Abstract
Wounding of plants leads to endogenous rise of jasmonic acid (JA) accompanied with the expression of a distinct set of genes. Among them are those coding for the allene oxide cyclase (AOC) that catalyses a regulatory step in JA biosynthesis, and for 1-deoxy-D-xylulose 5-phosphate synthase 2 (DXS2), an enzyme involved in isoprenoid biosynthesis. To address the question how roots and shoots of Medicago truncatula respond to mechanostimulation and wounding, M. truncatula plants were analysed in respect to JA levels as well as MtAOC1 and MtDXS2-1 transcript accumulation. Harvest-caused mechanostimulation resulted in a strong, but transient increase in JA level in roots and shoots followed by a transient increase in MtAOC1 transcript accumulation. Additional wounding of either shoots or roots led to further increased JA and MtAOC1 transcript levels in shoots, but not in roots. In situ hybridization revealed a cell-specific transcript accumulation of MtAOC1 after mechanostimulation in companion cells of the vascular tissue of the stem. AOC protein, however, was found to occur constitutively in vascular bundles. Further, transcript accumulation of MtDXS2-1 was similar to that of MtAOC1 in shoots, but its transcript levels were not enhanced in roots. Repeated touching of shoots increased MtAOC1 transcript levels and led to significantly shorter shoots and increased biomass. In conclusion, M. truncatula plants respond very sensitively to mechanostimulation with enhanced JA levels and altered transcript accumulation, which might contribute to the altered phenotype after repeated touching of plants.
Keywords: Allene oxide cyclase, cell specific expression, 1-deoxy-D-xylulose 5-phosphate synthase 2, jasmonic acid, mechanostimulation, Medicago truncatula, wounding
Introduction
Wounding by mechanical injuries or herbivore attack is a common event in the life of a plant and elicits a complex series of spatial and temporal responses. Different signalling pathways are activated, which involve various signalling molecules, such as jasmonic acid (JA), nitric oxide, ethylene, ABA, systemin, reactive oxygen species, and possibly electrical or hydraulic signals (Orozco-Cárdenas et al., 2001; Stratmann, 2003; Chen et al., 2004; Howe, 2004; Wasternack, 2006). For systemin and JA, signalling properties are well established, whereby tomato (Solanum lycopersicon=Lycopersicon esculentum) serves as the best-studied model in that respect (Schilmiller and Howe, 2005). A characteristic feature of the local wound response of plants is a transient increase of JA in the first hour followed by activation of defence genes such as those coding for proteinase inhibitors, enzymes of phytoalexin synthesis, amino acid metabolism or vegetative storage proteins, thionins, and defensins (Ryan, 2000; Howe, 2004). Local wounding, however, is followed by systemic activation of defence genes in leaves. Among others, systemin, JA, and/or related compounds were suggested to act as systemic signals, too. Grafting experiments using tomato mutants affected in JA biosynthesis and in JA signalling led to strong arguments for JA as an essential component of the systemic signal (Li et al., 2000; Li et al., 2002). The preferred generation of JA in vascular bundles (Stenzel et al., 2003a) and the occurrence of the biosynthetic enzymes in companion cells and even in sieve elements of the vascular bundles (Hause et al., 2003) support this assumption. Similar data were found with Arabidopsis thaliana (Truman et al., 2007). Much less is known on signalling and responses in shoots and roots following wounding of either shoots or roots.
JA and its derivatives, commonly named jasmonates, are lipid-derived signals. They are synthesized via the octadecanoid pathway, where 12-oxophytodienoic acid (OPDA) is a central intermediate. The initial reaction is the 13-lipoxygenase-catalysed insertion of molecular oxygen into position 13 of α-linolenic acid. The resulting (13-S)-hydroperoxy linolenic acid (13-HPOT) is the substrate for at least seven different pathways (Feussner and Wasternack, 2002). Only the conversion of 13-HPOT by an allene oxide synthase (AOS) specifically acting with 13-HPOT leads to the formation of an unstable allene oxide that is further processed by an allene oxide cyclase (AOC), leading exclusively to the cis-(+)-enantiomer (9S,13S) of OPDA. It carries that enantiomeric structure which is present in the naturally occurring jasmonates. In the subsequent steps occurring in peroxisomes, OPDA is reduced by an OPDA reductase, followed by shortening the carboxylic acid side chain by three cycles of β-oxidation (Castillo et al., 2004; Afitlhile et al., 2005; Delker et al., 2007).
Among the biosynthetic enzymes, the AOC is regarded to be crucial for JA biosynthesis due to the establishment of the naturally occurring enantiomeric structure of JA (Wasternack and Hause, 2002). AOC has been cloned and is encoded by a single-copy gene in tomato (Ziegler et al., 2000) and Hordeum vulgare (Maucher et al., 2004), and by small gene families in Arabidopsis thaliana (Stenzel et al., 2003b) and Medicago truncatula (Isayenkov et al., 2005) and many other plant species (see www.ncbi.nlm.nih.gov). All AOC-cDNAs carry a transit peptide for plastid targeting and localization in chloroplasts was confirmed immunohistochemically (Ziegler et al., 2000; Stenzel et al., 2003b; Isayenkov et al., 2005).
Mechanical wounding is qualitatively similar but not identical to wounding by herbivores in terms of local and systemic responses (Korth and Dixon, 1997; Kessler and Baldwin, 2002; Mithöfer et al., 2005). Herbivore attack, in contrast to mechanical wounding, is accompanied by oral secretions. These contain compounds such as volicitin which are able to induce plant defence genes (Halitschke et al., 2003; Engelberth et al., 2004). Both stresses, however, result in the production of secondary compounds such as isoprenoids (Ament et al., 2004; Arimura et al., 2005; Leitner et al., 2005). In many organisms, including bacteria and plants, the rate-limiting step for isoprenoid biosynthesis is catalysed by 1-deoxy-D-xylulose 5-phosphate synthase (DXS) (Eisenreich et al., 1998; Carretero-Paulet et al., 2002; Estevez et al., 2001). In the first step of the plastid-located methyl-D-erythritol 4-phosphate (MEP) pathway, DXS converts D-glyceraldehyde 3-phosphate and pyruvate to 1-deoxy-D-xylulose 5-phosphate. The corresponding gene has been shown to be inducible by jasmonates (Van der Fits and Memelink, 2000; Sanchez-Hernandez et al., 2006; Arimura et al., 2007).
Usually, the early and transient elevation of JA in response to wounding is followed by the activation of genes coding for JA biosynthetic enzymes (Wasternack and Hause, 2002). In tomato, Arabidopsis, and M. truncatula, genes encoding AOC are also JA-inducible (Strassner et al., 2002; Stenzel et al., 2003a; Isayenkov et al., 2005) suggesting a feed forward regulatory loop. Most of the stress responses including wounding have been shown to be organ-specific in terms of transcript accumulation (Swindell, 2006). As far as is known, however, there are few data on the response of roots to their wounding. Compared with that following nematode infection, an accumulation of transcripts in roots after mechanical injury was monitored (Veronico et al., 2006). By contrast, a JA-induced de novo nicotine synthesis in roots of Nicotiana species was detected after leaf wounding and points to a systemic response of roots to wounding of shoots (Zhang and Baldwin, 1997; Baldwin, 1998; Shi et al., 2006).
In this paper, the question how roots and shoots of M. truncatula respond to mechanostimulation and wounding is addressed. The levels of JA and transcripts of MtAOC1 as well as of DXS2-1 were monitored. The data suggest a high sensitivity of M. truncatula to mechanostimulation performed by harvesting the plants that leads to the accumulation of JA and the transcripts under analysis. Moreover, effects of repeated touching of plants on their phenotype were analysed.
Materials and methods
Plant material and treatments
Plants of Medicago truncatula Gaertn. var. Jemalong (obtained from Austra Hort Pty, Australia), were grown in a phytochamber (Percival, CLF, Emersacker, Germany) at 23 °C, with a 16 h light period and in pots filled with expanded clay (Lecaton, 2–5 mm particle size; Fibo Exclay Deutschland, Pinneberg). Every pot was fertilized once per week with 10 ml Long Ashton fertilizer (Hewitt, 1966). Plants were harvested after 5 weeks by careful removal of the expanded clay. Wounding of roots and leaves was performed by squeezing the complete root system and all leaflets with tweezers. Subsequently, roots of all plants were covered with wet filter paper to avoid drought stress. After the indicated time periods, roots and shoots were frozen separately in liquid nitrogen. Material from three to five different plants was pooled to minimize biological differences. All experiments were done at least in triplicate. To monitor the effect of touching, plants were grown as described above. One-half of the plants remained untouched for the whole growth period of 5 weeks. Complete shoots of the plants of the second half were touched by hand three times per week for 10 s each.
Quantitative analysis of JA
About 1 g fresh weight of plant material frozen in liquid nitrogen was homogenized in a mortar and extracted with 5 ml 80% (v/v) methanol. To quantify JA, [2H6]JA was added in an appropriate amount before extraction. Ion exchange chromatography on DEAE Sephadex A-25 cartridges, reversed phase HPLC, and gas chromatography–mass spectrometry/selected ion monitoring analyses were performed as described (Hause et al., 2002).
Quantitative reverse-transcription (RT)-PCR
Total RNA was isolated from 100 mg of complete root systems as well as of whole shoots using the Plant RNeasy Extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA synthesis was performed with the first-strand cDNA synthesis kit for RT-PCR (Promega, Madison, WI, USA) using 1 μg of total RNA in a volume of 10 μl with 0.5 μg oligo(dT)15 primers (MWG BIOTECH, Ebersbach, Germany). After cooling on ice, 9 μl of a mixture containing 4 μl M-MLV RT 5× reaction buffer, 4 μl dNTP mix, and 1 μl M-MLV reverse transcriptase RNase minus, point mutant was added and incubated at 40 °C for 10 min and then at 42 °C for 50 min.
All PCR reactions were performed in three technical replicates. TaqMan™ probes and primers, both for MtEF1a (elongation factor 1-a as constitutive expression control) and MtAOC1, were purchased from Applied Biosystems (Assays-by Design service, Foster City, CA, USA) as described (Isayenkov et al., 2004, 2005). Real-time PCR was carried out using the ABI Prism 7000 sequence detection system with optical tubes and caps from Applied Biosystems. Template cDNA (20 ng) was added to a mixture of 10 μl 2× TaqMan Master Mix buffer and 1 μl 20× TaqMan probe with primers (Applied Biosystems) to reach a total volume of 20 μl. The cycling conditions and method of calculation were used according to Isayenkov et al. (2005).
For quantitative determination of transcripts of DXS2-1 (Walter et al., 2002), 3 μl of 1:10 diluted cDNA (15 ng of reverse transcribed total RNA) or 3 μl of diluted control reaction were mixed with SYBR Green PCR Mastermix (Applied Biosystems, Warrington, UK), 1 pmol of forward primer, and 1 pmol of reverse primer in a final volume of 10 μl in three independent replicates. The following primers and annealing temperature were used: forward primer, 5′-CAC CTT GGA TAC ATA AAT CAT TAA GTC TCT-3′; reverse primer, 5′-CCG AAT CTC TTC TCT CAA CCA AGA-3′; 60 °C. To normalize DXS2-1 expression for differences in the efficiency of cDNA synthesis, transcript levels of the constitutively expressed MtEF1a were measured using the following primers and temperature: forward primer, 5′-AGA AGG AAG CTG CTG AGA TGA AC-3′; reverse primer, 5′-TGA CTG TGC AGT AGT ACT TGG TG-3′; 60 °C. The efficiency of each primer pair was in the range of 0.95–1.0. Real-time PCR was done using the Mx 3005P QPCR system (Stratagene, La Jolla, CA, USA) with the following protocol: denaturation (95 °C for 10 min), amplification (40 cycles of 95 °C for 30 s, primer-specific annealing temperature for 1 min, and 72 °C for 30 s), and melting curve (95 °C for 1 min, 60 °C for 30 s, heating up to 95 °C with a heating rate of 0.1 °C s−1). Data were evaluated with the MxPro software (Stratagene). To correct for well-to-well fluorescent fluctuations, normalization of the SYBR Green–dsDNA complex signal to the passive reference dye ROX, which is included in the SYBR Green PCR Mastermix, was performed. Relative DXS2-1 expression levels were calculated by the comparative Ct method including normalization to the constitutively expressed gene and to a control sample.
Immunocytochemistry and in situ hybridization
Immunocytochemical analysis of stems was performed as described (Isayenkov et al., 2005). Small pieces of stems were fixed with 4% (w/v) paraformaldehyde/0.1% (v/v) Triton X-100 in phosphate-buffered saline (135 mM, NaCl, 3 mM KCl, 1.5 mM KH2PO4, and 8 mM Na2HPO4) for 2 h at room temperature. After dehydration in a graded series of ethanol, the specimens were infiltrated with polyethylene glycol 1500 (Merck KgaA, Darmstadt, Germany) at 50 °C. Cross-sections 10 μm thick were used for immunolabelling. The rabbit polyclonal antibody raised against recombinant LeAOC (Ziegler et al., 2000) was used at a dilution of 1:1000. The use of pre-immune serum at the same dilutions served as a control and revealed no signals. As secondary antibody, goat anti-rabbit IgG conjugated with AlexaFluor488 (Molecular Probes, Leiden, The Netherlands) was used according to the manufacturer's instructions. Counterstaining was performed with DAPI (4,6-diamidino-2-phenylindol; Sigma-Aldrich, Steinheim, Germany). Sections were analysed by epifluorescence microscopy using a Zeiss ‘AxioImager’ (Zeiss, Jena, Germany) equipped with a CCD camera or by confocal laser scanning microscopy using a LSM510 META (Zeiss).
For in situ hybridization, cross-sections (16 μm thick) of the same embedding were collected in sieves, rinsed in 0.1 M TRIS-HCl, pH 8.0, and incubated with 10 μg ml−1 proteinase K (Sigma-Aldrich) in 0.05 M TRIS-HCl, pH 7.5, and 5 mM EDTA for 30 min at 37 °C. After incubation with 1% (w/v) bovine serum albumin and 2 mg ml−1 glycine in 0.1 M TRIS-HCl, pH 8.0, for 30 min, sections were equilibrated in 0.1 M triethanolamine, pH 8.0, and then acetylated for 10 min with 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0. After acetylation, sections were dehydrated in a graded series of ethanol and air-dried. For hybridization, a solution consisting of 50% (v/v) formamide, 4× SSC, 350 μg ml−1 tRNA, 0.5% (w/v) blocking reagent (Roche Diagnostics, Mannheim, Germany) and 40 U ml−1 RNase inhibitor (Fermentas, St Leon-Rot, Germany), and containing denaturated digoxigenin (DIG)-labelled sense or antisense RNA (DIG RNA labelling kit; Roche Diagnostics) was applied and sections were incubated at 45 °C overnight. After subsequent washing steps with 50% formamid/4× SSC, 4× SSC, and 0.2× SSC at 45 °C for 10 min each, sections were incubated with 20 μg ml−1 RNase A at 37 °C for 30 min, followed by washing with 0.2× SSC at 45 °C for 5 min. After a short equilibration in TBS (0.1 M TRIS-HCl, pH 7.5, 0.15 M NaCl) and blocking of sections with 1% blocking reagent in TBS for 30 min at room temperature, immunological detection of DIG-labelled RNA hybrids was performed using a 1:2000 diluted anti-DIG-fab fragment conjugated with alkaline phosphatase (Roche Diagnostics) according to the supplier's protocol. The colorimetric reaction was performed for 3 h at 37 °C with detection buffer (0.1 M TRIS-HCl, pH 9.5, 0.1 M NaCl, and 50 mM MgCl2) containing 0.4 M nitroblue tetrazolium, 0.5 M 5-bromo-4-chloro-3-indolyl-phosphate, and 10 mM levamisol (Sigma-Aldrich). The reaction was stopped by washing the sections in TE (10 mM TRIS-HCl, pH 8.0, and 1 mM EDTA). After transfer of the sections to slides, micrographs were taken using a Zeiss ‘AxioImager’ microscope. All micrographs were processed through the Photoshop 8.0.1 program (Adobe).
Determination of chlorophyll content and phenotypic analysis
For chlorophyll determination, leaf material was homogenized in liquid nitrogen. Fifty milligrams of each sample were extracted twice with 1.5 ml absolute methanol. The supernatant (in total 3 ml) was collected and diluted 1:1 with methanol. The total chlorophyll content in 1 ml diluted extract was measured spectrophotometrically (DU 640 Beckmann Spectrophotometer; Beckmann Instruments, Munich, Germany) against methanol at 664.5 nm wavelength, showing adsorption maxima of the extract. Determination was carried out in three independent replicates. As phenotypic markers of growth, the weight of shoots and roots and length of the longest shoot were determined for each plant.
Results
JA content and transcript accumulation of MtAOC in shoots and roots of M. truncatula
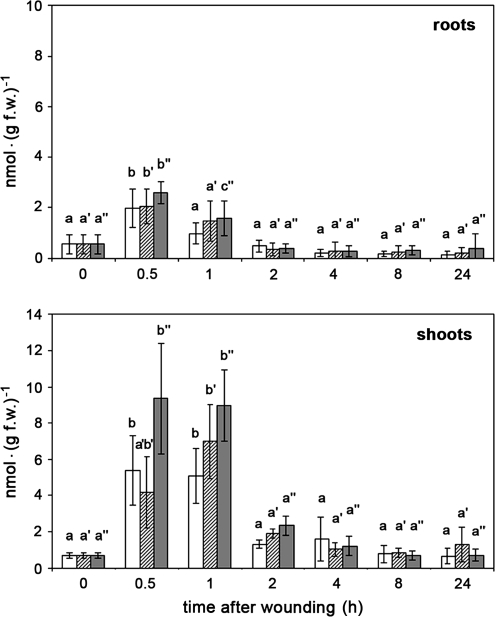
For plants grown in solid medium such as soil or expanded clay, harvest of roots may represent a mechanostimulation. Therefore, the effect of harvest was monitored in comparison with additional wounding of roots and shoots on the corresponding JA content. Five-week-old plants were harvested carefully to exclude wounding of roots during removal of the growth substrate. Surprisingly, both untreated shoots and roots exhibited a significant and transient increase in JA content (Fig. 1). Whereas roots showed a 4-fold increase with a maximum at 30 min, JA levels of shoots were raised nearly 8-fold between 30 min and 1 h after harvest. Additional wounding of roots performed by squeezing the whole roots system with tweezers, did not result in an additional increase of JA levels, either in roots wounded on intact plants or in roots wounded after separation from the shoot (Fig. 1, upper part). This is slightly different in shoots. Here, harvest of plants and wounding of roots cause a similar increase in JA content, whereas wounding of all leaves of the separated shoots tended to result in slightly higher JA levels (Fig. 1, lower part). For all treatments, however, the JA levels dropped down to basal levels of untreated tissues after 4 h.
Fig. 1.
Harvest- and wound-induced accumulation of JA in roots and shoots of 5-week-old M. truncatula plants. Plants were carefully removed from expanded clay and stayed untreated (white columns) or were wounded on roots (cross-hatched columns). From a third batch of plants shoots and roots were separated from each other and both were wounded (dark grey columns). At each time point, up to five plants were pooled, and roots and shoots were separately extracted for quantification of JA. The means ±SD are given for five biological replicates and are tested for each treatment with one-way ANOVA followed by Tukey HSD test (P <0.05). Means sharing the same letters are not significantly different.
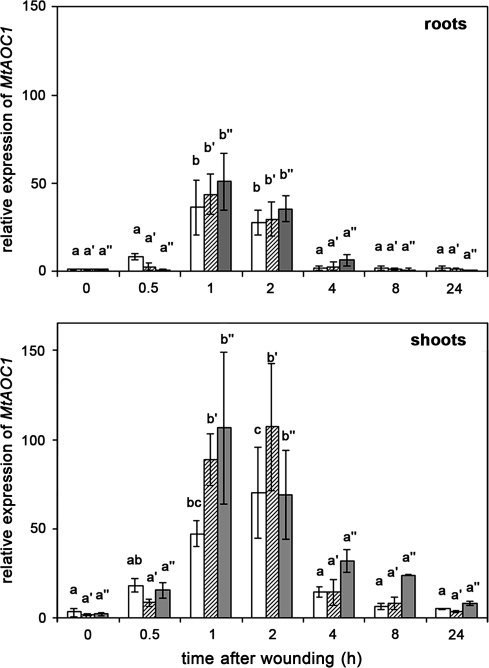
To inspect whether the increase in JA levels by harvest and wounding was accompanied by altered expression of JA biosynthesis genes, quantitative determination of transcript accumulation of MtAOC1 was recorded by qRT-PCR. MtAOC1 mRNA levels were altered with similar kinetics for roots and shoots by all three types of treatments (Fig. 2). In unwounded roots, harvest already caused a significant accumulation of MtAOC1 transcripts with a transient maximum between 1 h and 2 h after harvest. There was no significant increase in transcript accumulation after additional wounding, either in roots of whole plants or in roots separated from shoots at the time of wounding. This was slightly different in shoots, where transcripts accumulated to a much higher level and additional wounding of all leaves caused a minor enhancement of transcript levels in comparison with non-wounded plants. Similar to JA levels, MtAOC1 transcripts dropped down to basic levels of untreated plants 4 h after treatment.
Fig. 2.
Harvest- and wound-induced transcript accumulation of MtAOC1 in roots and shoots of 5-week-old M. truncatula plants. Relative MtAOC1 transcript levels were determined by real-time RT-PCR analysis using TaqMan probes. Plants were carefully removed from expanded clay and stayed untreated (white columns) or were wounded on roots (cross-hatched columns). From a third batch of plants, shoots and roots were separated from each other and both were wounded (dark gray columns). At each time point, up to five plants were pooled, and total RNA was extracted from roots and shoots separately. Real-time RT-PCR was carried out in triplicate for each sample. The mean MtAOC1 transcript level of plants directly after harvest (time point 0) was set to 1. Data are presented as mean values ±SD of at least three biological replicates. Different letters designate statistically different values separately for each treatment (ANOVA with Tukey HSD test, P < 0.05).
Cell-specific occurrence of MtAOC1 transcripts and protein
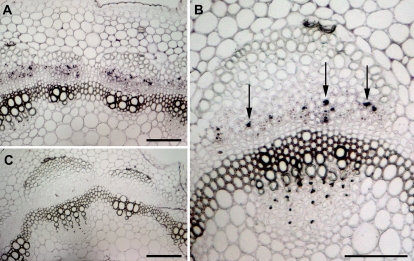
To examine where MtAOC1 transcripts and protein accumulate after harvest, MtAOC1 mRNA and protein were localized by in situ hybridizations and immunocytology, respectively, both of them in stem tissues at the maximum of transcript accumulation (Fig. 3). Two hours after harvest, MtAOC1 transcripts could be visualized in the phloem (Fig. 3A, B). The close-up revealed that transcripts occurred preferentially in companion cells, which are characterized by their typical pair-wise position next to sieve elements. Hybridization with a sense probe (Fig. 3C) as well as hybridization of plant material embedded directly after harvest (data not shown) did not exhibit specific labelling.
Fig. 3.
Localization of MtAOC1 transcripts in stems of M. truncatula 2 h after wounding of roots. In situ hybridization was performed on cross-sections 16 μm thick. (A) Hybridization with DIG-labelled antisense RNA for MtAOC1 shows staining in the phloem of the vascular bundles. (B) The close-up of one vascular bundle visualizes the occurrence of the signals in companion cells (arrows). (C) Control performed using DIG-labelled sense RNA shows no staining in the complete section. Scale bars represent 100 μm in all micrographs.
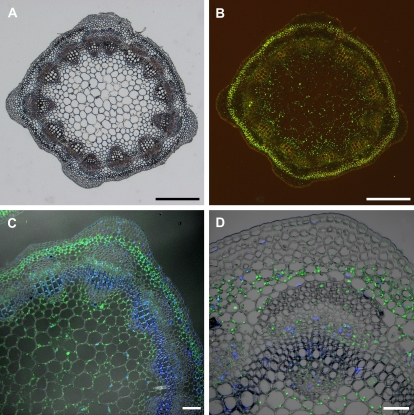
To analyse cell-specific occurrence of MtAOC protein in stems, cross-sections and immunohistological staining by an antibody specifically recognizing MtAOC were used (Isayenkov et al., 2005) (Fig. 4). MtAOC was clearly detectable in parenchymatic cells of the vascular bundles, the pith, and of the cortex. Analyses of immunolabelled sections by confocal laser scanning microscopy revealed the localization of MtAOC in plastids of the corresponding cells (Fig. 4C, D). There were no differences in cell-specific occurrence and labelling intensity detectable after the different treatments as harvest and additional wounding (data not shown). No signal was detectable in any of the cells when the tissues were probed with a pre-immune serum (Isayenkov et al., 2005, and data not shown).
Fig. 4.
Immunolocalization of AOC protein in cross-sections of stem of untreated M. truncatula plants. AOC was immunolabelled by an antibody against tomato AOC followed by a secondary antibody coupled to AlexaFluor488. The occurrence of AOC is visible by the green fluorescence signal. To visualize DNA-containing organelles, sections were counterstained with DAPI (C, D). (A) Bright-field image of a cross-section. (B) The same section as in (A) illuminated for green fluorescence. The green label indicative of AOC is visible in the pith, phloem tissue, and cortex. (C, D) Confocal laser scanning micrographs of the immunolabelled sections showing the occurrence of AOC in plastids of parenchymatic cells of pith, phloem, and cortex. The blue staining visualizes the nuclei due to the fluorescence of DAPI. Scale bars represent 500 μm in A and B, 100 μm in C, and 50 μm in D.
Transcript accumulation of MtDXS2-1 in roots and shoots of M. truncatula
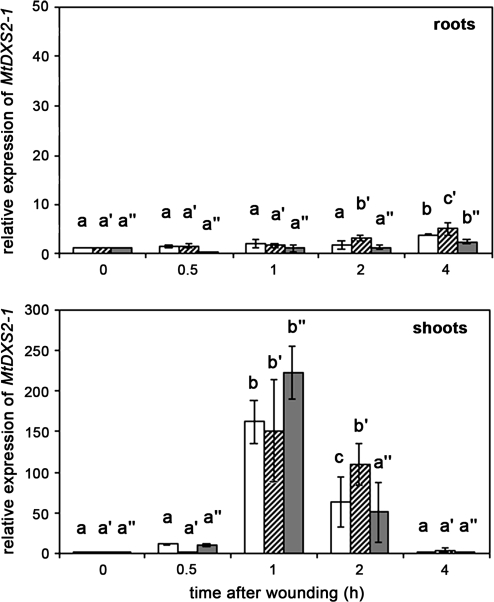
To analyse whether the harvest- and wound-induced increase in JA-levels lead to expression of a JA-responsive gene, quantitative RT-PCR was performed to determine transcript levels of MtDXS2-1. In contrast to MtAOC1, transcript levels of MtDXS2-1 remained nearly unaffected in roots, independent of the treatment. A slight increase was detectable at 4 h after treatment (Fig. 5, upper part). By contrast, a strong, but transient increase in transcript levels occurred in shoots 1 h after harvest of plants (Fig. 5, lower part). There was no additional increase in MtDXS2-1 mRNA levels upon wounding of roots or shoots. Transcript levels in shoots dropped down to basal levels of untreated plants 4 h after onset of treatment.
Fig. 5.
Harvest- and wound-induced transcript accumulation of MtDXS2-1 in roots and shoots of 5-week-old M. truncatula plants. Relative MtDXS2-1 transcript levels were determined using real-time RT-PCR analysis. Plants were carefully removed from expanded clay and stayed untreated (white columns) or were wounded on roots (cross-hatched columns). From a third batch of plants shoots and roots were separated from each other and both were wounded (dark grey columns). At each time point, up to five plants were pooled, and total RNA was extracted from roots and shoots separately. Real-time RT-PCR was carried out in triplicate for each sample. The mean MtDXS2-1 transcript level of plants directly after harvest (time point 0) was set to 1. Data are presented as mean values ±SD from at least four biological replicates. Different letters designate statistically different values for each treatment (ANOVA with Tukey HSD test, P < 0.01).
Repeated mechanostimulation causes alteration of the phenotype of M. truncatula
The harvest-induced rise in JA levels as well as in MtAOC1 and MtDXS2-1 transcript levels of roots and preferentially of shoots suggests that M. truncatula is highly sensitive in respect to JA formation and JA responses upon mechanostimulation. Therefore, the effects of repeated touching of shoots were analysed. Plants were grown for 5 weeks either without any touching (Fig. 6A) or shoots were slightly touched three times per week for 10 s (Fig. 6B). Touched plants exhibited a stunted phenotype and elevated chlorophyll content. This is reflected in various growth parameters listed in Table 1. The shoot length of touched plants decreased significantly, whereas shoot weight and chlorophyll content increased significantly. Although the root weight was not changed, the increased shoot weight led to an increase in the total biomass of touched plants in comparison to non-touched plants.
Fig. 6.
Effect of repeated touching of shoots on plant growth of M. truncatula. Plants were grown without touching (A) or with repeated touch stimulation performed by touching shoots for 10 s three times per week (B). (This figure is available in colour at JXB online).
Table 1.
Effect of repeated touching of shoots on plant growth of M. truncatula
| Control | Touch treatment | P-value | |
| Shoot length (cm) | 20.7±3.0 | 15.2±2.3 | 2.35. 10−6 |
| Shoot weight (g) | 1.85±0.54 | 2.35±0.45 | 0.0025 |
| Root weight (g) | 2.04±0.49 | 2.23±0.32 | 0.1533 |
| Total biomass (g) | 3.88±0.86 | 4.58±0.67 | 0.0059 |
| Chlorophyll (E664.5nm g−1) | 301±58 | 418±53 | 0.00018 |
Plants were grown without movement of shoots (control) or shoots were slightly touched for 10 s three times per week. After 5 weeks, the fresh weight of shoots and roots, shoot length, as well as chlorophyll content of leaves were determined. Data are given as mean ±SD (n=20). P-values were calculated using Student's t-test.
To check whether touching results in similar responses as harvest, JA levels and MtAOC1 transcript levels were monitored 60 min after touching the shoots for 10 s. The control set of plants remained untouched. The JA content of shoots and roots did not differ significantly between touched and untouched plants due to high variations in biological replicates (data not shown). However, relative MtAOC1 transcript levels of shoots of touched plants exhibited a 4.4-fold increase compared with the untouched plants (0.104±0.031 versus 0.024±0.002; P < 0.01). Even in the roots of touched plants there was a 2.4-fold increase of MtAOC1 transcript level detectable in comparison with untouched plants (0.048±0.011 versus 0.020±0.002; P < 0.01).
Discussion
The activation of herbivore- and wound-induced defence responses involves a complex network of plant signalling cascades (Rojo et al., 2003; Stratmann, 2003; Schaller et al., 2004). Thereby, jasmonates represent the best characterized class of signals mediating the elicitation of these responses (Schilmiller and Howe, 2005; Delker et al., 2007; Farmer, 2007). Upon wounding, JA levels increase locally, but jasmonates are also involved in the regulation of systemic response (Howe, 2004) as shown by grafting experiments with a JA-insensitive tomato and a mutant, which is defective in JA biosynthesis (Li et al., 2002; Ryan and Moura, 2002). Much less is known, however, on how roots respond to wounding in terms of jasmonate biosynthesis. Regarding root wounding, only transcript accumulations in wounded roots after nematode infection were reported (Veronico et al., 2006). To get insights into the root response to wounding and especially the possible role of jasmonates in that process, the accumulation of JA in shoots and roots of M. truncatula upon mechanostimulation (harvest) and additional wounding was analysed.
In the work presented here, wounding was performed on roots of complete plants or on shoots and roots separated from each other. Unexpectedly, JA accumulated already after mechanostimulation caused by harvest in both parts of the plant, independently on whether they had been separated from each other or not. Also in aeroponically grown M. truncatula plants, mechanical disturbances (performed by shaking the plants) lead to increased JA levels (K Pawlowski, personal communication). Subsequent wounding did not lead to an additional increase in JA levels. This is surprising, because up to now elevated jasmonate levels were only detected after wounding, herbivory, pathogen attack, or osmotic stress (Wasternack, 2006).
Usually, the wound-induced elevation of jasmonate levels occurs transiently followed by activation of genes coding for JA biosynthetic enzymes (Wasternack and Hause, 2002). In tomato, Arabidopsis and M. truncatula, most genes encoding enzymes of JA biosynthesis are JA-inducible (Strassner et al., 2002; Stenzel et al., 2003a; Isayenkov et al., 2005). As shown here, upon mechanostimulation by harvest of M. truncatula, the rise in JA precedes also the accumulation of MtAOC1 transcripts. Similar to the increase in JA levels, additional wounding did not lead to significantly enhanced levels of MtAOC1 transcripts either in shoots or in roots. This prompted the analysis of tissue-specific occurrence of MtAOC1 transcripts and AOC protein in stems upon mechanostimulation. MtAOC1 transcripts have been detected in vascular tissues only. The detailed analysis revealed the preferential occurrence of MtAOC1 transcripts in companion cells where AOC protein is also located. This is similar to tomato, where AOC is located exclusively in vascular tissues, in sieve elements, and in companion cells (Hause et al., 2003). In tomato, the biosynthesis of jasmonates upon wounding is restricted to the veins of a leaf and has been shown to be reminiscent of the location of JA-inducible proteins specifically in the vascular bundle (Stenzel et al., 2003a). The immunological detection of AOC protein in stems shown here for M. truncatula revealed, however, the occurrence of AOC in parenchymatic cells of the vascular bundles, of the pith, and of the cortex. This was independent of the treatment, but differed from leaves and roots, which showed AOC protein exclusively restricted to vascular bundles (Isayenkov et al., 2005). The protein constitutively present seems to be active in biosynthesis of JA after mechanostimulation. This could be realized by activation of the enzyme or by increased substrate availability as proposed for tomato (Hause et al., 2000; Stenzel et al., 2003a). Obviously, vascular tissues of M. truncatula stems carry the capacity to form jasmonates and—regarding MtAOC1 as a gene induced upon endogenous rise of JA—produce jasmonates after mechanostimulation.
Rises in levels of JA as well as MtAOC1 transcripts in response to mechanostimulation occurred to a much higher extent in shoots than in roots. The lower response level of roots in term of JA-induced transcript accumulation is also reflected in the transcript accumulation of MtDXS2-1. In this case, enhanced transcript levels could be monitored in shoots only. The present data point to a regulation of MtDXS2-1 transcript accumulation by wound-induced jasmonates in shoots, because the rise in JA precedes the transient maximum of MtDXS2-1 transcript levels. The lower level of JA in roots, however, might be insufficient to induce the MtDXS2-1 expression. This is supported by the fact that the promoter of MtDXS2-1 is not activated in roots by wounding but by application of at least 100 μM JA for 24 h (D Floss and B Hause, unpublished results). The recorded member of the DXS gene family, DXS2, codes for the key enzyme involved in the MEP pathway leading to isoprenoids (Estevez et al., 2001), and has been shown to be up-regulated in response to wounding of leaves and JA treatment (Bede et al., 2006; Sanchez-Hernandez et al., 2006). The resulting release of wound-induced terpene volatiles is therefore controlled through a JA-regulated MEP pathway and contributes to the well-described indirect defence mechanisms of plants against herbivores (Leitner et al., 2005; Arimura et al., 2007; Gao et al., 2007).
The data shown here suggest that even the harvest represents a mechanostimulation of plants leading to increased JA levels in roots and shoots. This raises the question how M. truncatula plants react to repeated mechanostimulation of shoots such as repeated touching over several weeks. This ‘touch response’ is well described for Arabidopsis in terms of phenotypic alterations such as delay in flowering and an inhibition of inflorescence elongation (Braam, 2005). In Arabidopsis, over 2.5% of total genes were touch-inducible including genes related to calcium and kinase signalling, cell wall modifications, and disease resistance responses (Lee et al., 2005). Among these touch-induced genes are also those coding for AOS and AOC3. It is tempting to speculate that touch-responsive genes might be regulated at least partially by increased jasmonate levels. Due to the fact that M. truncatula is regarded to be a model system for plant functional genomics and is frequently used for studying biological processes that are unique and pertinent to legumes (Suzuki et al., 2005), effects of mechanostimulation occurring upon harvest of plants should be considered in experiments regarding global transcript accumulations.
Touch-treated M. truncatula plants showed an altered phenotype in comparison with non-touched plants. They exhibited a stunted phenotype reflected by decreased shoot length and increased biomass, and an elevated chlorophyll content. These features represent morphological traits that are characteristic of plants treated with JA or plants exhibiting a constitutive high level of JA (Wasternack and Hause, 2002). The Arabidopsis mutant cev1, which has constitutively elevated JA levels, produces plants that have stunted roots and shoots and accumulate anthocyanins (Ellis et al., 2002). Furthermore, growth inhibition preferentially of roots is mediated by JA and has been used for numerous screens to identify mutants affected in JA signalling (Berger, 2002; Yan et al., 2007). For touch-treated M. truncatula plants, an increase in JA levels has not been detected, but MtAOC1 transcript levels were significantly increased both in shoots and in roots. Due to the accumulation of MtAOC1 transcripts upon endogenous rise of JA, this increased transcript level may visualize a subtle JA increase, which, after repeated touching, might lead to the phenotype described.
In conclusion, the data presented here revealed that M. truncatula plants respond very sensitively to mechanostimulation. Mechanostimulation by harvest leads to a rapid increase in JA levels followed by increased transcript accumulation of JA-responsive genes. Even repeated mechanostimulation performed by touching obviously changed the phenotype of plants. It will be interesting to analyse whether repeated touching of plants will influence other features of the plants such as biotic interactions with pathogens or symbionts. It could be reasonable that enhanced JA levels over time due to repeated mechanostimulation influence such interactions.
Acknowledgments
The authors thank Otto Miersch for the GC-MS measurements and the synthesis of [2H6]JA, Martin Wolfram for determination of chlorophyll contents, and Daniela Floss for providing primers for MtDXS2-1. Claus Wasternack and Dieter Strack are acknowledged for critically reading the manuscript.
Glossary
Abbreviations
- AOC
allene oxide cyclase
- AOS
allene oxide synthase
- DXS
1-deoxy-D-xylulose 5-phosphate synthase
- 13-HPOT
(13-S)-hydroperoxy linolenic acid
- JA
jasmonic acid
- MEP
methyl-D-erythritol 4-phosphate
- OPDA
12-oxophytodienoic acid
References
- Afitlhile MM, Fukushige H, Nishimura M, Hildebrand DF. A defect in glyoxysomal fatty acid β-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiology and Biochemistry. 2005;43:603–609. doi: 10.1016/j.plaphy.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiology. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G-i, Garms S, Maffei M, Bossi S, Schulze B, Leitner M, Mithöfer A, Boland W. Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta. 2007;227:453–463. doi: 10.1007/s00425-007-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G-i, Kost C, Boland W. Herbivore-induced, indirect plant defences. Biochimica et Biophysica Acta – Molecular and Cell Biology of Lipids. 2005;1734:91–111. doi: 10.1016/j.bbalip.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proceedings of the National Academy of Sciences, USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede J, Musser R, Felton G, Korth K. Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Molecular Biology. 2006;60:519–531. doi: 10.1007/s11103-005-4923-y. [DOI] [PubMed] [Google Scholar]
- Berger S. Jasmonate-related mutants of Arabidopsis as tools for studying stress signaling. Planta. 2002;214:497–504. doi: 10.1007/s00425-001-0688-y. [DOI] [PubMed] [Google Scholar]
- Braam J. In touch: plant responses to mechanical stimuli. New Phytologist. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- Carretero-Paulet L, Ahumada I, Cunillera N, Rodriguez-Concepcion M, Ferrer A, Boronat A, Campos N. Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiology. 2002;129:1581–1591. doi: 10.1104/pp.003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo MC, Martinez C, Buchala A, Metraux J-P, Leon J. Gene-specific involvement of β-oxidation in wound-activated responses in Arabidopsis. Plant Physiology. 2004;135:85–94. doi: 10.1104/pp.104.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaig BC, Melotto M, He SY, Howe GA. Regulation of plant arginase by wounding, jasmonate, and the phytotoxin coronatine. Journal of Biological Chemistry. 2004;279:45998–46007. doi: 10.1074/jbc.M407151200. [DOI] [PubMed] [Google Scholar]
- Delker C, Zolman BK, Miersch O, Wasternack C. Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal β-oxidation enzymes: additional proof by properties of pex6 and aim1. Phytochemistry. 2007;68:1642–1650. doi: 10.1016/j.phytochem.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chemistry and Biology. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. The Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Alborn H, Schmelz E, Tumlinson J. Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences, USA. 2004;101:1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez JM, Cantero A, Reindl A, Reichler S, Leon P. 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. Journal of Biological Chemistry. 2001;276:22901–22909. doi: 10.1074/jbc.M100854200. [DOI] [PubMed] [Google Scholar]
- Farmer EE. Plant biology: jasmonate perception machines. Nature. 2007;448:659–660. doi: 10.1038/448659a. [DOI] [PubMed] [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Annual Reviews in Plant Biology. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Gao L-L, Anderson JP, Klingler JP, Nair RM, Edwards OR, Singh KB. Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Molecular Plant-Microbe Interaction. 2007;20:82–93. doi: 10.1094/MPMI-20-0082. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hu D, Schmidt D, Baldwin I. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiology. 2003;131:1894–1902. doi: 10.1104/pp.102.018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Hause G, Kutter C, Miersch O, Wasternack C. Enzymes of jasmonate biosynthesis occur in tomato sieve elements. Plant and Cell Physiology. 2003;44:643–648. doi: 10.1093/pcp/pcg072. [DOI] [PubMed] [Google Scholar]
- Hause B, Maier W, Miersch O, Kramell R, Strack D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiology. 2002;130:1213–1220. doi: 10.1104/pp.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Stenzel I, Miersch O, Maucher H, Kramell R, Ziegler J, Wasternack C. Tissue-specific oxylipin signature of tomato flowers: allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. The Plant Journal. 2000;24:113–126. doi: 10.1046/j.1365-313x.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. 2nd edn. Farnham Royal: Commonwealth Agricultural Bureaux; 1966. [Google Scholar]
- Howe GA. Jasmonates as signals in the wound response. Journal of Plant Growth Regulation. 2004;23:223–237. [Google Scholar]
- Isayenkov S, Fester T, Hause B. Rapid determination of fungal colonization and arbuscule formation in roots of Medicago truncatula using real-time (RT) PCR. Journal of Plant Physiology. 2004;161:1379–1383. doi: 10.1016/j.jplph.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Isayenkov S, Mrosk C, Stenzel I, Strack D, Hause B. Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiology. 2005;139:1401–1410. doi: 10.1104/pp.105.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin I. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review in Plant Biology. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Korth KL, Dixon RA. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiology. 1997;115:1299–1305. doi: 10.1104/pp.115.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Polisensky DH, Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytologist. 2005;165:429–444. doi: 10.1111/j.1469-8137.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- Leitner M, Boland W, Mithöfer A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytologist. 2005;167:597–606. doi: 10.1111/j.1469-8137.2005.01426.x. [DOI] [PubMed] [Google Scholar]
- Li C, Schilmiller AL, Liu G, et al. Role of β-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. The Plant Cell. 2005;17:971–986. doi: 10.1105/tpc.104.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li C, Lee G, Howe G. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proceedings of the National Academy of Sciences, USA. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucher H, Stenzel I, Miersch O, Stein N, Prasa M, Zierold U, Schweizer P, Dorer C, Hause B, Wasternack C. The allene oxide cyclase of barley (Hordeum vulgare L.): cloning and organ-specific expression. Phytochemistry. 2004;65:801–811. doi: 10.1016/j.phytochem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Wanner G, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiology. 2005;137:1160–1168. doi: 10.1104/pp.104.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas M, Narváez-Vásquez J, Ryan C. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. The Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Solano R, Sánchez-Serrano J. Interactions between signaling compounds involved in plant defense. Journal of Plant Growth Regulation. 2003;22:82–98. [Google Scholar]
- Ryan C. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica and Biophysica Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Ryan C, Moura D. Systemic wound signaling in plants: a new perception. Proceedings of the National Academy of Sciences, USA. 2002;99:6519–6520. doi: 10.1073/pnas.112196499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Hernandez C, Lopez MG, Delano-Frier JP. Reduced levels of volatile emissions in jasmonate-deficient spr2 tomato mutants favour oviposition by insect herbivores. Plant, Cell and Environment. 2006;29:546–557. doi: 10.1111/j.1365-3040.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Schaller F, Schaller A, Stintzi A. Biosynthesis and metabolism of jasmonates. Journal of Plant Growth Regulation. 2004;23:179–199. [Google Scholar]
- Schilmiller AL, Howe GA. Systemic signaling in the wound response. Current Opinion in Plant Biology. 2005;8:369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Shi Q, Li C, Zhang F. Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin. Journal of Experimental Botany. 2006;57:2899–2907. doi: 10.1093/jxb/erl051. [DOI] [PubMed] [Google Scholar]
- Stenzel I, Hause B, Maucher H, Pitzschke A, Miersch O, Ziegler J, Ryan C, Wasternack C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato: amplification in wound signaling. The Plant Journal. 2003a;33:577–589. doi: 10.1046/j.1365-313x.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, Ziegler J, Feussner I, Wasternack C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Molecular Biology. 2003b;51:895–911. doi: 10.1023/a:1023049319723. [DOI] [PubMed] [Google Scholar]
- Strassner J, Schaller F, Frick U, Howe G, Weiler E, Amrhein N, Macheroux P, Schaller A. Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductase reveals differential roles for ocatdecanoid biosynthesis in the local versus the systemic wound response. The Plant Journal. 2002;32:585–601. doi: 10.1046/j.1365-313x.2002.01449.x. [DOI] [PubMed] [Google Scholar]
- Stratmann J. Long distance run in the wound response: jasmonic acid is pulling ahead. Trends in Plant Science. 2003;8:247–250. doi: 10.1016/S1360-1385(03)00106-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Reddy MSS, Naoumkina M, Aziz N, May GD, Huhman DV, Sumner LW, Blount JW, Mendes P, Dixon RA. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:696–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- Swindell WR. The association among gene expression responses to nine abiotic stress treatments in Arabidopsis thaliana. Genetics. 2006;174:1811–1824. doi: 10.1534/genetics.106.061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proceedings of the National Academy of Sciences, USA. 2007;104:1075–1080. doi: 10.1073/pnas.0605423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Fits L, Memelink J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science. 2000;289:295–297. doi: 10.1126/science.289.5477.295. [DOI] [PubMed] [Google Scholar]
- Veronico P, Giannino D, Melillo MT, Leone A, Reyes A, Kennedy MW, Bleve-Zacheo T. A novel lipoxygenase in pea roots: its function in wounding and biotic stress. Plant Physiology. 2006;141:1045–1055. doi: 10.1104/pp.106.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter MH, Hans J, Strack D. Two distantly related genes encoding 1-deoxy-D-xylulose 5-phosphate synthases: differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. The Plant Journal. 2002;31:243–254. doi: 10.1046/j.1365-313x.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Oxylipins: biosynthesis, signal transduction and action. In: Hedden P, Thomas S, editors. Plant hormone signaling Annual Plant Reviews. Oxford: Blackwell Publishing; 2006. pp. 185–228. [Google Scholar]
- Wasternack C, Hause B. Jasmonates and octadecanoids: signals in plant stress response and development. In: Moldave K, editor. Progress in nucleic acid research and molecular biology. New York, NY: Academic Press; 2002. pp. 165–221. [DOI] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. A downstream mediator in the growth repression limb of the jasmonate pathway. The Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-P, Baldwin I. Transport of [2-14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta. 1997;203:436–441. [Google Scholar]
- Ziegler J, Stenzel I, Hause B, Maucher H, Hamberg M, Grimm R, Ganal M, Wasternack C. Molecular cloning of allene oxide cyclase: the enzyme establishing the stereochemistry of octadecanoids and jasmonates. Journal of Biological Chemistry. 2000;275:19132–19138. doi: 10.1074/jbc.M002133200. [DOI] [PubMed] [Google Scholar]