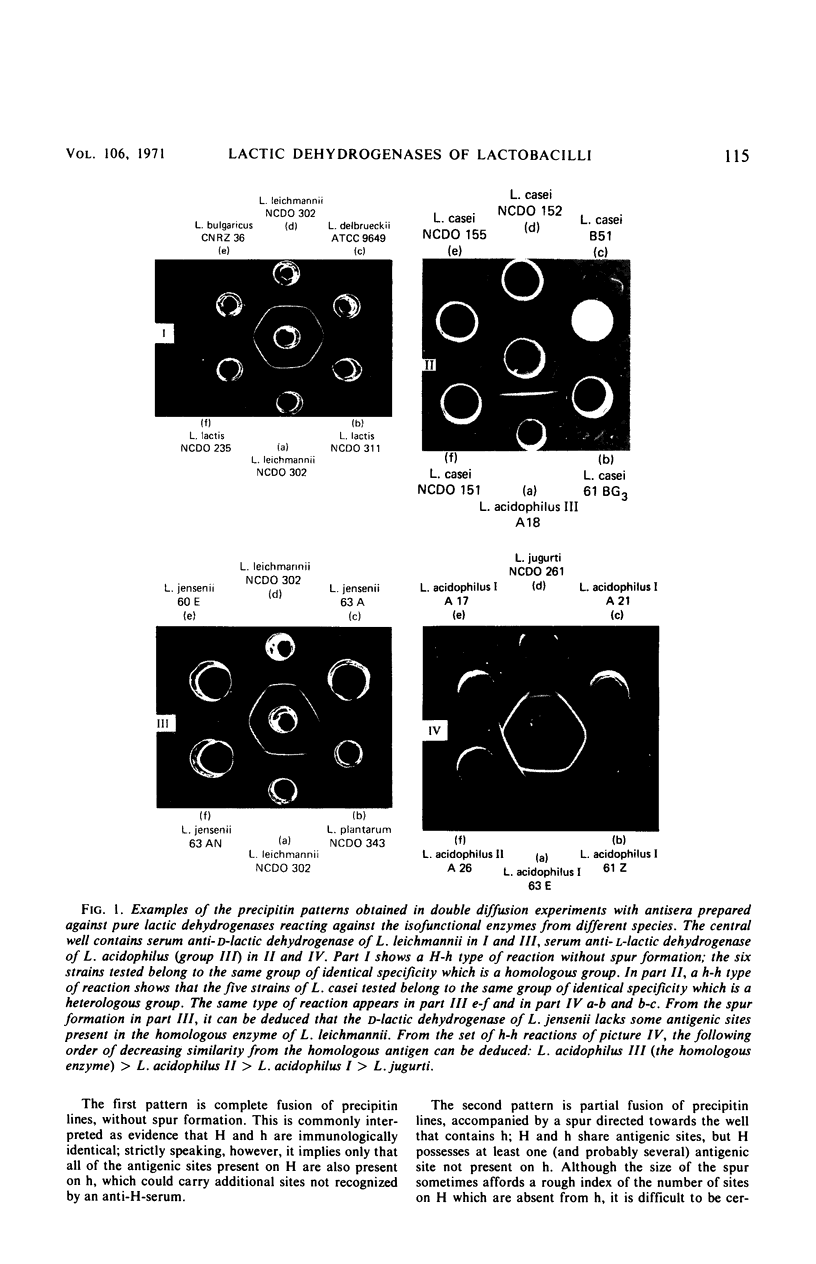
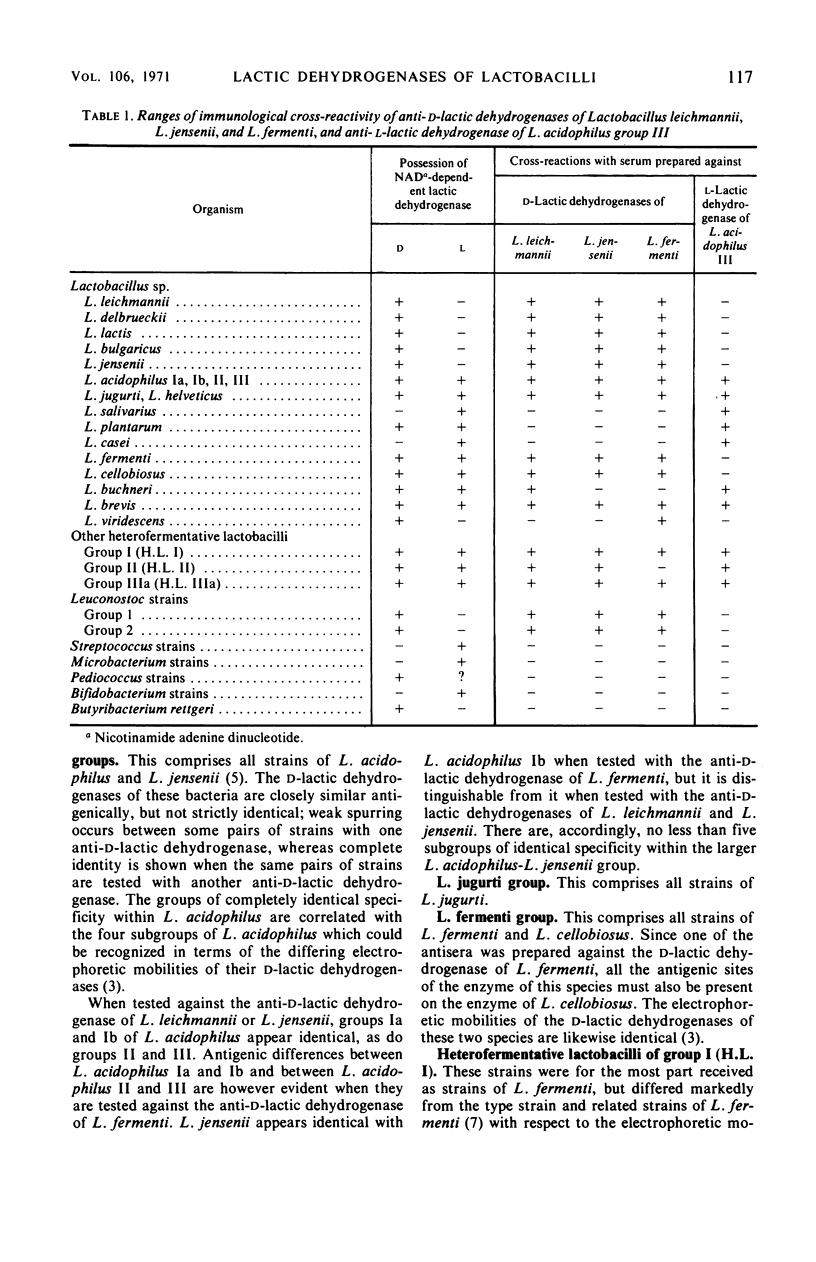
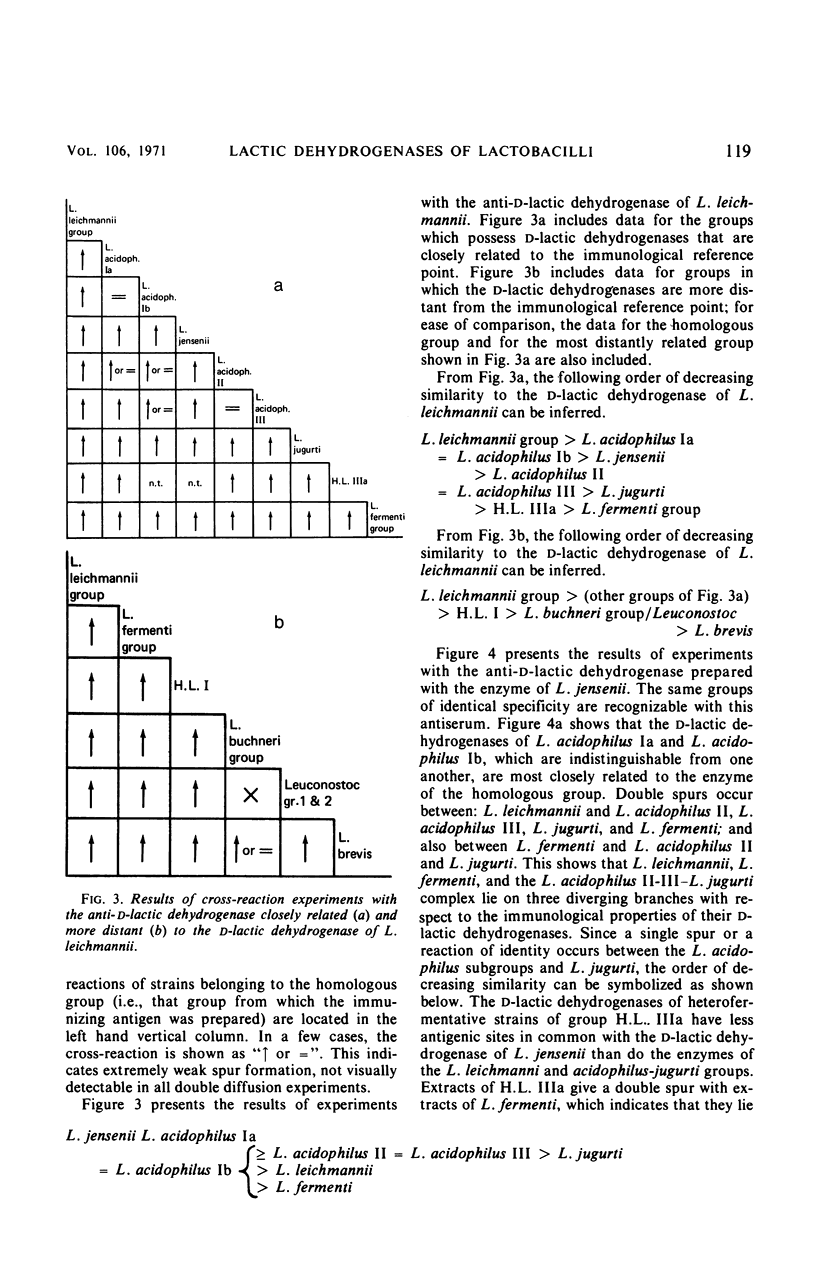
Abstract
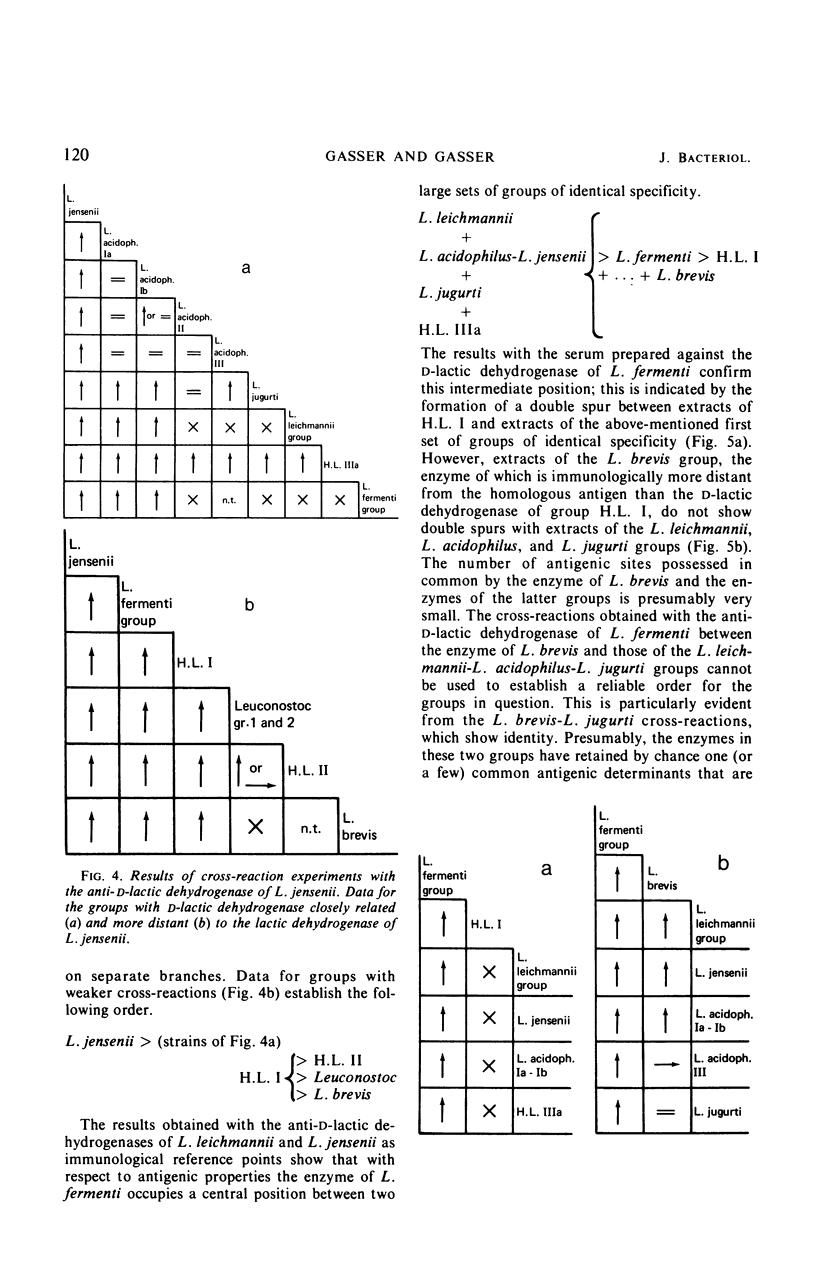
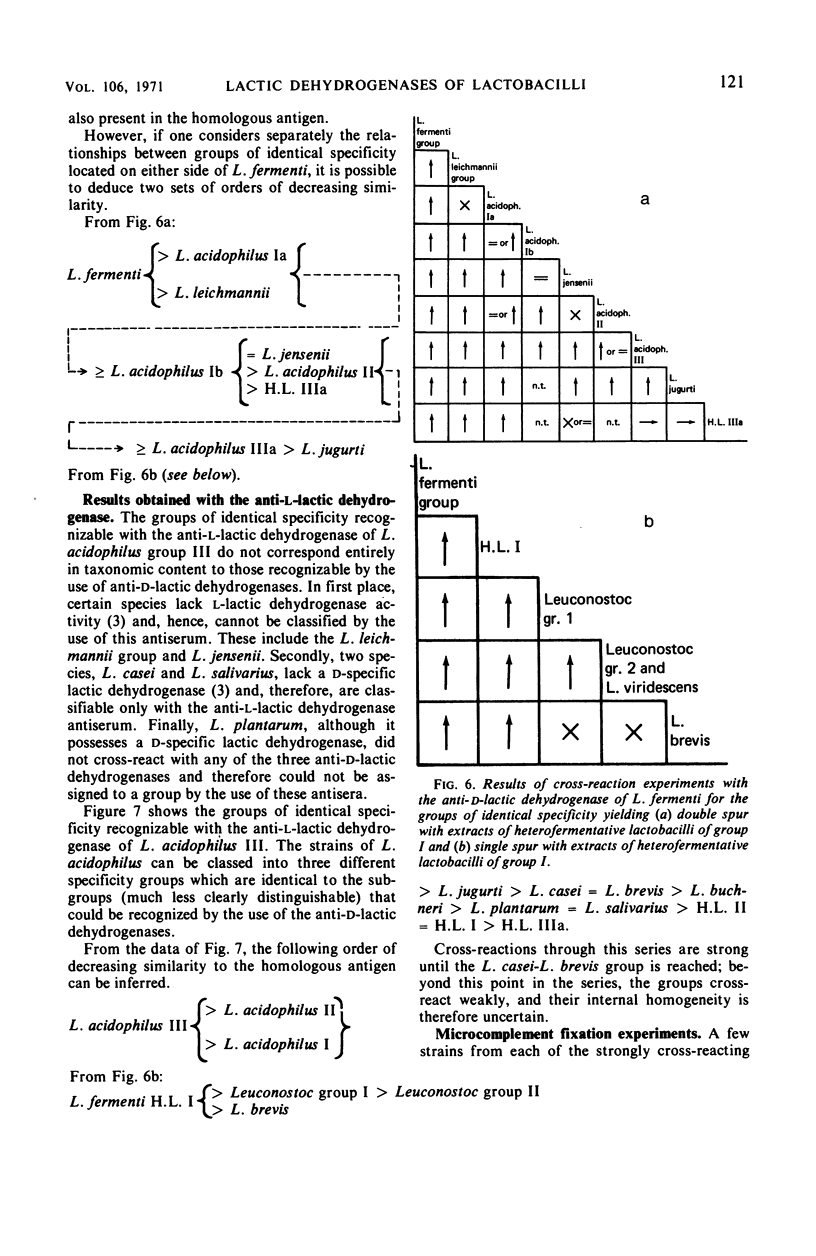
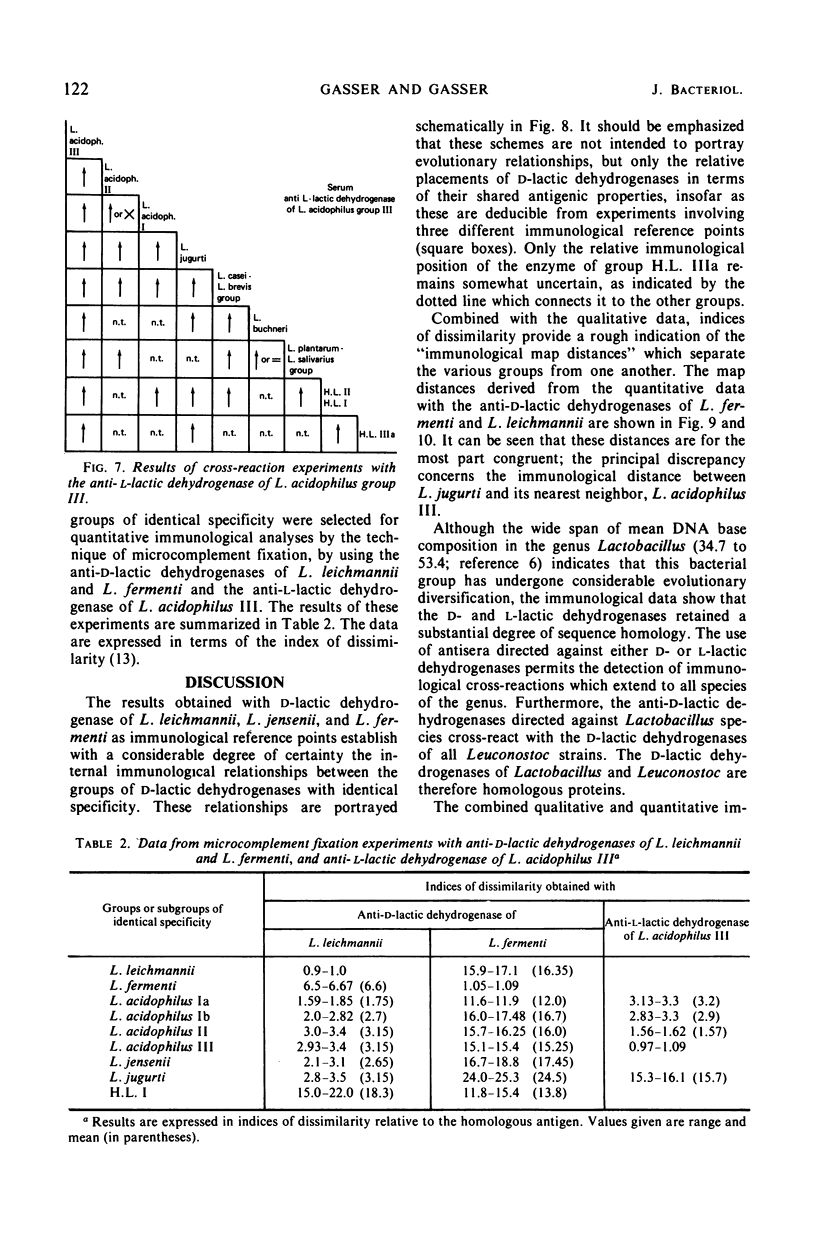
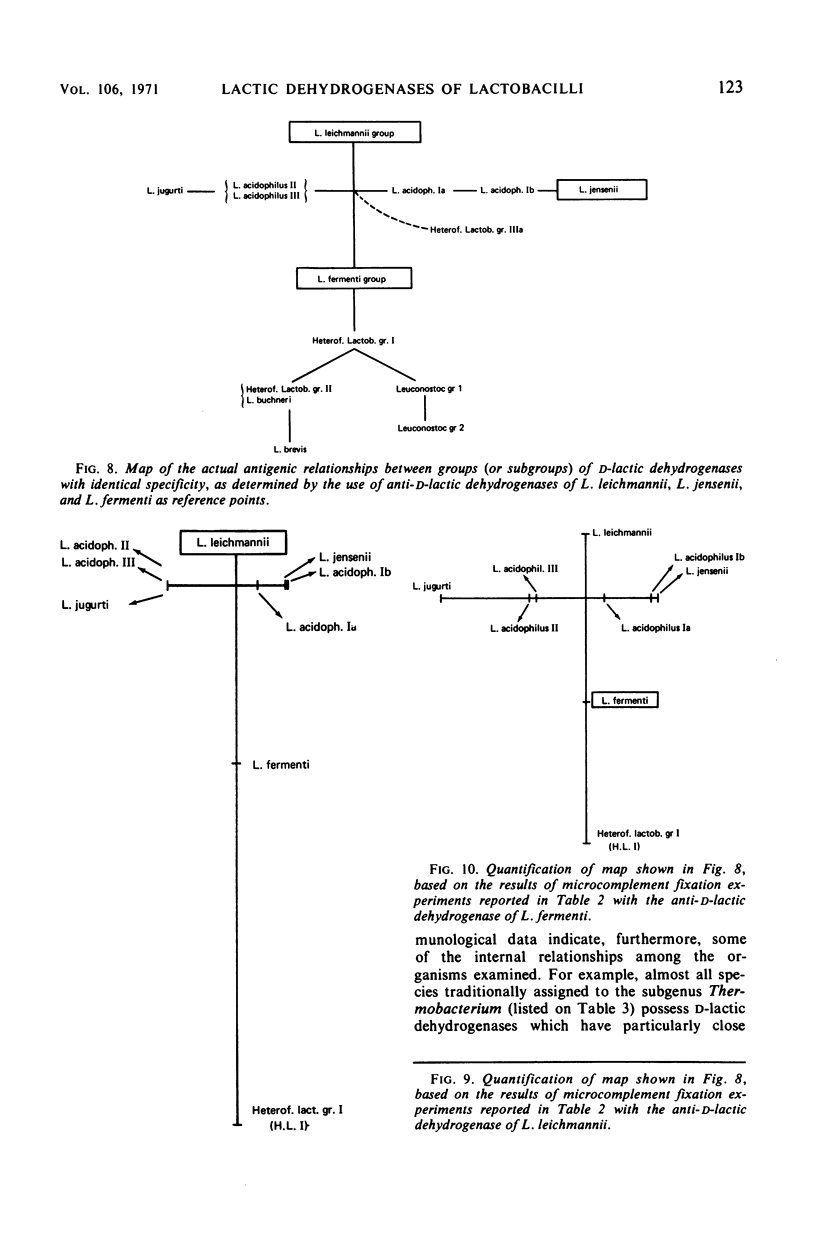
Antisera were prepared against pure nicotinamide adenine dinucleotide-dependent d-lactic dehydrogenases of Lactobacillus leichmannii, L. jensenii, and L. fermenti. When tested against these three antisera, crude extracts of almost all species of Lactobacillus containing a d-lactic dehydrogenase give cross-reactions. Extensive pairwise comparisons between cross-reacting crude extracts by double diffusion experiments permit the recognition of groups of identical antigenic specificity among the lactic dehydrogenases of the various nomenspecies of Lactobacillus. The same groups are revealed by each of the three antisera. By analyses of spur formation, the groups of identical antigenic specificity can be arranged in order of decreasing similarity to the homologous d-lactic dehydrogenase used as the reference point. From the combined results obtained with the three antisera, a map of the antigenic relationships among the d-lactic dehydrogenases of lactobacilli can be constructed. Microcomplement fixation experiments with two of the three anti-d-lactic dehydrogenases antisera support the conclusions drawn from double diffusion experiments and provide a quantitative estimation of the antigenic relationships among the various d-lactic dehydrogenases. An antiserum was also prepared against the pure l-lactic dehydrogenase of L. acidophilus group III. It cross-reacts with extracts of almost all lactobacilli containing an l-lactic dehydrogenase. With respect to species that contain both d- and l-lactic dehydrogenases, this antiserum reveals the same groups of identical antigenic specificity as do the antisera directed against d-lactic dehydrogenases. Other than the genus Lactobacillus, only extracts of Leuconostoc cross-react with anti-d-lactic dehydrogenase. No extrageneric cross-reactions were obtained with the anti-l-lactic dehydrogenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Prager E. M., Wilson A. C. Immunological prediction of sequence differences among proteins. Chemical comparison of chicken, quail, and phesant lysozymes. J Biol Chem. 1969 Apr 25;244(8):2085–2094. [PubMed] [Google Scholar]
- Gasser F., Doudoroff M., Contopoulos R. Purification and properties of NAD-dependent lactic dehydrogenases of different species of lactobacillus. J Gen Microbiol. 1970 Aug;62(2):241–250. doi: 10.1099/00221287-62-2-241. [DOI] [PubMed] [Google Scholar]
- Gasser F. Electrophoretic characterization of lactic dehydrogenases in the genus Lactobacillus. J Gen Microbiol. 1970 Aug;62(2):223–239. doi: 10.1099/00221287-62-2-223. [DOI] [PubMed] [Google Scholar]
- Gasser F., Mandel M., Rogosa M. Lactobacillus jensenii sp.nov., a new representative of the subgenus Thermobacterium. J Gen Microbiol. 1970 Aug;62(2):219–222. doi: 10.1099/00221287-62-2-219. [DOI] [PubMed] [Google Scholar]
- LAPRESLE C., KAMINSKI M., TANNER C. E. Immunochemical study of the enzymatic degradation of human serum albumin: an analysis of the antigenic structure of a protein molecule. J Immunol. 1959 Feb;82(2):94–102. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- SEBALD M., GASSER F., WERNER H. TENEUR GC PERCENTAGE ET CLASSIFICATION. APPLICATION AU GROUPE DES BIFIDOBACT'ERIES ET 'A QUELQUES GENRES VOISINS. Ann Inst Pasteur (Paris) 1965 Aug;109:251–269. [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Wittenberger C. L., Fulco J. G. Purification and allosteric properties of a nicotinamide adenine dinucleotide-linked D(-)-specific lactate dehydrogenase from Butyribacterium rettgeri. J Biol Chem. 1967 Jun 25;242(12):2917–2924. [PubMed] [Google Scholar]




