Abstract
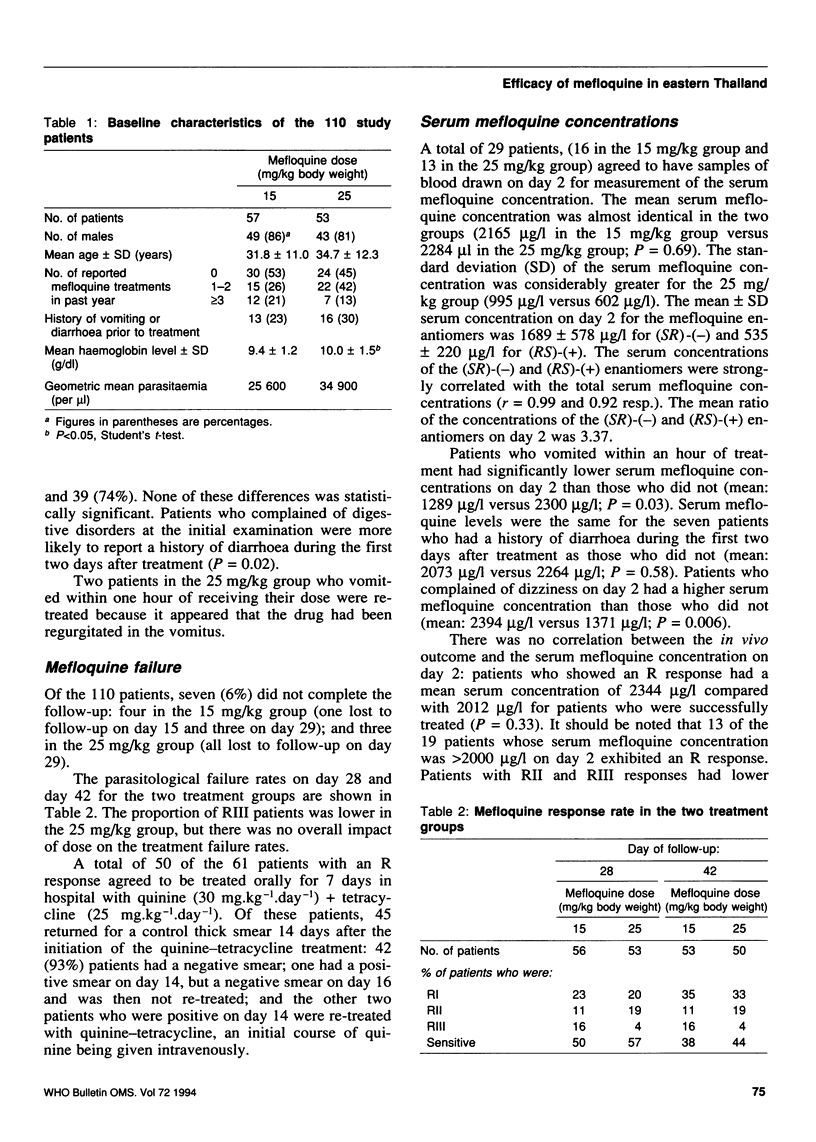
Reported are the results of a randomized trial of a single dose of mefloquine (15 mg/kg or 25 mg/kg body weight) for the treatment of uncomplicated multidrug-resistant falciparum malaria. Of the 110 adult patients enrolled in the study 57 were randomly assigned to the 15 mg/kg group and 53 to the 25 mg/kg group. The baseline characteristics of the patients did not differ significantly in the two groups, except that those in the 15 mg/kg group had lower haemoglobin levels. Adverse effects following treatment were commoner in the 25 mg/kg group, but not significantly so. Seven patients (6%) did not complete the 42-day follow-up. The parasitological failure rates in the 15 and 25 mg/kg groups were, respectively, 50% (28/56) and 43% (25/53) on day 28, and 62% (33/53) and 56% (28/50) on day 42. Treatment failures were not correlated with the serum mefloquine concentrations on day 2, and 13 out of 19 patients with serum mefloquine concentrations > 2000 micrograms/l on day 2 showed an R response during the follow-up. The mean ratio between the concentrations of the (SR)-(-) and (RS)-(+) enantiomers of mefloquine on day 2 was 3.37, indicating that there are differences in their pharmacokinetics. Re-treatment of patients who showed an R response with seven days of quinine (30 mg.kg-1.day-1)+tetracycline (25 mg.kg-1.day-1) was successful in 93% of the cases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basco L. K., Gillotin C., Gimenez F., Farinotti R., Le Bras J. Absence of antimalarial activity or interaction with mefloquine enantiomers in vitro of the main human metabolite of mefloquine. Trans R Soc Trop Med Hyg. 1991 Mar-Apr;85(2):208–209. doi: 10.1016/0035-9203(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Bergqvist Y., Hellgren U., Churchill F. C. High-performance liquid chromatographic assay for the simultaneous monitoring of mefloquine and its acid metabolite in biological samples using protein precipitation and ion-pair extraction. J Chromatogr. 1988 Nov 18;432:253–263. doi: 10.1016/s0378-4347(00)80650-7. [DOI] [PubMed] [Google Scholar]
- Boudreau E. F., Fleckenstein L., Pang L. W., Childs G. E., Schroeder A. C., Ratnaratorn B., Phintuyothin P. Mefloquine kinetics in cured and recrudescent patients with acute falciparum malaria and in healthy volunteers. Clin Pharmacol Ther. 1990 Oct;48(4):399–409. doi: 10.1038/clpt.1990.168. [DOI] [PubMed] [Google Scholar]
- Carroll F. I., Blackwell J. T. Optical isomers of aryl-2-piperidylmethanol antimalarial agents. Preparation, optical purity, and absolute stereochemistry. J Med Chem. 1974 Feb;17(2):210–219. doi: 10.1021/jm00248a015. [DOI] [PubMed] [Google Scholar]
- Doberstyn E. B., Phintuyothin P., Noeypatimanondh S., Teerakiartkamjorn C. Single-dose therapy of falciparum malaria with mefloquine or pyrimethamine-sulfadoxine. Bull World Health Organ. 1979;57(2):275–279. [PMC free article] [PubMed] [Google Scholar]
- Fontanet A. L., Johnston D. B., Walker A. M., Rooney W., Thimasarn K., Sturchler D., Macdonald M., Hours M., Wirth D. F. High prevalence of mefloquine-resistant falciparum malaria in eastern Thailand. Bull World Health Organ. 1993;71(3-4):377–383. [PMC free article] [PubMed] [Google Scholar]
- Gimenez F., Farinotti R., Thuillier A., Hazebroucq G., Wainer I. W. Determination of the enantiomers of mefloquine in plasma and whole blood using a coupled achiral-chiral high-performance liquid chromatographic system. J Chromatogr. 1990 Aug 3;529(2):339–346. doi: 10.1016/s0378-4347(00)83840-2. [DOI] [PubMed] [Google Scholar]
- Harinasuta T., Bunnag D., Wernsdorfer W. H. A phase II clinical trial of mefloquine in patients with chloroquine-resistant falciparum malaria in Thailand. Bull World Health Organ. 1983;61(2):299–305. [PMC free article] [PubMed] [Google Scholar]
- Hellgren U., Kihamia C. M., Bergqvist Y., Rombo L. Standard and reduced doses of mefloquine for treatment of Plasmodium falciparum in Tanzania: whole blood concentrations in relation to adverse reactions, in vivo response, and in vitro susceptibility. Am J Trop Med Hyg. 1991 Aug;45(2):254–262. doi: 10.4269/ajtmh.1991.45.254. [DOI] [PubMed] [Google Scholar]
- Meek S. R., Doberstyn E. B., Gaüzère B. A., Thanapanich C., Nordlander E., Phuphaisan S. Treatment of falciparum malaria with quinne and tetracycline or combined mefloquine/sulfadoxine/pyrimethamine on the Thai-Kampuchean border. Am J Trop Med Hyg. 1986 Mar;35(2):246–250. doi: 10.4269/ajtmh.1986.35.246. [DOI] [PubMed] [Google Scholar]
- Ngiam T. L., Go M. L. Stereospecific inhibition of cholinesterases by mefloquine enantiomers. Chem Pharm Bull (Tokyo) 1987 Jan;35(1):409–412. doi: 10.1248/cpb.35.409. [DOI] [PubMed] [Google Scholar]
- Nosten F., Imvithaya S., Vincenti M., Delmas G., Lebihan G., Hausler B., White N. Malaria on the Thai-Burmese border: treatment of 5192 patients with mefloquine-sulfadoxine-pyrimethamine. Bull World Health Organ. 1987;65(6):891–896. [PMC free article] [PubMed] [Google Scholar]
- Nosten F., ter Kuile F., Chongsuphajaisiddhi T., Luxemburger C., Webster H. K., Edstein M., Phaipun L., Thew K. L., White N. J. Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet. 1991 May 11;337(8750):1140–1143. doi: 10.1016/0140-6736(91)92798-7. [DOI] [PubMed] [Google Scholar]
- ter Kuile F. O., Nosten F., Thieren M., Luxemburger C., Edstein M. D., Chongsuphajaisiddhi T., Phaipun L., Webster H. K., White N. J. High-dose mefloquine in the treatment of multidrug-resistant falciparum malaria. J Infect Dis. 1992 Dec;166(6):1393–1400. doi: 10.1093/infdis/166.6.1393. [DOI] [PubMed] [Google Scholar]


