Abstract
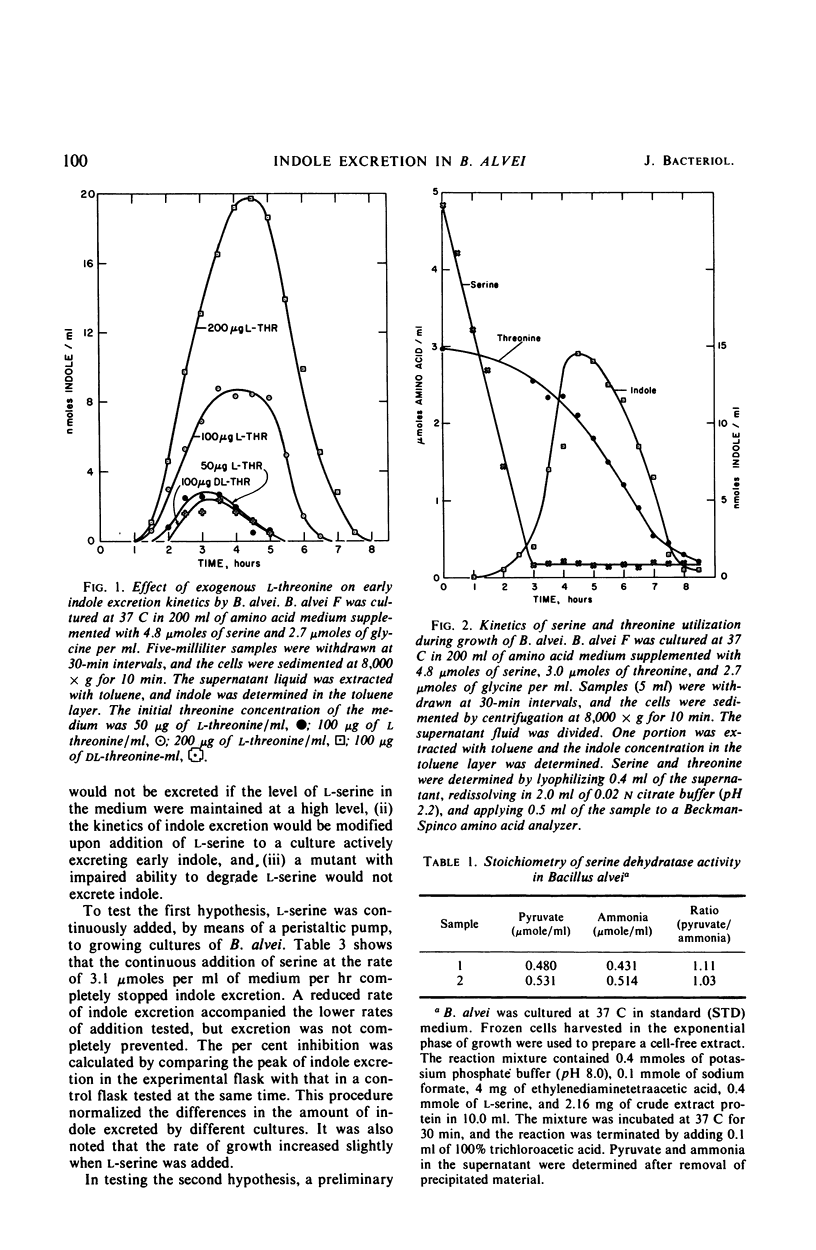
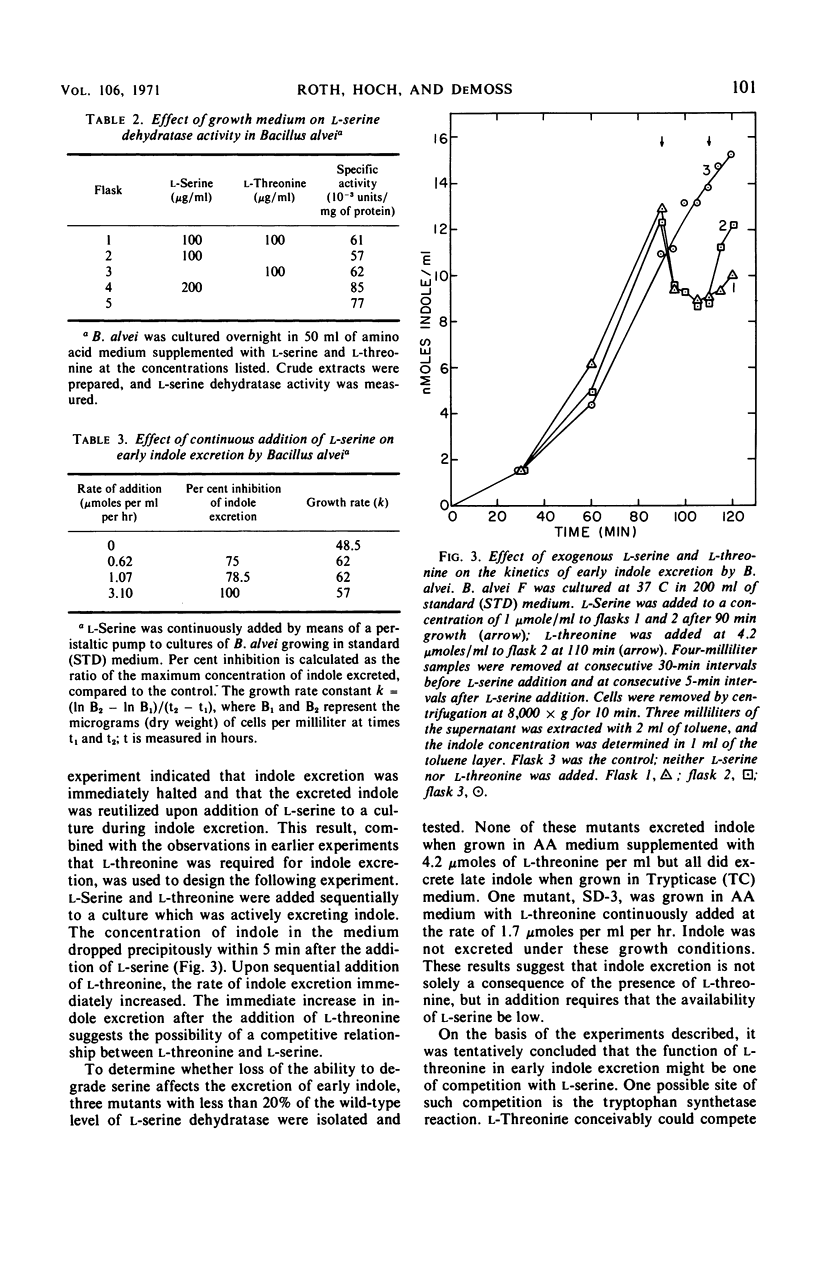
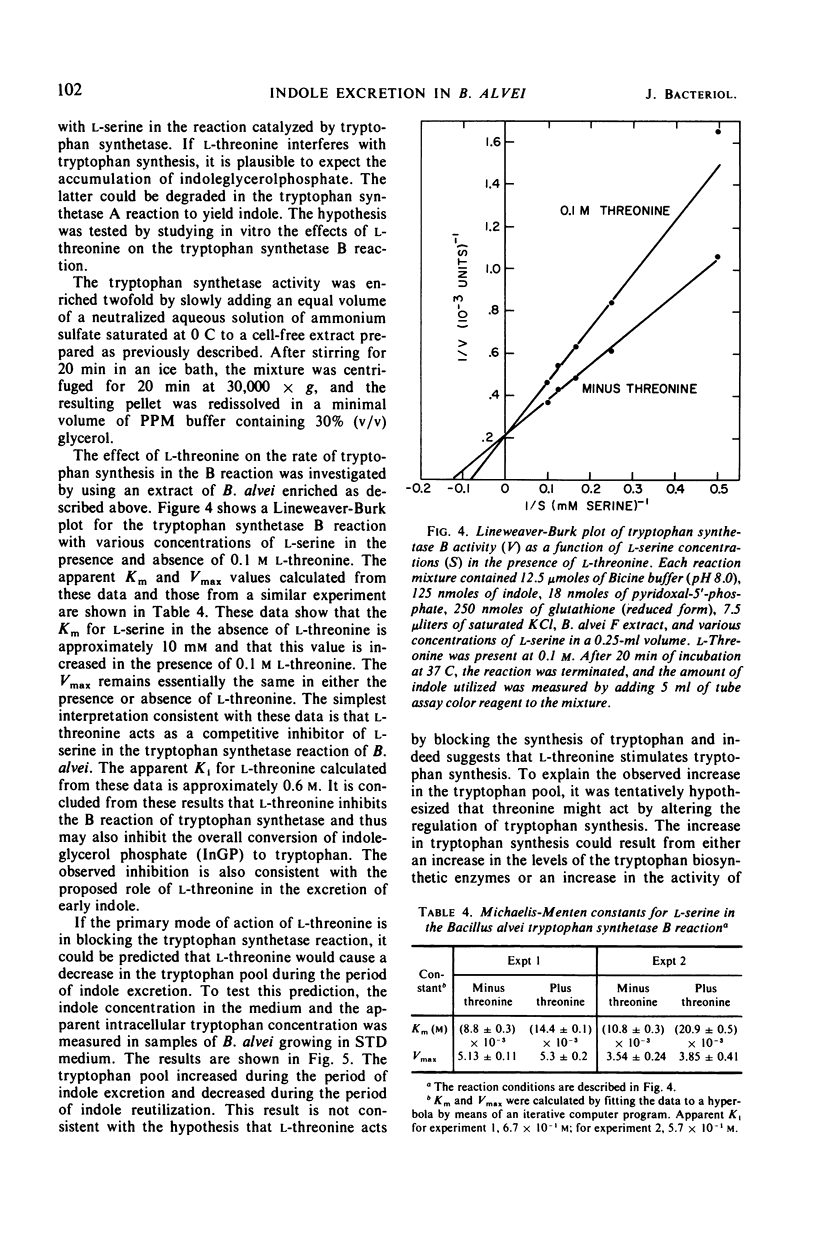
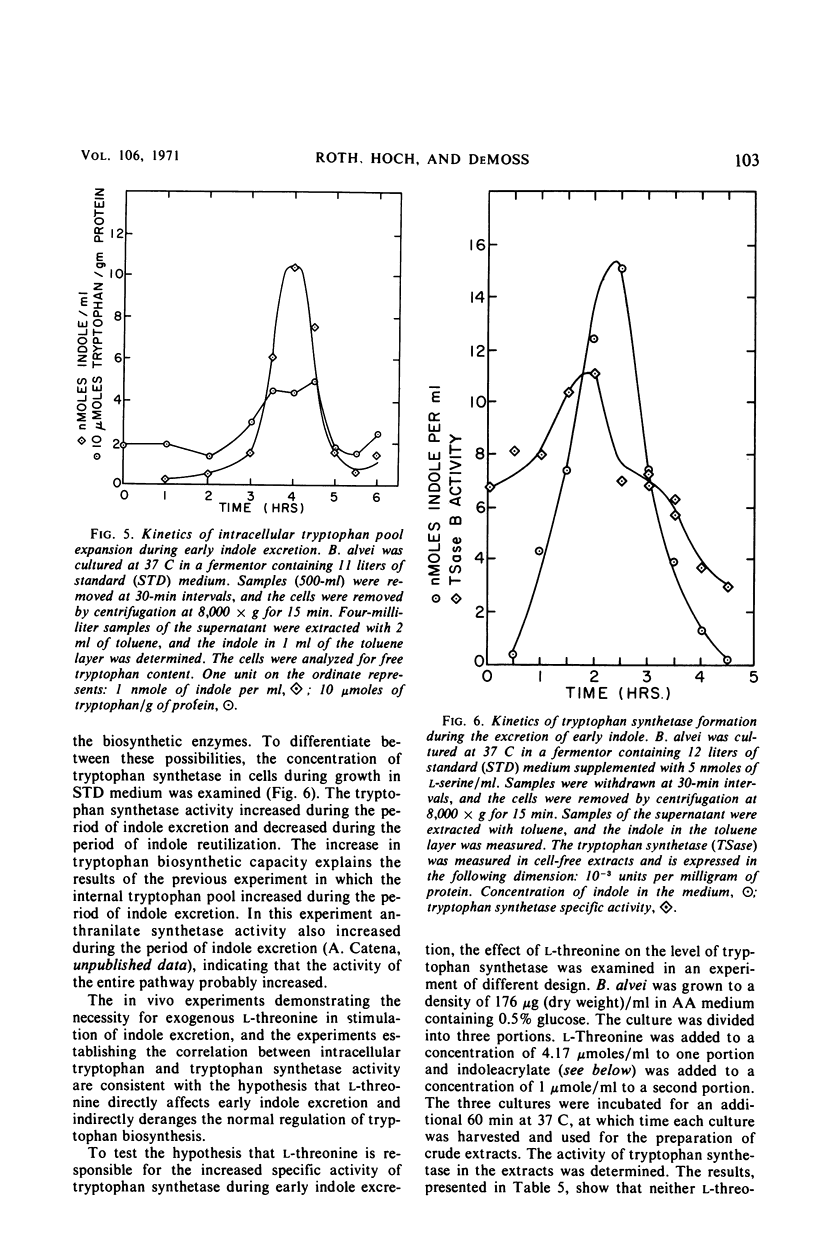
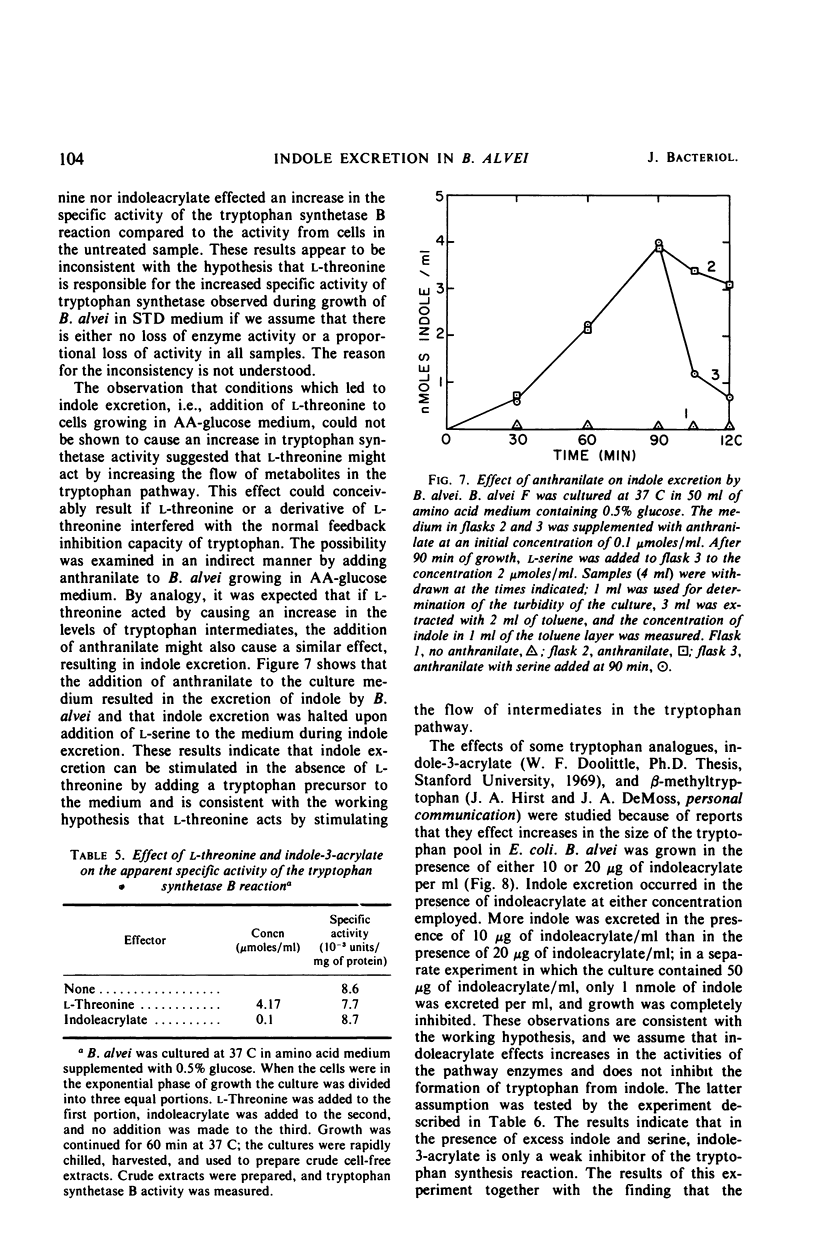
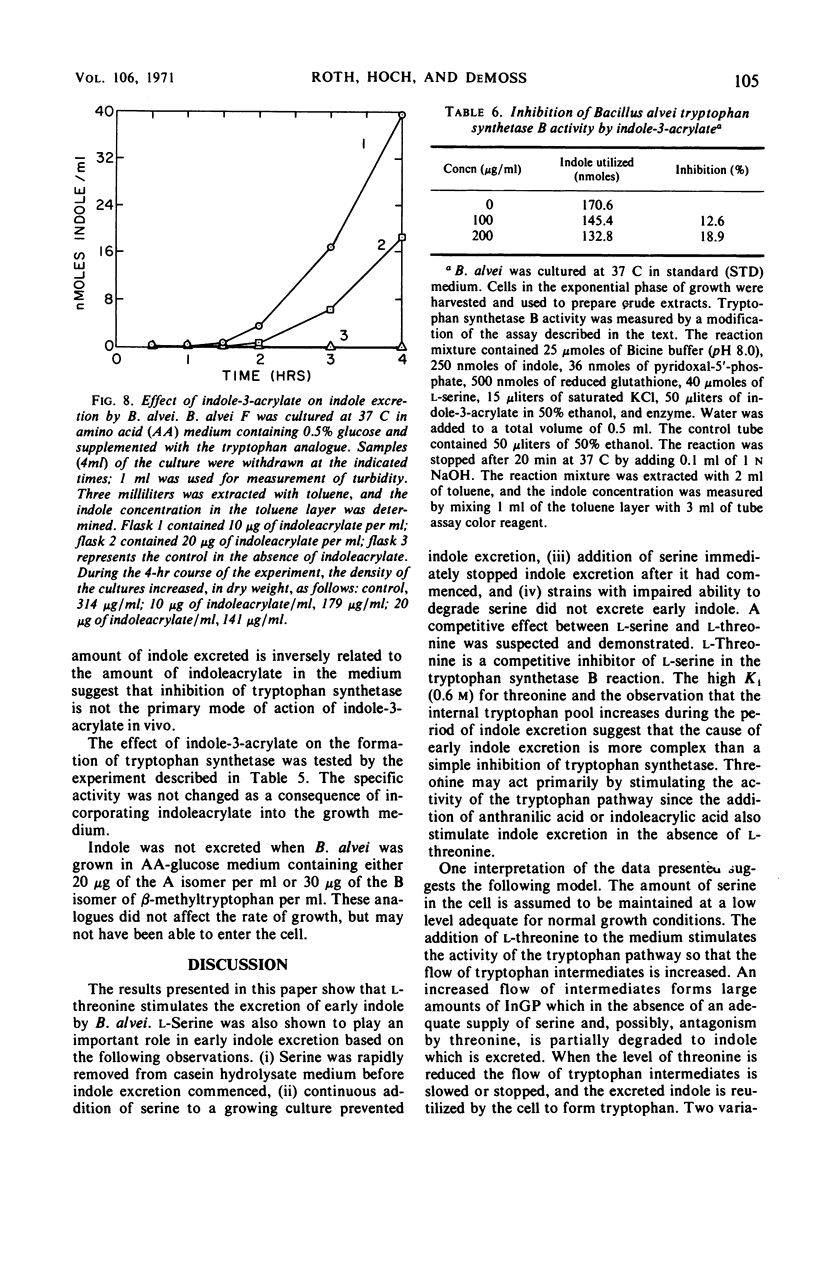
Bacillus alvei excretes indole during early exponential growth in acid-hydrolyzed casein medium. l-Threonine is the amino acid responsible for “early” indole excretion, and the amount of indole excreted is directly related to the amount of l-threonine in the medium. “Early-indole” excretion can be prevented by the continuous addition of serine (3.1 μmoles per ml per hr) or by substituting a mutant with an impaired ability to degrade serine. The addition of serine to a culture during the period of indole excretion halts the excretion and stimulates indole utilization. Threonine is a competitive inhibitor of serine (Ki = 0.6 m) in the tryptophan synthetase B reaction. The internal tryptophan concentration increases during the period of indole excretion, suggesting that threonine acts by increasing the activity of the tryptophan pathway. This view is supported by experiments demonstrating that anthranilic acid and indoleacrylic acid also stimulate indole excretion. A metabolic explanation is offered and discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeMoss R. D., Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969 Apr;98(1):167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK L. H., DEMOSS R. D. Specific enzymic method for the estimation of L-tryptophan. Arch Biochem Biophys. 1957 Apr;67(2):387–397. doi: 10.1016/0003-9861(57)90293-x. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., STEIN W. H., MOORE S. The amino acid composition of ribonuclease. J Biol Chem. 1954 Dec;211(2):941–950. [PubMed] [Google Scholar]
- Hoch J. A., DeMoss R. D. Physiological role of tryptophanase in control of tryptophan biosynthesis in Bacillus alvei. J Bacteriol. 1966 Feb;91(2):667–672. doi: 10.1128/jb.91.2.667-672.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Demoss R. D. Physiological Effects of a Constitutive Tryptophanase in Bacillus alvei. J Bacteriol. 1965 Sep;90(3):604–610. doi: 10.1128/jb.90.3.604-610.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



