Abstract
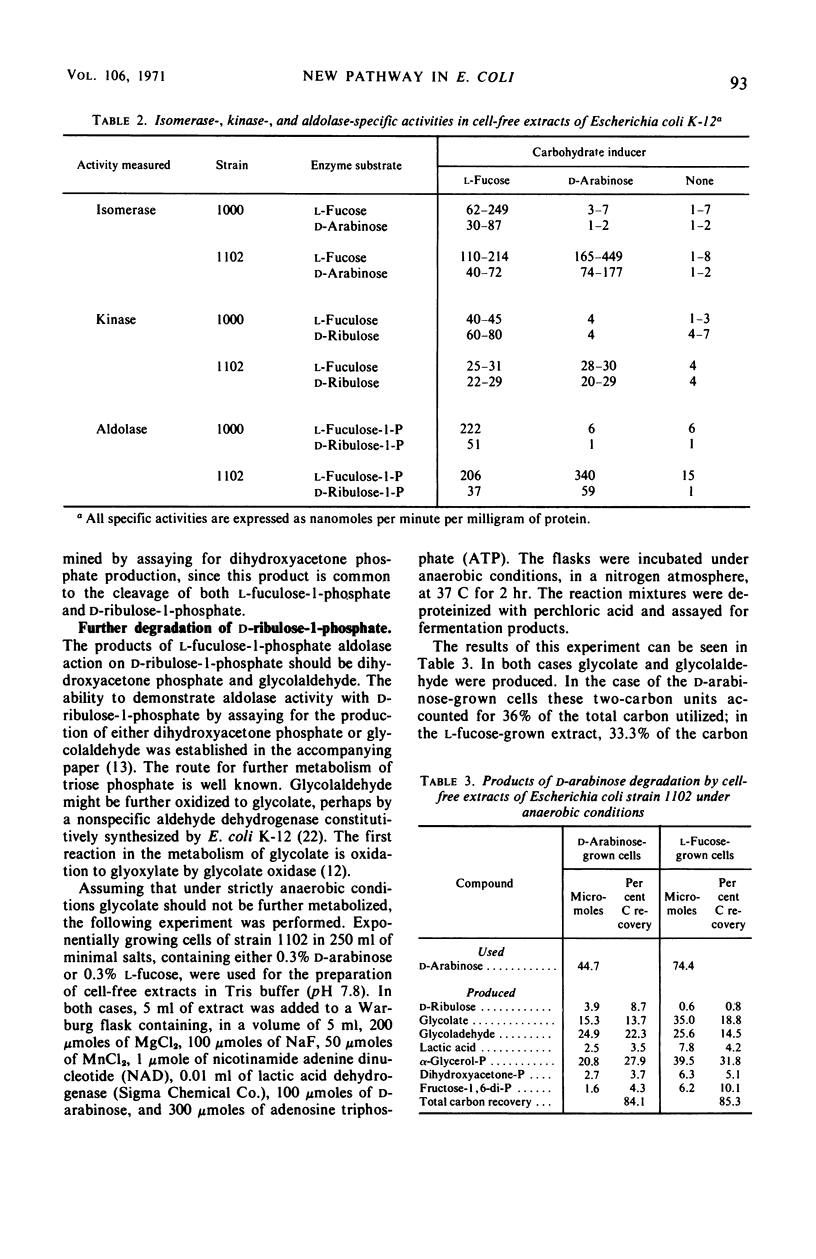
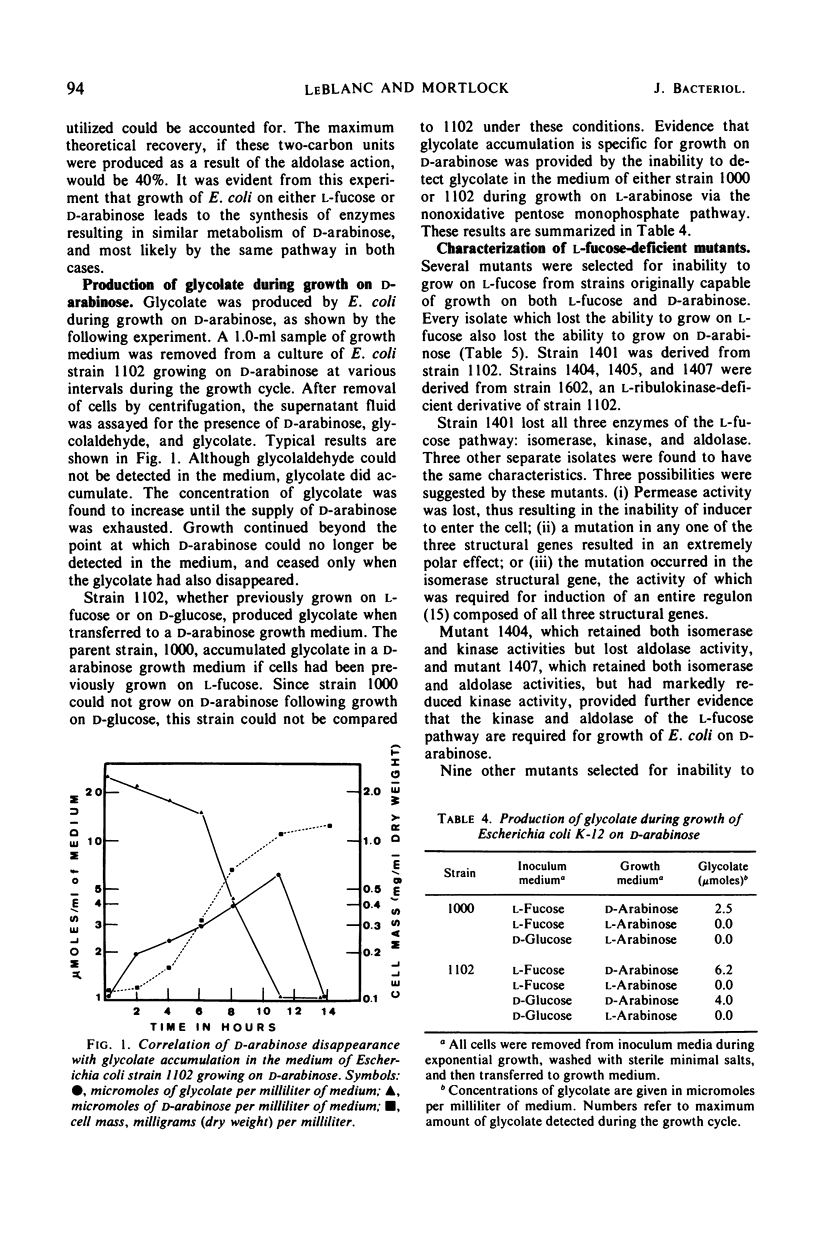
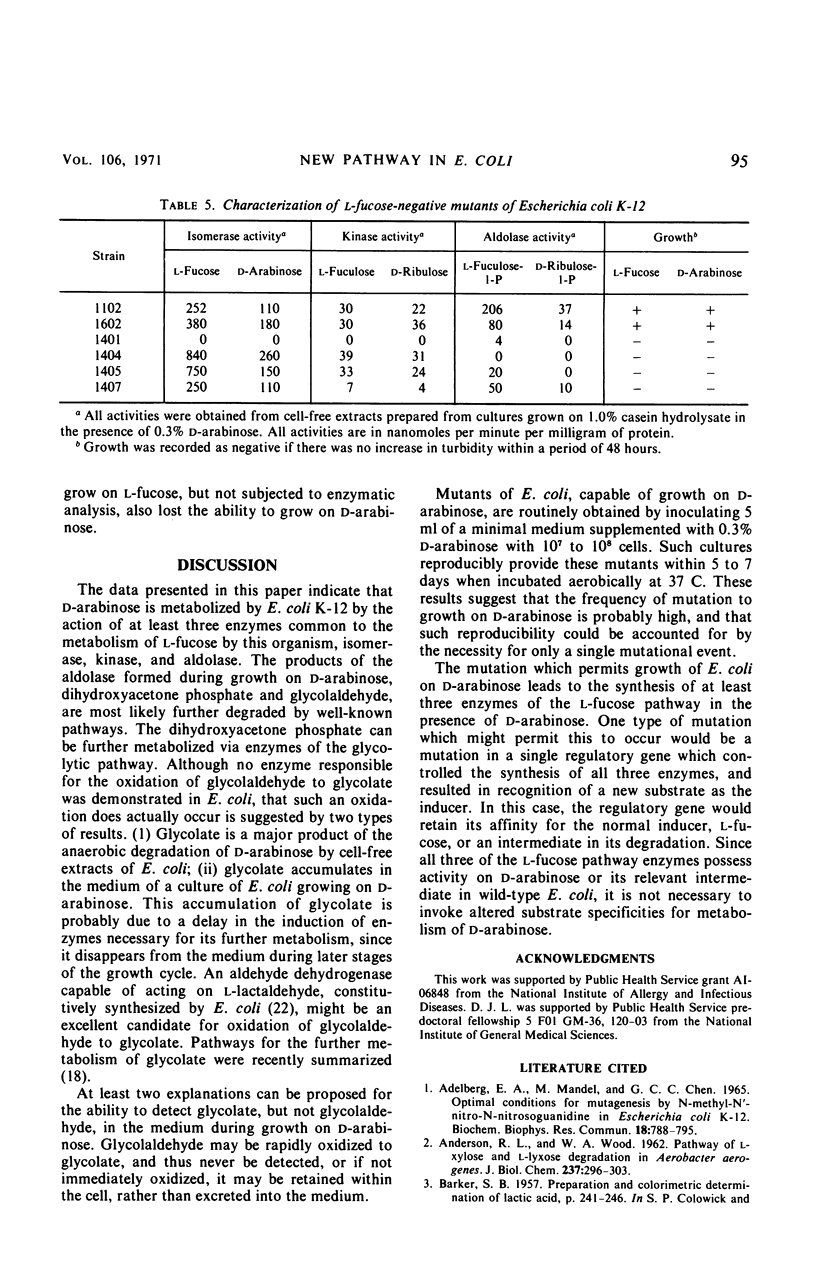
Several growth characteristics of Escherichia coli K-12 suggest that growth on l-fucose results in the synthesis of all the enzymes necessary for growth on d-arabinose. Conversely, when a mutant of E. coli is grown on d-arabinose, all of the enzymes necessary for immediate growth on l-fucose are present. Three enzymes of the l-fucose pathway in E. coli, l-fucose isomerase, l-fuculokinase, and l-fuculose-l-phospháte aldolase possess activity on d-arabinose, d-ribulose, and d-ribulose-l-phosphate, respectively. The products of the aldolase, with d-ribulose-l-phosphate as substrate, are dihydroxyacetone phosphate and glycolaldehyde. l-Fucose, but not d-arabinose, is capable of inducing these activities in wild-type E. coli. In mutants capable of utilizing d-arabinose as sole source of carbon and energy, these activities are induced in the presence of d-arabinose and in the presence of l-fucose. Mutants unable to utilize l-fucose, selected from strains capable of growth on d-arabinose, are found to have lost the ability to grow on d-arabinose. Enzymatic analysis of cell-free extracts, prepared from cultures of these mutants, reveals that a deficiency in any of the l-fucose pathway enzymes results in the loss of ability to utilize d-arabinose. Thus, the pathway of d-arabinose catabolism in E. coli K-12 is believed to be: d-arabinose ⇌ d-ribulose → d-ribulose-l-phosphate ⇌ dihydroxyacetone phosphate plus glycolaldehyde. Evidence is presented which suggests that the glycolaldehyde is further oxidized to glycolate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R. L., WOOD W. A. Pathway of L-xylose and L-lyxose degradation in Aerobacter aerogenes. J Biol Chem. 1962 Feb;237:296–303. [PubMed] [Google Scholar]
- COHEN S. Studies on D-ribulose and its enzymatic conversion to D-arabinose. J Biol Chem. 1953 Mar;201(1):71–84. [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- KORNBERG H. L., SADLER J. R. The metabolism of C2-compounds in micro-organisms. VIII. A dicarboxylic acid cycle as a route for the oxidation of glycollate by Escherichia coli. Biochem J. 1961 Dec;81:503–513. doi: 10.1042/bj0810503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Mortlock R. P. Metabolism of D-arabinose: origin of a D-ribulokinase activity in Escherichia coli. J Bacteriol. 1971 Apr;106(1):82–89. doi: 10.1128/jb.106.1.82-89.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R., Cohen S. S. D-phosphoarabinoisomerase and D-ribulokinase in Escherichia coli. J Biol Chem. 1966 Oct 10;241(19):4304–4315. [PubMed] [Google Scholar]
- MORTLOCK R. P., WOOD W. A. METABOLISM OF PENTOSES AND PENTITOLS BY AEROBACTER AEROGENES. I. DEMONSTRATION OF PENTOSE ISOMERASE, PENTULOKINASE, AND PENTITOL DEHYDROGENASE ENZYME FAMILIES. J Bacteriol. 1964 Oct;88:838–844. doi: 10.1128/jb.88.4.838-844.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K. Regulation of glyoxylate metabolism in Escherichia coli K-12. J Bacteriol. 1969 Jun;98(3):1098–1108. doi: 10.1128/jb.98.3.1098-1108.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALLERONI N. J., DOUDOROFF M. Metabolism of carbohydrates by Pseudomonas saccharophila. III. Oxidation of D-arabinose. J Bacteriol. 1957 Aug;74(2):180–185. doi: 10.1128/jb.74.2.180-185.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T. Purification and properties of lactaldehyde dehydrogenase from Escherichia coli. J Biol Chem. 1969 Oct 10;244(19):5233–5238. [PubMed] [Google Scholar]



