Abstract
Representatives of both Streptococcus faecalis and Lactobacillus casei produce isofunctional malic enzymes. All 10 strains of S. faecalis tested could be induced to synthesize malic enzyme and readily adapted to growth on malate. Although 17 of 21 L. casei strains could be induced to produce malic enzyme, only 9 of 14 strains tested grew at the expense of malate. A comparison of catalytic and regulatory properties suggested that the malic enzymes from S. faecalis and L. casei were very similar. Immunological analyses showed that the numerous similarities in function actually reflected partial protein homologies; however, two distinct forms of the malic enzyme were detected among different strains of L. casei by immunochemical and serological procedures. The division of L. casei into two subgroups based on the immunological type of malic enzyme synthesized corresponds to two subspecies currently recognized by microbial taxonomists.
Full text
PDF
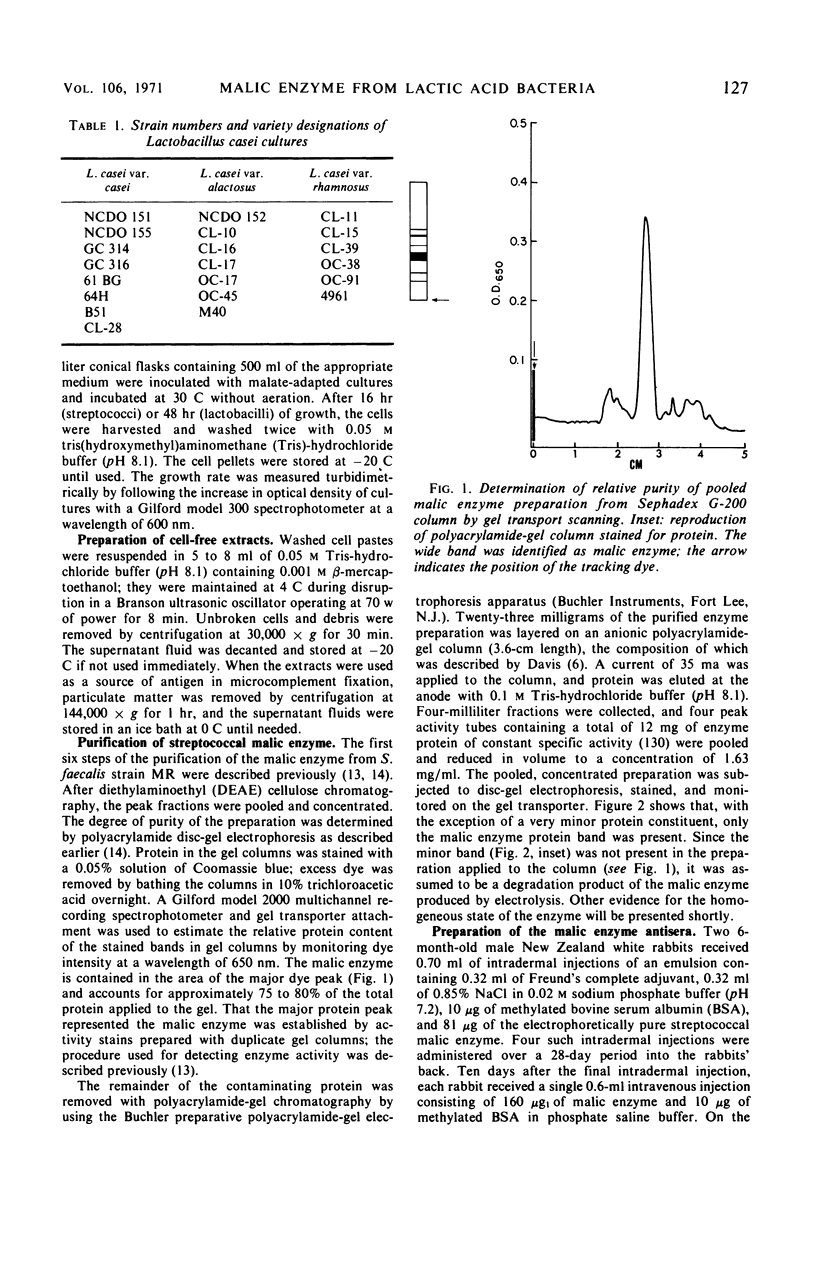





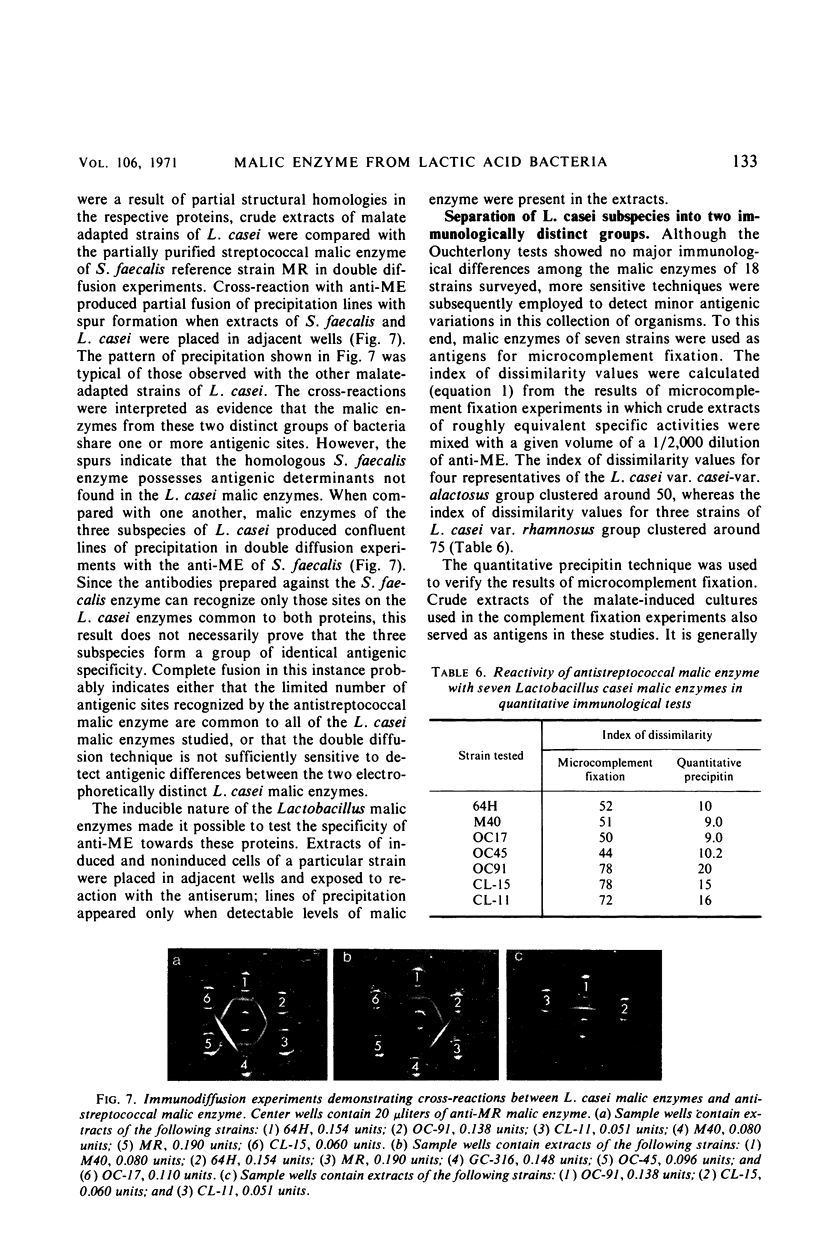




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., AQVIST S. E., COOKE J. P., JONSSON B. A comparative study of the structures of bovine and ovine pancreatic ribonucleases. J Biol Chem. 1959 May;234(5):1118–1123. [PubMed] [Google Scholar]
- Arnheim N., Prager E. M., Wilson A. C. Immunological prediction of sequence differences among proteins. Chemical comparison of chicken, quail, and phesant lysozymes. J Biol Chem. 1969 Apr 25;244(8):2085–2094. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- HOOK W. A., MUSCHEL L. H. ANTICOMPLEMENTARY EFFECTS AND COMPLEMENT ACTIVITY OF HUMAN SERA. Proc Soc Exp Biol Med. 1964 Oct;117:292–297. doi: 10.3181/00379727-117-29561. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Stenmark S. L. Comparative allostery of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthetase as a molecular basis for classification. J Bacteriol. 1970 Mar;101(3):763–769. doi: 10.1128/jb.101.3.763-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Taxonomic implications of temperature dependence of the allosteric inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase in Bacillus. J Bacteriol. 1970 May;102(2):489–497. doi: 10.1128/jb.102.2.489-497.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORKES S., DEL CAMPILLO A., OCHOA S. Biosynthesis of dicarboxylic acids by carbon dioxide fixation. IV. Isolation and properties of an adaptive "malic" enzyme from Lactobacillus arabinosus. J Biol Chem. 1950 Dec;187(2):891–905. [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus. II. Evidence for allosteric inhibition of an inducible malate dehydrogenase (decarboxylating) by ATP and glycolytic intermediate products. Biochim Biophys Acta. 1969 Apr 22;178(2):205–212. doi: 10.1016/0005-2744(69)90390-8. [DOI] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: physiological properties and purification of an inducible malic enzyme. J Bacteriol. 1969 May;98(2):705–711. doi: 10.1128/jb.98.2.705-711.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: regulation of malic enzyme synthesis by an inducible malate permease. J Bacteriol. 1970 Apr;102(1):130–137. doi: 10.1128/jb.102.1.130-137.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLAND F. J. A correlation of antigenic characteristics among certain bacteria of the Lactobacillus group. J Infect Dis. 1950 Jan-Feb;86(1):63–80. doi: 10.1093/infdis/86.1.63. [DOI] [PubMed] [Google Scholar]
- PERRIN D. Immunological studies with genetically altered beta-galactosidases. Ann N Y Acad Sci. 1963 May 8;103:1058–1066. doi: 10.1111/j.1749-6632.1963.tb53757.x. [DOI] [PubMed] [Google Scholar]
- REICHLIN M., HAY M., LEVINE L. ANTIBODIES TO HUMAN A1 HEMOGLOBIN AND THEIR REACTION WITH A2, S, C, AND H HEMOGLOBINS. Immunochemistry. 1964 Apr;1:21–30. doi: 10.1016/0019-2791(64)90052-7. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., WISEMAN R. F., MITCHELL J. A., DISRAELY M. N., BEAMAN A. J. Species differentiation of oral lactobacilli from man including description of Lactobacillus salivarius nov spec and lactobacillus Cellobiosus nov spec. J Bacteriol. 1953 Jun;65(6):681–699. doi: 10.1128/jb.65.6.681-699.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M., Love L. L. Direct quantitative gas chromatographic separation of C2-C6 fatty acids, methanol, and ethyl alcohol in aqueous microbial fermentation media. Appl Microbiol. 1968 Feb;16(2):285–290. doi: 10.1128/am.16.2.285-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]









