., 1997). When pulse–chase was performed in the presence of BFA, an initial stabilization of zein–Nef was detected after a 2 h chase, which was, however, followed by rapid degradation (Fig. 3C). Therefore, BFA has little effect on the degradation of zein–Nef. The decrease in zein–Nef recovered at 0 h chase upon BFA treatment compared with the untreated control is due to the general inhibition of protein synthesis caused by the drug (Mellor et al., 1994).
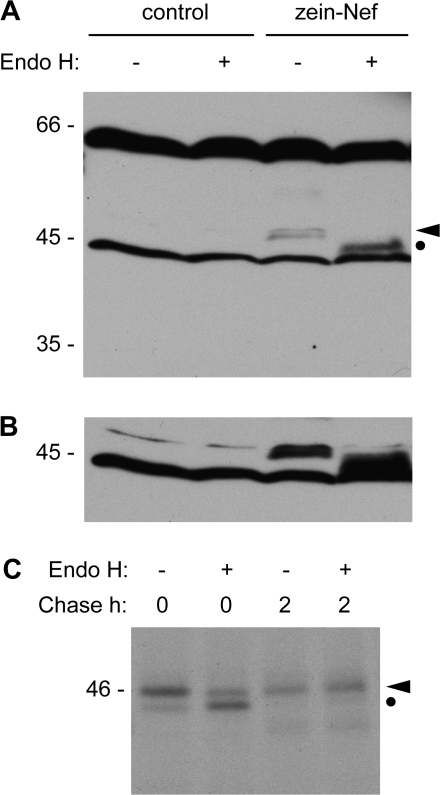
Protein disposal by ER quality control, in many cases, involves proteolysis by the ubiquitin–proteasome system, although alternative mechanisms exist (Sitia and Braakman, 2003; Donoso et al., 2005; Kruse et al., 2006). Proteasome inhibitors can be used to demonstrate degradation by this pathway, but they have substrate-specific effects because the relative contribution of the different active sites of the proteasome depends on the protein to be degraded (Kisselev et al., 2006). Thus, in plant cells the widely used compound clasto-lactacystin-β-lactone partially inhibits quality control degradation of the ricin A subunit but has no effect on a structurally defective form of phaseolin, in spite of the fact that both proteins undergo BFA-insensitive degradation (Di Cola et al., 2001; Nuttall et al., 2003). Treatment with clasto-lactacystin-β-lactone had no detectable effect on the degradation of zein–Nef during pulse–chase experiments (not shown). The pathway of degradation was therefore investigated taking advantage of the presence of two fortuitous N-glycosylation sites in Nef. These are irrelevant in wild-type Nef because N-glycosylation occurs in the ER lumen, but when the protein is introduced into the ER it is glycosylated (Marusic et al., 2007). It was reasoned that if zein–Nef was also glycosylated, one could investigate whether its glycans are modified by enzymes of the Golgi complex. Unmodified glycans can be removed in vitro by endoglysosidase H, but modifications occurring in the medial and late Golgi complex confer resistance to the enzyme. Total protein extracts from leaves of transgenic tobacco expressing zein–Nef or from wild-type tobacco were digested with endoglycosidase H and analysed by SDS–PAGE and protein blot with anti-Nef antibody. Digestion with endoglycosidase H caused a shift in the molecular mass of zein–Nef (Fig. 4A, glycosylated and deglycosylated zein–Nef are marked by the arrowhead and dot, respectively; notice that the relatively long exposure needed to clearly detect the small amount of zein–Nef present in transgenic tobacco resulted in the detection of additional cross-reacting polypeptides present also in control wild-type plants). Longer exposure of the blot indicated that zein–Nef was totally susceptible to the enzyme (Fig. 4B). This indicates that either zein–Nef does not traffic through the Golgi complex before degradation or that its conformation does not allow access of Golgi enzymes to its glycan moiety. To distinguish between the two possibilities, pulse–chase of protoplasts was performed in the presence of BFA, zein–Nef was immunoprecipitated with anti-Nef antibody and treated in vitro with endoglycosidase H. Zein–Nef acquired full resistance to endoglycosidase H during the 2 h chase and was already partially resistant at the end of the pulse (Fig. 4C). Because BFA artificially intermixes the ER and the Golgi complex, the results indicate that the conformation of zein–Nef allows access to glycan-processing enzymes. Therefore, in normal conditions the protein does not undergo glycan processing because it does not encounter Golgi enzymes; it follows that zein–Nef is degraded before having the possibility of trafficking through the Golgi complex. It was previously shown that a mutated, assembly-defective form of phaseolin has the same behaviour (Pedrazzini et al., 1997). Figure 4C also indicates that the minor zein–Nef polypeptide with sightly lower molecular mass is unglycosylated zein–Nef (clearly visible at 0 h chase; compare with the 0 h chase samples in Fig. 3C).
Fig. 4.
Zein–Nef does not traffic through the Golgi complex. (A) Proteins were extracted with reducing buffer from young leaves of tobacco plants expressing zein–Nef or from wild-type tobacco (control). After incubation in the presence (+) or absence (–) of endoglycosidase H (Endo H), proteins were analysed by SDS–PAGE and protein blot with anti-Nef antibody. (B) Longer exposure of the blot shown in (A). (C) Protoplasts isolated from plants expressing zein–Nef were treated with brefeldin A and subjected to pulse-labelling with [35S]Met and [35S]Cys for 1 h followed by chase for 0 h or 2 h; protoplasts were homogenated with reducing buffer and immunoprecipitation was performed with anti-Nef antibody. The immunoprecipitates were incubated in the presence (+) or absence (–) of endoglycosidase H and analysed by SDS–PAGE and fluorography. In (A) and (C), the positions of glycosylated (arrowhead) and deglycosylated (dot) zein–Nef are indicated on the right. In each panel, numbers on the left indicate the position of molecular mass markers, in kilodaltons.
It is concluded that, unlike zeolin, zein–Nef is rapidly degraded, most probably by ER quality control, supporting the hypothesis that Nef is not folded properly in the ER environment (Marusic et al., 2007). If this is the case, the present results also suggest that the zein fragment is not able to stabilize a defective protein subjected to ER quality control degradation. To test this hypothesis further, a new construct was produced based on the known detailed information on phaseolin structural maturation, as illustrated below.
Assembly-defective phaseolin is not rescued by the N-terminal portion of γ-zein
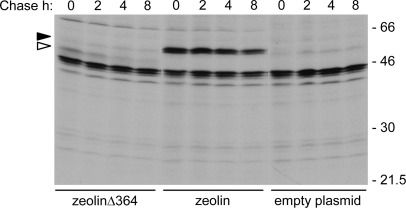
Wild-type phaseolin is a homotrimer that requires assembly to traffic correctly from the ER to vacuoles; deletions that inhibit phaseolin trimerization lead to its degradation by quality control (Pedrazzini et al., 1997; Frigerio et al., 2001). A new version of zeolin (zeolinΔ364), in which the deletion mutant Δ364 was used instead of full-length phaseolin, was therefore produced. This phaseolin mutant lacks the three C-terminal α-helical segments necessary for assembly into trimers; the very similar mutant Δ363 remains monomeric in vivo and is degraded by ER quality control (Pedrazzini et al., 1997). Apart from the deletion of residues 365–421 of phaseolin, zeolinΔ364 is identical to zeolin. The construct was tested by transient expression in tobacco protoplasts followed by pulse–chase and immunoprecipitation with anti-phaseolin antiserum. As controls, protoplasts were transformed either with the empty vector or with the vector encoding zeolin.
Foreign protein expression is less efficient in transiently transfected protoplasts than in transgenic plants; as a consequence, fluorographs need longer exposure and contaminants of immunoprecipitations become visible (Frigerio et al., 2001; Pompa and Vitale, 2006). Thus, a number of irrelevant radioactive polypeptides were immunoselected by the anti-phaseolin antiserum when the empty vector plasmid is expressed (Fig. 5, empty plasmid). In spite of this, zeolin synthesis was easily detectable and the protein had the expected stability during the chase (Fig. 5, zeolin). ZeolinΔ364 was recovered in much lower amounts than zeolin at the end of the pulse and was in large part degraded upon 4 h chase (Fig. 5, zeolinΔ364). Therefore, the zein domain is unable to rescue efficiently from degradation a structurally defective form of a plant secretory protein, indicating that quality control can be dominant over stable PB formation.
Fig. 5.
ZeolinΔ364 is unstable. Protoplasts isolated from young leaves of wild-type tobacco were transiently transformed with plasmid encoding zeolinΔ364, zeolin, or with empty plasmid as a control. Transformed protoplasts were subjected to pulse-labelling with [35S]Met and [35S]Cys for 1 h followed by chase for the indicated times. Proteins were immunoprecipitated from protoplast homogenates in reducing conditions with anti-phaseolin antiserum and analysed by SDS–PAGE and fluorography. The positions of zeolin (black arrowhead) and zeolinΔ364 (white arrowhead) are marked on the left. Numbers on the right indicate the positions of molecular mass markers, in kilodaltons.
Fusion with the entire zeolin sequence stabilizes Nef in the plant secretory pathway
The experiments reported above indicate that the zein domains used to produce zeolin are not able to promote stable accumulation when fused ahead of Nef, and that this is probably due to folding defects of the chimeric protein in the ER. In an effort to overcome degradation by quality control, tobacco was transformed with two new constructs in which either the position of the zein portion with respect to Nef was changed (by placing it at the C-terminus, as in zeolin) or the whole zeolin sequence was fused to Nef.
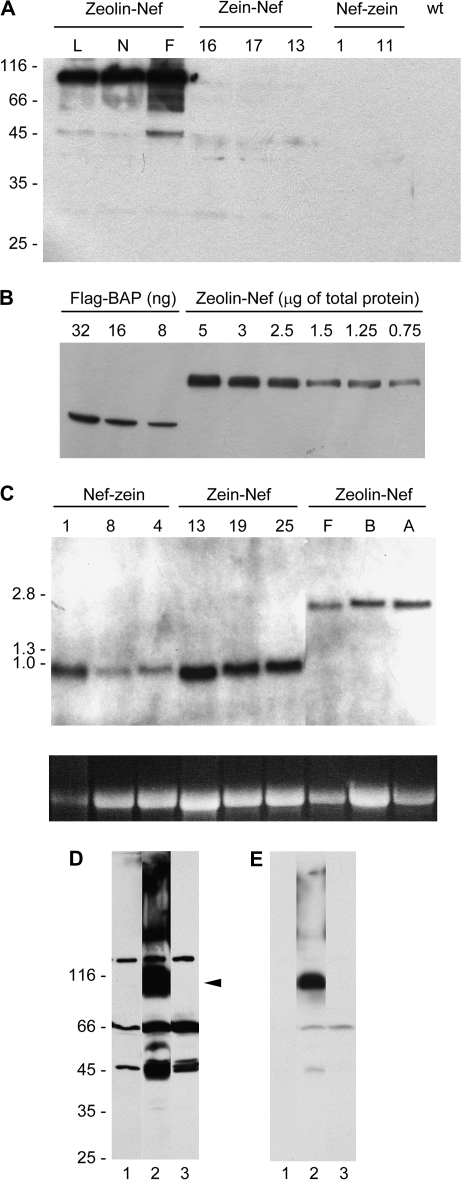
The first construct, Nef–zein, was composed of the signal peptide of the tobacco PR1 protein (Marusic et al., 2007) followed by the Nef sequence without the Met initiator codon, a Flag epitope to allow additional possibility of detection with antibodies, the (GGGGS)3 linker, and finally the same 89 amino acids of γ-zein that constitute the C-terminal sequence of zeolin (Fig. 1). Analysis of independent transgenic lines indicated that, in leaves, on average, Nef–zein mRNA accumulated to slightly lower levels than zein–Nef mRNA (Fig. 6C). Protein blot indicated that Nef–zein failed to accumulate at higher levels than zein–Nef and was actually virtually undetectable (Fig. 6A). Therefore, placing the zein sequence at the C-terminus did not improve accumulation of the Nef fusion.
Fig. 6.
Zeolin–Nef accumulates to much higher amounts than zein–Nef or Nef-zein. (A) Proteins were extracted with reducing buffer from young leaves of independent transgenic lines of tobacco expressing zeolin–Nef (lines L, N, and F), zein–Nef (16, 17, and 13), or Nef-zein (1 and 11) or from wild-type tobacco (wt). Analysis was by SDS–PAGE followed by protein blot using anti-γ-zein antiserum. (B) Different amounts of commercial Flag–BAP or total protein extracts from young leaves of tobacco plants expressing zeolin–Nef (transgenic line F, see A) were analysed by SDS–PAGE followed by protein blot using anti-Flag antibodies. (C) RNA was extracted from plants transformed as in (A) (three independent transgenic lines each, identified by numbers or letters) and hybridized with a probe corresponding to the zein portion of zeolin DNA. The image at the bottom shows ethidium bromide staining of 28S RNA in each lane, as a control for gel loading differences. In (A), (B), and (C), letters and numbers at the top identify the different independent transgenic lines. (D) Proteins were extracted with reducing buffer from young leaves of wild-type tobacco (lane 1) or tobacco expressing zeolin–Nef (lane 2) or zein–Nef (lane 3). Analysis was by SDS–PAGE followed by protein blot using anti-Nef antibody. The position of intact zeolin–Nef (arrowhead) is marked. (E) Shorter exposure of the protein blot shown in (D). Numbers on the left indicate the positions of molecular mass markers, in kilodaltons (A and D) or kilobase pairs (C).
The second construct, zeolin–Nef, was composed of the whole zeolin sequence followed by the (GGGGS)3 linker, the LVPRG thrombin cleavage site, the Nef sequence without the initial Met codon, and finally the Flag epitope (Fig. 1). The mRNA levels of zeolin–Nef and Nef–zein in tobacco leaves were similar (Fig. 6C) but the fusion with zeolin caused a marked improvement in recombinant protein accumulation; a polypeptide of about 90 kDa was detected by the anti-γ-zein antiserum and, compared with zein–Nef, accumulated to levels that are at least one order of magnitude higher (Fig. 6A). Therefore, the addition of the entire zeolin sequence markedly increases the accumulation of Nef.
Quantification of intact zeolin–Nef was performed by comparison of progressive dilutions of leaf extracts of plant F with known amounts of the commercial standard protein Flag–BAP, using anti-Flag antibodies (Fig. 6B). The results indicated that zeolin–Nef represents around 1.5% of total soluble proteins extracted in the presence of 2-mercaptoethanol (average of three independent extractions and quantitations; SD = 0.12).
A proportion of zeolin–Nef enters traffic along the secretory pathway, resulting in the release and vacuolar sorting of phaseolin
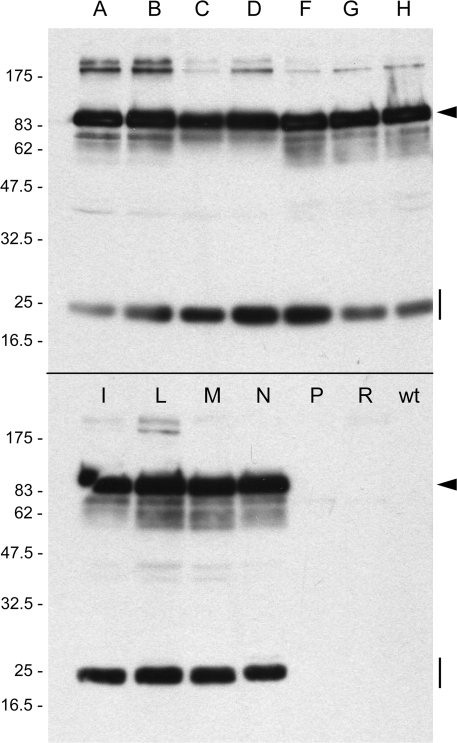
Zeolin is very stable, but a small proportion of polypeptides undergo post-translational cleavage that removes the phaseolin portion of the recombinant molecule (Mainieri et al., 2004). This processing requires intracellular traffic and indicates a quantitatively minor failure in the stable incorporation into PB. The destiny of the zein portion is unknown, but the released phaseolin is in part secreted and in part sorted to the vacuole. Zeolin accumulates to >3% of total leaf soluble protein at developmental stages similar to those analysed here (Mainieri et al., 2004), suggesting that it is more stable than zeolin–Nef. It was therefore investigated whether a relevant proportion of zeolin–Nef undergoes traffic and processing events. Protein blots using anti-Nef antibodies were first performed. As already shown in Fig. 4A, upon long blot exposure some polypeptides were detected in wild-type tobacco leaf extracts and therefore constitute unspecific contaminants (Fig. 6D, lane 1). In extracts of plants expressing zeolin–Nef, minor components around 45 kDa not present in wild-type tobacco were also detected, besides the abundant 90 kDa intact zeolin–Nef and larger oligomers (Fig. 6D, lane 2, and see Fig. 6E for a shorter exposure of the same blot). The 45 kDa components were very similar in molecular mass to zein–Nef (Fig. 6D, compare lanes 2 and 3), and were also detected as minor components by the anti-γ-zein antiserum (Fig. 6A), strongly suggesting release of the phaseolin sequence from a minor proportion of zeolin–Nef. To verify this hypothesis, protein blotting was performed using anti-phaseolin or anti-Flag antibodies. The analysis was made on several independent transgenic lines (Fig. 7, lanes A–N) and one wild-type plant (Fig. 7, wt). In all zeolin–Nef transgenic lines, the anti-phaseolin antiserum recognized intact zeolin–Nef and a polypeptide of 25 kDa (Fig. 7, arrowheads and vertical lines, respectively; the minor components with apparent molecular mass above 175 kDa are probably undenatured oligomers—see also Fig. 6D), whereas anti-Flag antibodies recognized only intact zeolin–Nef (not shown). No polypeptides were detected in wild-type tobacco extracts (Fig. 7, wt). The results shown in Fig. 7 also indicated that there is very low variability in accumulation of zeolin–Nef in independent transformation events.
Fig. 7.
Phaseolin fragments accumulate in plants that synthesize zeolin–Nef. Proteins were extracted with reducing buffer from young leaves of independent lines of tobacco transformed with the vector encoding zeolin–Nef (the different trangenic lines are identified by single letters) or from wild-type tobacco (wt). Analysis was by SDS–PAGE followed by protein blot using anti-phaseolin antiserum. Equal amounts of protein extract (40 μg) were loaded in each lane. The two lanes between N and wt contain extracts from plants that were positive by antibiotic selection but negative for recombinant protein expression. The positions of intact zeolin (arrowhead) and phaseolin fragment (vertical line) are marked on the right. Numbers on the left indicate the positions of molecular mass markers, in kilodaltons.
The fact that the polypeptide of 25 kDa is detected by anti-phaseolin but not by anti-Nef, anti-zein, or anti-Flag antiserum, confirms that phaseolin is released from a proportion of zeolin–Nef and strongly suggests that it is sorted to vacuoles of leaf cells; wild-type phaseolin is fragmented into components in the 20–25 kDa range upon vacuolar delivery when expressed in vegetative tissues (Bagga et al., 1992; Pedrazzini et al., 1997). This was confirmed by pulse–chase analysis of zeolin–Nef; the typical vacuolar fragmentation products of phaseolin in the 20–25 kDa range were produced during the chase and could be immunoprecipitated by anti-phaseolin but not anti-γ-zein or anti-Nef antibodies (Fig. 8A, the vertical bar indicates phaseolin fragments and the arrowhead intact zeolin–Nef; the other polypeptides constantly present throughout the pulse–chase are endogenous tobacco proteins recognized by the different antisera). The formation of phaseolin fragments was fully inhibited by BFA, indicating that the release of phaseolin occurs after zeolin–Nef has entered traffic and is a post-Golgi event (Fig. 8B). Shorter exposure of the fluorograph showed more clearly that BFA partially inhibits synthesis and stabilizes intact zeolin–Nef (Fig. 8C). In the absence of the inhibitor, zeolin–Nef is markedly more stable than zein–Nef (compare Fig. 8A and 3B) but not as stable as zeolin; the latter does not undergo marked degradation even after 24 h chase (Pompa and Vitale, 2006).
Fig. 8.
A proportion of zeolin–Nef enters secretory traffic resulting in the production of phaseolin vacuolar fragments. Protoplasts isolated from young leaves of transgenic tobacco expressing zeolin–Nef were subjected to pulse-labelling with [35S]Met and [35S]Cys for 1 h followed by chase for the indicated times. Protoplasts were homogenated with reducing buffer. (A) Proteins were immunoprecipitated with anti-phaseolin, anti-γ-zein, or anti-Nef antibodies, as indicated on top of the panel. (B) Pulse–chase was performed in the presence (+) or absence (–) of BFA; immunoprecipitation was with anti-phaseolin antiserum. (C) Shorter exposure of the fluorograph in (B), showing only the region containing intact zeolin–Nef. In all panels, analysis was by SDS–PAGE and fluorography. The positions of zeolin–Nef (arrowhead) and phaseolin fragmentation products (vertical bar) are marked on the left. Numbers on the right indicate the positions of molecular mass markers, in kilodaltons.
Zeolin–Nef forms PB
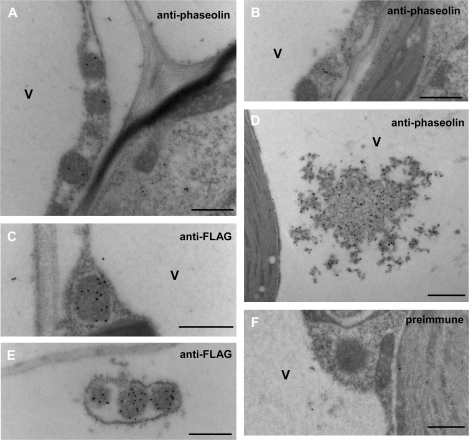
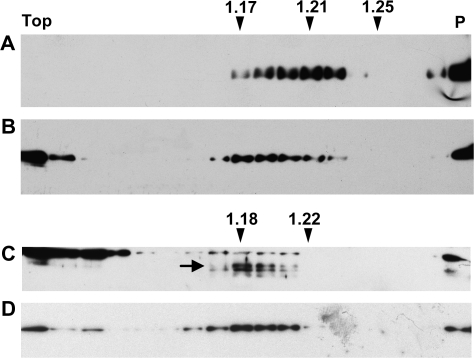
In spite of the traffic competence and subsequent fragmentation of a proportion of zeolin–Nef, the intact chimeric protein accumulates to relatively high levels. It was therefore investigated whether most of zeolin–Nef forms PB. Immuno-electron microscopy of leaf tissue isolated from zeolin–Nef plants detected spherical electron-dense structures with diameter of 0.2–0.5 μm that are recognized by anti-phaseolin and anti-Flag antiserum but not preimmune serum (Fig. 9A, C, E, F). These spherical structures were never found in the vacuole, and sometimes an electron-transparent area was present between them and the surrounding ER membrane with attached ribosomes (Fig. 9C, more clearly in E), suggesting that their interaction with the luminal side of the ER membrane is not strong. Less electron-dense structures were also labelled and could represent PB in the early process of formation (Fig. 9B). Zeolin PB are on the average larger (0.5–1 μm; Mainieri et al., 2004) and should therefore have a higher protein–membrane ratio and higher density than zeolin–Nef PB. It was then reasonable to expect that intact zeolin–Nef would be contained in a subcellular fraction with a density intermediate between those of cisternal ER and zeolin PB, which in tobacco leaf cells is around 1.17–1.18 and 1.25, respectively (Pedrazzini et al., 1997; Mainieri et al., 2004). Subcellular fractionation was performed by isopycnic sucrose gradient centrifugation of leaf homogenates prepared in the absence of detergent. A peak of zeolin–Nef was detected around a density of 1.21, confirming our hypothesis (Fig. 10A; compare with B, where the position of the ER is highlighted by antiserum against the ER resident chaperone BiP). The compartment where the very small amount of intact zein–Nef is located was instead indistinguishable from the ER in the gradients (Fig. 10C, D; the arrow indicates zein–Nef), confirming that this chimeric protein is unable to form PB and is detectable only in the ER, a typical feature of proteins degraded by ER quality control (Pedrazzini et al., 1997). BiP was also present at the top of the gradients, as already observed in similar experiments (Pedrazzini et al., 1997; Mainieri et al., 2004), possibly reflecting partial release from the ER lumen upon homogenation.
Fig. 9.
Zeolin–Nef forms protein bodies. Thin sections prepared from young leaves of transgenic tobacco expressing zeolin–Nef were incubated with anti-phaseolin antiserum (A, B, D), anti-Flag antibodies (C, E), or preimmune serum (F), followed by secondary goat anti-rabbit 15 nm gold complex. V, Vacuole. Bars = 500 nm (A, B, C, E, F) or 1000 nm (D).
Fig. 10.
Density of zeolin–Nef protein bodies. Young leaves from transgenic tobacco expressing zeolin–Nef (A, B) or zein–Nef (C, D) were homogenized in the absence of detergent and the presence of sucrose. The homogenates were fractionated by centrifugation on isopycnic sucrose gradient. Proteins in each gradient fraction were analysed by SDS–PAGE and protein blot, using anti-Nef (A, C) or anti-BiP (B, D) antibodies. In (C), the position of zein–Nef is indicated by an arrow; the other bands represent immunoreactive endogenous tobacco proteins, revealed because of the long exposure needed to detect zein–Nef. Numbers at top indicate density (grams per millilitre). The top of the gradients is on the left (Top); the pellet precipitated at the bottom of the tubes, probably representing unbroken tissue, is on the right (P).
Phaseolin accumulated in vacuoles of tobacco leaf cells can be detected by immuno-electron microscopy as unstructured electron-dense aggregates (Pedrazzini et al. 1997). Similar aggregates within the vacuolar lumen, besides PB, were recognized by the anti-phaseolin antiserum in zeolin–Nef-expressing plants (Fig. 9D), consistent with the pulse–chase results. These vacuolar aggregates, as expected, were not recognized by anti-Flag or anti-γ-zein antisera, and were not observed in preparations from zein–Nef plants (not shown). The concentration of gold particles was markedly higher in vacuolar phaseolin than in PB-located zeolin–Nef (Fig. 9); pulse–chase analysis indicated that the vacuolar proportion of zeolin–Nef is mostly, if not exclusively, constituted of phaseolin fragments (Fig. 8), which at steady state are not more abundant than intact zeolin–Nef (Fig. 7). It can be concluded that most likely the fusion with Nef and the zein domains, and the assembly into PB, do not allow full protein surface exposure of phaseolin in the fusion molecule, masking epitopes that are recognized by the anti-phaseolin antiserum only when zeolin–Nef is denatured or the phaseolin portion is released in vivo upon trafficking.
Discussion
Newly synthesized secretory proteins fold and assemble in the ER, and the intermediates of these structural maturation events are much less resistant to proteases than mature polypeptides; it is therefore reasonable to hypothesize that the low hydrolytic activity of this compartment is related to its nursery role within the secretory pathway. The ER, however, also has the function of controlling successful folding and assembly of the newly synthesized proteins and delivering to degradation polypeptides that do not meet this requirement (Vitale and Denecke, 1999; Sitia and Braakman, 2003). This is possibly the reason why recombinant proteins constructed to be retained in the ER can fail to accumulate to high amounts (Patel et al., 2007; Yang et al. 2007), and this has been directly shown using the model protein phaseolin; the addition of the ER retention signal KDEL to an assembly-defective form of this seed storage protein is unable to rescue it from degradation by ER quality control (Frigerio et al., 2001). This property of the ER may be a serious drawback when the aim is to increase accumulation of non-secretory proteins. The ER environment favours the formation of disulphide bonds, whereas this does not occur in the cytosol. Moreover, N-glycosylation also occurs within the ER. These protein modifications may negatively affect the process of folding of non-secretory proteins. Indeed, the first effort to introduce a cytosolic protein into the plant ER resulted in glycosylation of a fortuitous N-glycosylation site with consequent 100-fold inhibition of enzymatic activity of the recombinat protein (Iturriaga et al., 1989).
Nef is naturally cytosolic or attached to the cytosolic face of membranes via a lipid anchor, and was highly unstable when introduced into the secretory pathway via addition of a signal peptide (Marusic et al., 2007). Nef has two fortuitous N-glycosylation sites, but their inactivation by point mutation did not improve stability of the secretory construct in transient expression experiments, suggesting folding defects independent of glycosylation (Marusic et al., 2007).
The work presented here has tried to take advantage of the present knowledge on PB formation to increase the stability of Nef in the ER. The results show that the γ-zein domains that lead to stable PB formation when fused to phaseolin in the chimeric protein zeolin are unable to rescue Nef. However, a fusion to the entire zeolin sequence avoids quality control degradation, leads to PB formation, and improvement in Nef stability. As a result, accumulation of zeolin–Nef exceeds 1% of total leaf protein; a value that is usually considered as high accumulation in vegetative tissues of transgenic plants, although it is not as high as the 3.5% reached by zeolin. A mutated cytosolic Nef with a G→A N-terminal substitution, to avoid N-myristoylation, accumulated to <0.5% of total protein in leaves of one transgenic tobacco plant, but analysis of several plants indicated a much lower average level (Marusic et al., 2007), whereas the accumulation of zeolin–Nef is highly consistent in different plants. Another mutated form of cytosolic Nef with a deletion of the first 18 amino acids accumulated to a maximum of 0.7% of total protein, but also in this case the average in different plants was much lower (Marusic et al., 2007).
Like zein–Nef, zeolin–Nef is glycosylated (data not shown). If glycosylation proves to have negative effects on the antigenic properties of Nef, it will be necessary to inactivate its glycosylation sites by point mutagenesis. If the technology were extended to proteins for which an enzymatic activity needs to be preserved, it would be necessary to establish on a case by case basis whether inclusion into PB negatively affects biological properties. For what regards covalent modifications, recombinant proteins assembled into PB are retained in the ER and therefore undergo the typical modifications of this compartment, similarly to proteins with added KDEL or HDEL.
Quality control and PB formation
Why does the γ-zein portion of zeolin fail to stabilize Nef to satisfactory levels? The detailed mechanisms of PB formation are still not clear. Not all proteins that are naturally part of seed PB are very stable when expressed individually in transgenic plants, indicating that certain PB components are fundamental for the process (Coleman et al., 1996; Napier et al., 1997). Among the abundant components of maize PB, γ-zein is sufficient for PB formation and is necessary for the stable accumulation of α-zeins (Coleman et al., 1996). γ-Zein polymerization into insoluble PB requires intra-chain disulphide bonds (Vitale et al., 1982), and the same holds true for zeolin PB (Pompa and Vitale, 2006), indicating that one or more of the six Cys residues of zeolin (out of the 15 of γ-zein) has a fundamental role. Phaseolin is devoid of Cys residues, whereas Nef has three residues that in the cytosol are most likely reduced but in the ER could in theory form incorrect disulphide bonds with the zein domains, inhibiting PB formation and leading to quality control degradation. However, it should be noticed that the zein portion is also unable to efficiently stabilize the assembly of the defective phaseolin Δ364 mutant, which does not contain Cys residues, indicating that perturbation of PB assembly can occur independently of direct interference by non-zein Cys residues. Altogether, the failure of zeolinΔ364, Nef–zein, and Zein–Nef to accumulate support the hypothesis that, whatever the specific defect, structurally defective polypeptides are not easily diverted from degradation by fusion to the zein domains. Assembly into PB, as judged by the loss of solubility of newly synthesized zeolin during pulse–chase labelling, does not occur immediately after translation; at the end of 1 h pulse labelling, about 50% of the completed polypeptides are still soluble, the process of insolubilization being almost completed only between 4 h and 8 h chase (Mainieri et al., 2004; Pompa and Vitale, 2006). Therefore, degradation may occur because the defective recombinant proteins are unable to form PB but, alternatively, it cannot be excluded that the quality control machinery rapidly recognizes as defective the Nef and Δ364 portions and sorts the whole polypeptide for degradation before the zein portion has time to promote assembly. It is not possible to distinguish between these two possibilities from the present experiments.
The dominant effect of zeolin
Zeolin–Nef is not recognized as a defective protein; it assembles into PB that are smaller than zeolin ones and it enters traffic leading to vacuolar fragmentation of phaseolin in a higher proportion than zeolin does, but this traffic is fully inhibited by BFA, indicating that it is not mediated by quality control. Because zein–Nef is rapidly degraded in a process that is only slightly retarded by BFA, the phaseolin portion of zeolin is making the difference between the two Nef fusions. Wild-type phaseolin does not form PB in the ER. However, it interacts with membranes very early after synthesis, a feature that is unusual in soluble secretory proteins and is dependent on the phaseolin hydrophobic signal for vacuolar sorting (Castelli and Vitale, 2005). Moreover, phaseolin is naturally a trimer and its assembly occurs in the ER. On the whole, the results presented in the present work open a new scenario in which, besides the necessary role of the Cys residues of zein (Pompa and Vitale, 2006), the phaseolin portion of zeolin may also contribute to zeolin PB formation. This may occur by promoting transient trimerization of zeolin and zeolin–Nef that is then disrupted by assembly into PB. If they are ever formed, these trimers must be transient or very unstable, unlike natural phaseolin trimers, because in vitro disassembly of zeolin PB by treatment with reducing agent does not produce zeolin trimers (Pedrazzini et al., 1997; Mainieri et al., 2004). Alternatively, phaseolin may promote early interactions of the chimeric proteins with the ER membrane. Both events may avoid quality control degradation early after synthesis and give enough time to start productive PB assembly. The fact that Δ364 phaseolin is unable to form trimers and is devoid of the phaseolin vacuolar sorting signal (which is at the C-terminus of wild-type phaseolin) is in agreement with these possible scenarios, although it does not prove either of the two. A consequence would be that the disulphide bonds are necessary but not sufficient for zeolin assembly into PB. In this case, the vacuolar storage protein phaseolin would somehow substitute (not on purpose) for the γ-zein C-terminal domain that is missing in zeolin, which indeed is homologous to members of another class of vacuolar storage proteins, the 2S albumins (Shewry et al., 1995).
Oligomer formation and stability of foreign proteins
The results presented here complement and extend recent studies indicating that the formation of large oligomers within the ER is an efficient strategy to improve the accumulation of foreign proteins in transgenic plants. The accumulation of the human immunodeficiency virus p24 protein introduced into the plant secretory pathway was enhanced an average 13-fold by fusing the antigen to a human immunoglobulin A (IgA) fragment containing the heavy chain constant α3 and α4 domains (Obregon et al., 2006). The IgA domains promoted the formation of disulphide-bonded dimers (probably through the same bonds that are formed in natural IgA). Unlike p24 inserted into the secretory pathway, the fusion protein was not secreted. The results of pulse–chase and immunoprecipitation experiments also suggested the formation of high molecular mass forms difficult to immunoprecipitate. Large oligomers were also formed when a fusion between the tetanus toxin C fragment and the heavy chain of a monoclonal antibody against the same fragment was co-expressed with the Ig light chains (Chargelegue et al., 2005). The oligomers were in this case due to antibody–antigen association, and the recombinant protein accumulated at 0.8% or more of total protein. The insertion of a polypeptide consisting of 27 repeats of the elastin pentapeptide VPGVG into three recombinant proteins that were otherwise almost undetectable when targeted to the plant ER allowed accumulation up to 0.75% of total soluble protein in tobacco leaves (Patel et al., 2007). The elastin pentapeptide (or similar variants) occurs in repeats in elastin and is responsible for the co-acervation properties of the protein at temperatures above 30 °C in controlled in vitro conditions. It is not known whether the increase in accumulation of the recombinant proteins is due to co-acervation occurring in vivo but this is one possibility. Co-acervation has been suggested to occur in mammalian tissues that secrete elastin, where the physiological temperature is around 37 °C (Vrhovski and Weiss, 1998). A fusion of a single-chain Ig variable fragment (scFv) to 100 repeats of the elastin pentapeptide, followed by the ER retention signal KDEL, accumulated in tobacco seeds to 40-fold higher levels than a similar construct devoid of the elastin portion, reaching 25% of total seed protein (Scheller et al., 2006). Large fragments of the Japanese cedar pollen allergen Cry j 1 inserted into rice glutelin (which in its wild-type form is a vacuolar storage protein) accumulated to up to 15% rice seed protein and were located into PB, most probably because of cysteine-mediated interactions with the storage prolamins, whereas accumulation of full-length Cry j 1 with added KDEL was about 100-fold lower (Yang et al., 2007).
Large oligomer formation thus seems to be a promising strategy to avoid degradation of foreign protein in the plant secretory pathway and even to enhance the accumulation of correctly folded polypeptides. As reported above, this can be achieved in different ways, including, as shown here, the formation of PB similar to the ones produced in seeds by prolamins. These ‘artificial’ PB also cast further light on the natural mechanism of PB formation and avoidance of quality control degradation.
Acknowledgments
We thank Andrea Pompa for technical assistance, and Emanuela Pedrazzini and Alessandra Barbante for useful discussions and suggestions. We are grateful to Ulrike Bechtold, Roger Hellens, and Phil Mullineaux for the gift of the pGreenII plasmid. This work was supported by the European Union Integrated Project ‘Pharma-Planta’ (LSHBCT-2003-503565) and the project Ingenio (Fondo Sociale Europeo del Ministero del Lavoro e delle Politiche Sociali e della Regione Lombardia).
Glossary
Abbreviations
- BFA
brefeldin A
- ER
endoplasmic reticulum
- Nef
negative factor
- PB
protein bodies
References
- Bagga S, Sutton D, Kemp JD, Sengupta-Gopalan C. Constitutive expression of the β-phaseolin gene in different tissues of transgenic alfalfa does not ensure phaseolin accumulation in non-seed tissue. Plant Molecular Biology. 1992;19:951–958. doi: 10.1007/BF00040527. [DOI] [PubMed] [Google Scholar]
- Bellucci M, Lazzari B, Viotti A, Arcioni S. Differential expression of a γ-zein gene in Medicago sativa, Lotus corniculatus and Nicotiana tabacum. Plant Science. 1997;127:161–169. [Google Scholar]
- Bellucci M, Alpini A, Paolocci F, Cong L, Arcioni S. Accumulation of maize γ-zein and γ-zein:KDEL to high levels in tobacco leaves and differential increase of BiP synthesis in transformants. Theoretical and Applied Genetics. 2000;101:796–804. [Google Scholar]
- Bentham M, Mazaleyrat S, Harris M. Role of myristoylation and N-terminal basic residues in membrane association of the human immunodeficiency virus type 1 Nef protein. Journal of General Virology. 2006;87:563–571. doi: 10.1099/vir.0.81200-0. [DOI] [PubMed] [Google Scholar]
- Castelli S, Vitale A. The phaseolin vacuolar sorting signal promotes transient, strong membrane association and aggregation of the bean storage protein in transgenic tobacco. Journal of Experimental Botany. 2005;56:1379–1387. doi: 10.1093/jxb/eri139. [DOI] [PubMed] [Google Scholar]
- Chargelegue D, Drake PM, Obregon P, Prada A, Fairweather N, Ma JK. Highly immunogenic and protective recombinant vaccine candidate expressed in transgenic plants. Infection and Immunity. 2005;73:5915–5922. doi: 10.1128/IAI.73.9.5915-5922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CE, Herman EM, Takasaki K, Larkins BA. The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. The Plant Cell. 1996;8:2335–2345. doi: 10.1105/tpc.8.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Jameel S. Biology of the HIV Nef protein. Indian Journal of Medical Research. 2005;121:315–332. [PubMed] [Google Scholar]
- Di Cola A, Frigerio L, Lord JM, Ceriotti A, Roberts LM. Ricin A chain without its partner B chain is degraded after retrotranslocation from the endoplasmic reticulum to the cytosol in plant cells. Proceedings of the National Academy of Sciences, USA. 2001;98:14726–14731. doi: 10.1073/pnas.251386098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso G, Herzog V, Schmitz A. Misfolded BiP is degraded by a proteasome-independent endoplasmic-reticulum-associated degradation pathway. Biochemical Journal. 2005;387:897–903. doi: 10.1042/BJ20041312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran PM. Foreign protein degradation and instability in plants and plant tissue cultures. Trends in Biotechnology. 2006;24:426–432. doi: 10.1016/j.tibtech.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM. Plant-based production of biopharmaceuticals. Current Opinion in Plant Biology. 2004;7:152–158. doi: 10.1016/j.pbi.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A. Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. The Plant Cell. 1998;10:1031–1042. doi: 10.1105/tpc.10.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Pastres A, Prada A, Vitale A. Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. The Plant Cell. 2001;13:1109–1126. doi: 10.1105/tpc.13.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli MI, Torren M, Ludevid D. Two structural domains mediate two sequential events in γ-zein targeting: protein endoplasmic reticulum retention and protein body formation. The Plant Cell. 1994;6:1911–1922. doi: 10.1105/tpc.6.12.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese SI, Woerz I, Homann S, Tibroni N, Geyer M, Fackler OT. Specific and distinct determinants mediate membrane binding and lipid raft incorporation of HIV-1(SF2) Nef. Virology. 2006;355:175–191. doi: 10.1016/j.virol.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Iturriaga G, Jefferson RA, Bevan MW. Endoplasmic reticulum targeting and glycosylation of hybrid proteins in transgenic tobacco. The Plant Cell. 1989;1:381–390. doi: 10.1105/tpc.1.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G. Membrane trafficking in plants. Annual Review of Cell and Developmental Biology. 2004;20:481–504. doi: 10.1146/annurev.cellbio.20.082503.103057. [DOI] [PubMed] [Google Scholar]
- Kaminchik J, Bashan N, Itach A, Sarver N, Gorecki M, Panet A. Genetic characterization of human immunodeficiency virus type 1 nef gene products translated in vitro and expressed in mammalian cells. Journal of Virology. 1991;65:583–588. doi: 10.1128/jvi.65.2.583-588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. Journal of Biological Chemistry. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- Kruse KB, Brodsky JL, McCracken AA. Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human α-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Molecular Biology of the Cell. 2006;17:203–212. doi: 10.1091/mbc.E04-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludevid D, Torrent M, Lasserre-Ramassamy S. Production of peptides and proteins by accumulation in plant endoplasmic reticulum-derived protein bodies. Patent EP1523558. 2005 [Google Scholar]
- Mainieri D, Rossi M, Archinti M, Bellucci M, De Marchis F, Vavassori S, Pompa A, Arcioni S, Vitale A. Zeolin: a new recombinant storage protein constructed using maize γ-zein and bean phaseolin. Plant Physiology. 2004;136:3447–3456. doi: 10.1104/pp.104.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusic C, Nuttall J, Buriani G, Lico C, Lombardi R, Baschieri S, Benvenuto E, Frigerio L. Expression, intracellular targeting and purification of HIV Nef variants in tobacco cells. BMC Biotechnology. 2007;7:12. doi: 10.1186/1472-6750-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Kimball SR, Jefferson LS. Brefeldin A inhibits protein synthesis through the phosphorylation of the alpha-subunit of eukaryotic initiation factor-2. FEBS Letters. 1994;350:143–146. doi: 10.1016/0014-5793(94)00756-x. [DOI] [PubMed] [Google Scholar]
- Napier JA, Richard G, Turner MF, Shewry PR. Trafficking of wheat gluten proteins in transgenic tobacco plants: γ-gliadin does not contain an endoplasmic reticulum-retention signal. Planta. 1997;203:488–494. doi: 10.1007/s004250050218. [DOI] [PubMed] [Google Scholar]
- Nuttall J, Vitale A, Frigerio L. C-terminal extension of phaseolin with a short methionine-rich sequence can inhibit trimerisation and result in high instability. Plant Molecular Biology. 2003;51:885–894. doi: 10.1023/a:1023041901029. [DOI] [PubMed] [Google Scholar]
- Obregon P, Chargelegue D, Drake PM, Prada A, Nuttall J, Frigerio L, Ma JK. HIV-1 p24-immunoglobulin fusion molecule: a new strategy for plant-based protein production. Plant Biotechnology Journal. 2006;4:195–207. doi: 10.1111/j.1467-7652.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- Patel J, Zhu H, Menassa R, Gyenis L, Richman A, Brandle J. Elastin-like polypeptide fusions enhance the accumulation of recombinant proteins in tobacco leaves. Transgenic Research. 2007;16:239–249. doi: 10.1007/s11248-006-9026-2. [DOI] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, Faoro F, Bollini R, Ceriotti A, Vitale A. Protein quality control along the route to the plant vacuole. The Plant Cell. 1997;9:1869–1880. doi: 10.1105/tpc.9.10.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa A, Vitale A. Retention of a bean phaseolin/maize gamma-zein fusion in the endoplasmic reticulum depends on disulfide bond formation. The Plant Cell. 2006;18:2608–2621. doi: 10.1105/tpc.106.042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez N, Ayala M, Lorenzo D, Palenzuela D, Herrera L, Doreste V, Perez M, Gavilond JV, Oramas P. Expression of a single-chain Fv antibody fragment specific for the hepatitis B surface antigen in transgenic tobacco plants. Transgenic Research. 2002;11:61–64. doi: 10.1023/a:1013967705337. [DOI] [PubMed] [Google Scholar]
- Scheller J, Leps M, Conrad U. Forcing single-chain variable fragment production in tobacco seeds by fusion to elastin-like polypeptides. Plant Biotechnology Journal. 2006;4:243–249. doi: 10.1111/j.1467-7652.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Napier JA, Tatham AS. Seed storage proteins: structures and biosynthesis. The Plant Cell. 1995;7:945–956. doi: 10.1105/tpc.7.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Stöger E, Vaquero C, Torres E, et al. Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Molecular Biology. 2000;42:583–590. doi: 10.1023/a:1006301519427. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ. Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnology Journal. 2007;5:2–15. doi: 10.1111/j.1467-7652.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJ. A biotechnological approach to improving the nutritive value of alfalfa. Journal of Animal Science. 1995;73:2752–2759. doi: 10.2527/1995.7392752x. [DOI] [PubMed] [Google Scholar]
- Takase K, Hagiwara K. Expression of human α-lactalbumin in transgenic tobacco. Journal of Biochemistry. 1998;123:440–444. doi: 10.1093/oxfordjournals.jbchem.a021956. [DOI] [PubMed] [Google Scholar]
- Titti F, Cafaro A, Ferrantelli F, et al. Problems and emerging approaches in HIV/AIDS vaccine development. Expert Opinion on Emerging Drugs. 2007;12:23–48. doi: 10.1517/14728214.12.1.23. [DOI] [PubMed] [Google Scholar]
- Vaquero C, Sack M, Schuster F, Finnern R, Drossard J, Schumann D, Reimann A, Fischer R. A carcinoembryonic antigen-specific diabody produced in tobacco. FASEB Journal. 2002;16:408–410. doi: 10.1096/fj.01-0363fje. [DOI] [PubMed] [Google Scholar]
- Verwoerd TC, van Paridon PA, van Ooyen AJ, van Lent JW, Hoekema A, Pen J. Stable accumulation of Aspergillus niger phytase in transgenic tobacco leaves. Plant Physiology. 1995;109:1199–1205. doi: 10.1104/pp.109.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Ceriotti A. Protein quality control mechanisms and protein storage in the endoplasmic reticulum. A conflict of interests? Plant Physiology. 2004;136:3420–3426. doi: 10.1104/pp.104.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Denecke J. The endoplasmic reticulum-gateway of the secretory pathway. The Plant Cell. 1999;11:615–628. doi: 10.1105/tpc.11.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Pedrazzini E. Recombinant pharmaceuticals from plants: the plant endomembrane system as bioreactor. Molecular Interventions. 2005;5:216–225. doi: 10.1124/mi.5.4.5. [DOI] [PubMed] [Google Scholar]
- Vitale A, Smaniotto E, Longhi R, Galante E. Reduced soluble proteins associated with maize endosperm protein bodies. Journal of Experimental Botany. 1982;33:439–448. [Google Scholar]
- Vrhovski B, Weiss AS. Biochemistry of tropoelastin. European Journal of Biochemistry. 1998;258:1–18. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- Wandelt CI, Khan MR, Craig S, Schroeder HE, Spencer D, Higgins TJ. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. The Plant Journal. 1992;2:181–192. doi: 10.1046/j.1365-313x.1992.t01-41-00999.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Suzuki K, Hirose S, Wakasa Y, Takaiwa F. Development of transgenic rice seed accumulating a major Japanese cedar pollen allergen (Cry j 1) structurally disrupted for oral immunotherapy. Plant Biotechnology Journal. 2007;5:815–826. doi: 10.1111/j.1467-7652.2007.00287.x. [DOI] [PubMed] [Google Scholar]