Abstract
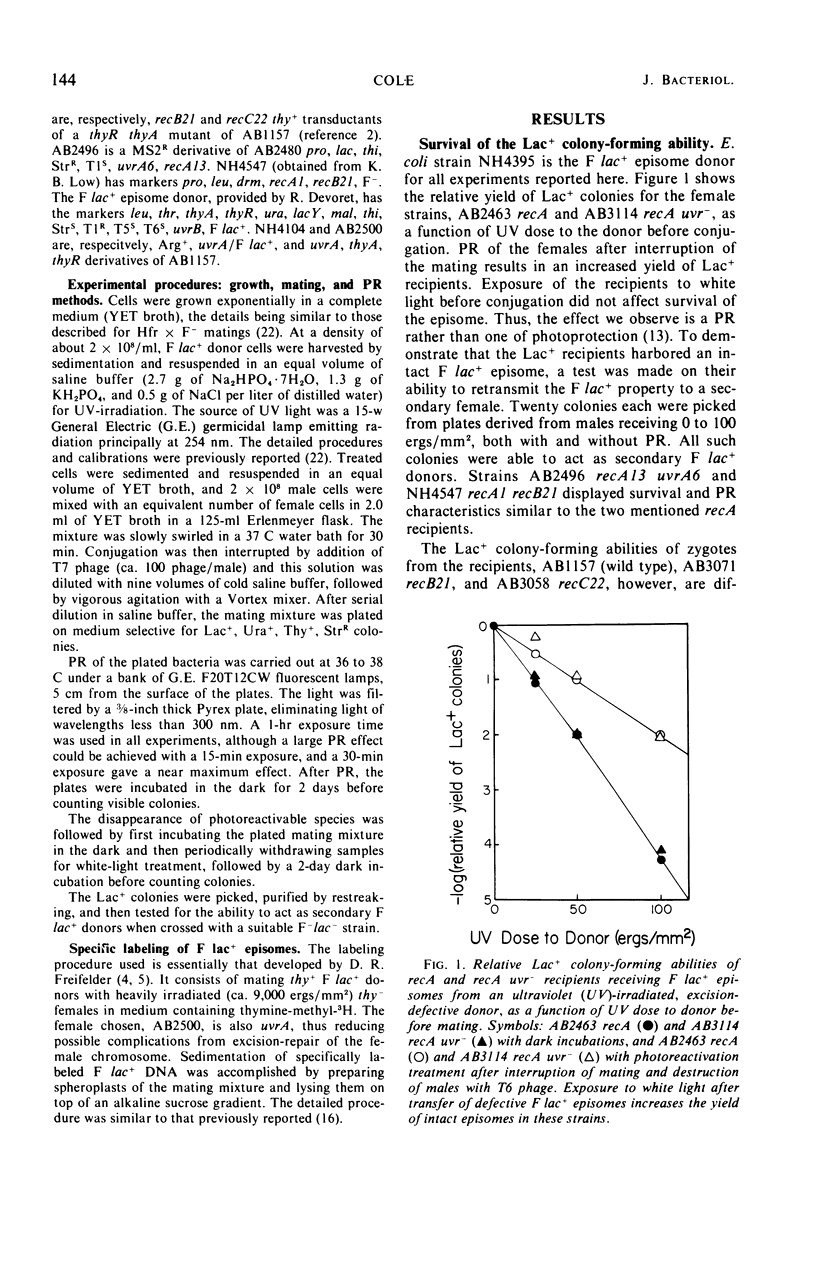
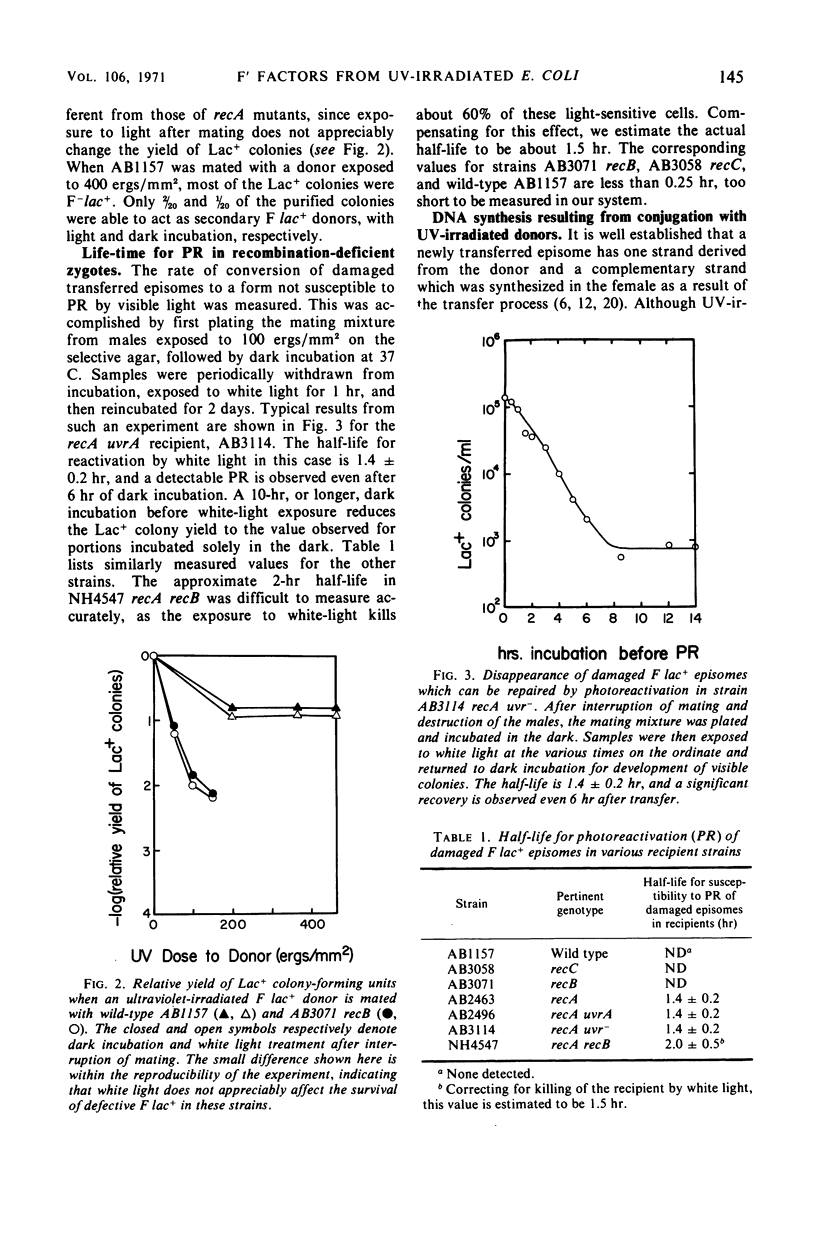
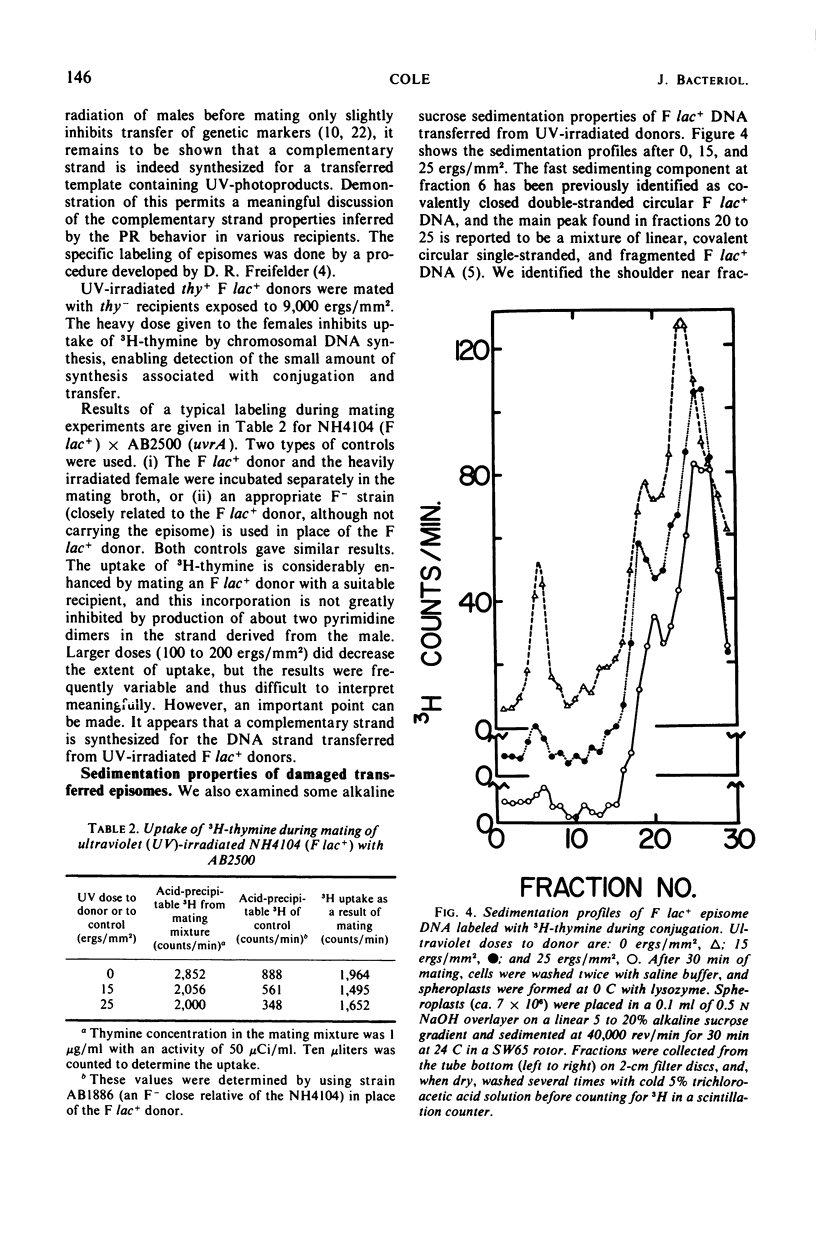
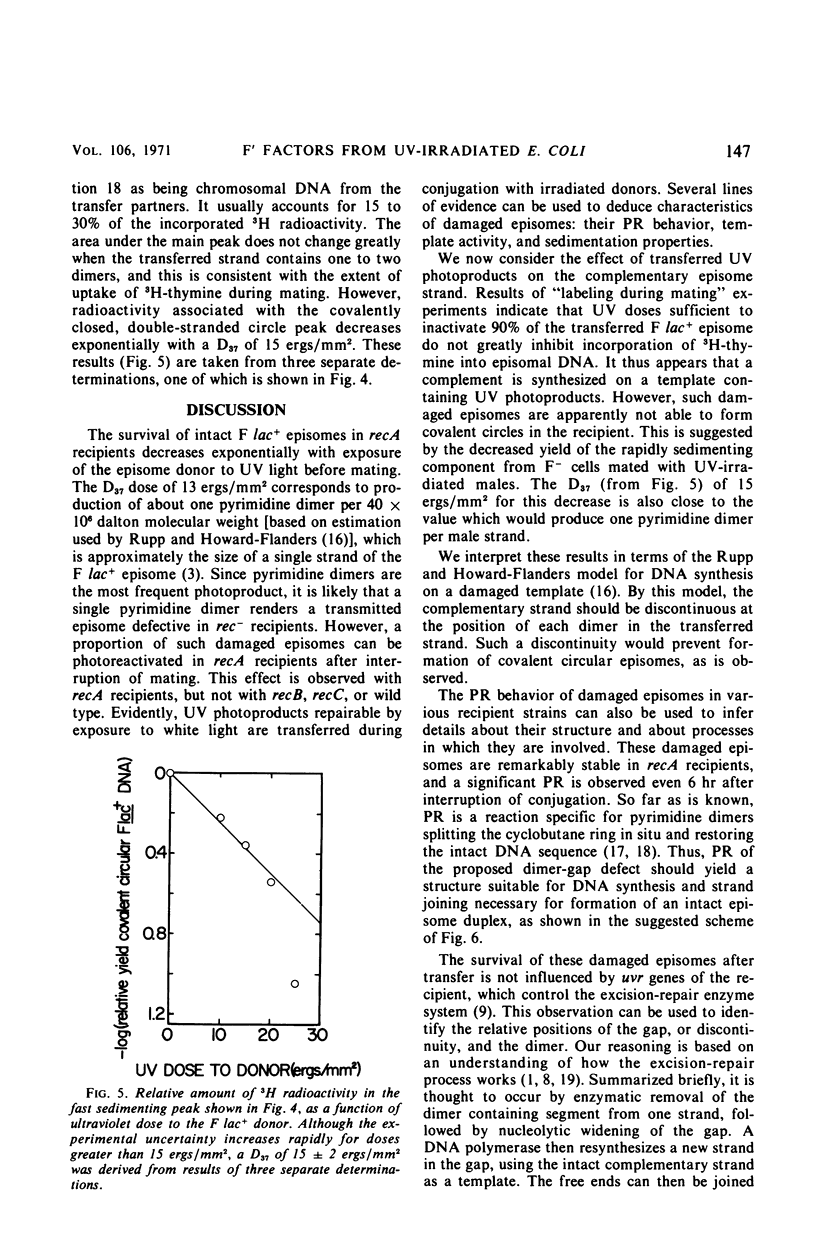
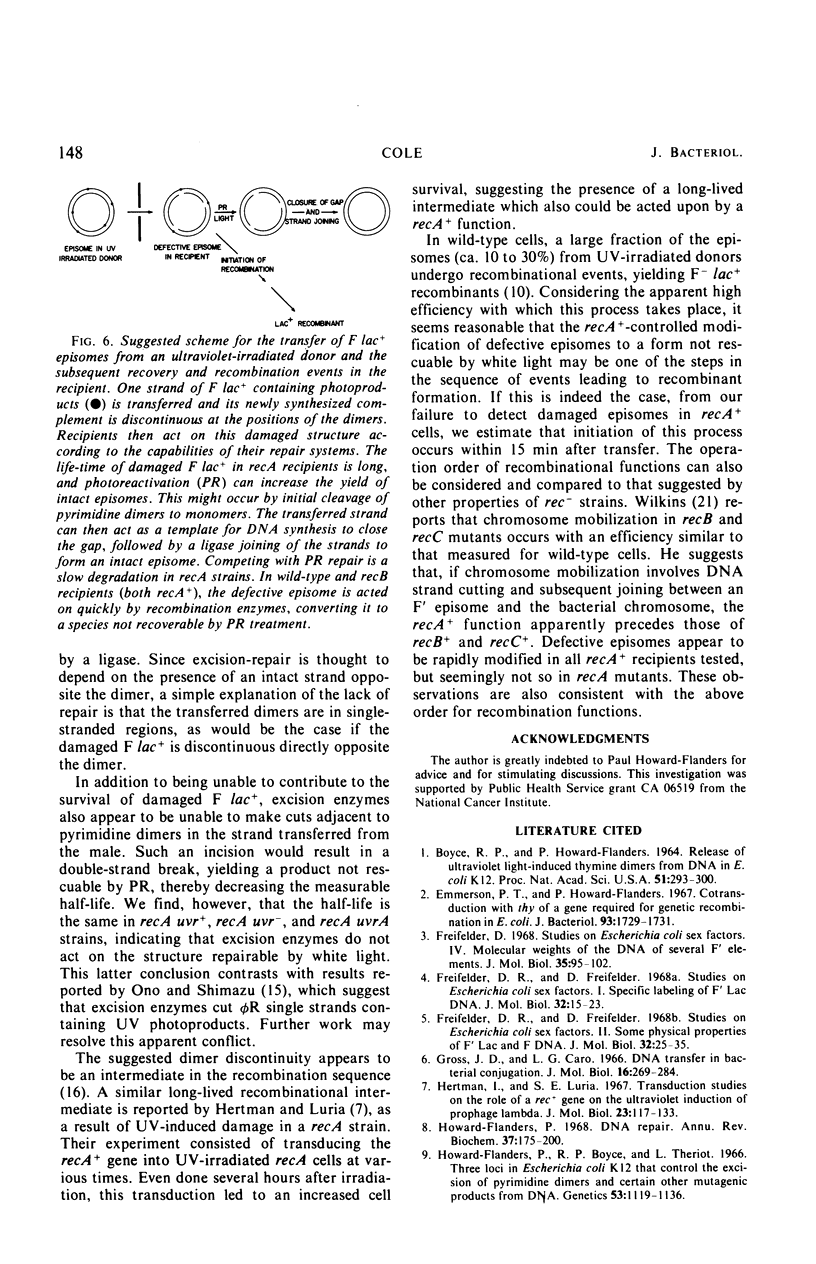
Ultraviolet (UV)-irradiated Escherichia coli K-12 F lac+ donors transfer damaged F′ factors when mated with female cells. Exposure of the zygotes to white light after mating can cause the photoreactivation of the damaged transferred F′ factors. In so far as the photoreactivation is specific for pyrimidine dimers, these experiments indicate the presence of UV-induced dimers in the transferred F′ factor deoxyribonucleic acid (DNA). These damaged F′ factors are remarkably stable in recA recipients, having a half-life for susceptibility to photoreactivation of 1.5 to 2 hr. This is judged by the formation of Lac+ colonies, all of which are male secondary donors. In crosses with wild type and uvrA, recB, or recC mutant recipients, however, the Lac+ colonies are predominantly recombinants and photoreactivation is not detected. The extent of DNA synthesis resulting from transfer of F′ episomes from irradiated donors suggests that a complementary strand is formed on the damaged template. Photoreactivation behavior and sedimentation properties are used to deduce properties of such damaged episomes. We conclude that the complementary strand is discontinuous directly opposite dimers in the transferred strand. This structure may be an intermediate in the recombinational event sequence in Rec+ recipients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T., Howard-Flanders P. Cotransduction with thy of a gene required for genetic recombination in Escherichia coli. J Bacteriol. 1967 May;93(5):1729–1731. doi: 10.1128/jb.93.5.1729-1731.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. I. Specific labeling of F'Lac DNA. J Mol Biol. 1968 Feb 28;32(1):15–23. doi: 10.1016/0022-2836(68)90141-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. II. Some physical properties of F'Lac and F DNA. J Mol Biol. 1968 Feb 28;32(1):25–35. doi: 10.1016/0022-2836(68)90142-3. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies on Escherichia coli sex factors. IV. Molecular weights of the DNA of several F' elements. J Mol Biol. 1968 Jul 14;35(1):95–102. doi: 10.1016/s0022-2836(68)80039-7. [DOI] [PubMed] [Google Scholar]
- Gross J. D., Caro L. G. DNA transfer in bacterial conjugation. J Mol Biol. 1966 Apr;16(2):269–284. doi: 10.1016/s0022-2836(66)80172-9. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Rupp W. D., Wilkins B. M., Cole R. S. DNA replication and recombination after UV irradiation. Cold Spring Harb Symp Quant Biol. 1968;33:195–207. doi: 10.1101/sqb.1968.033.01.023. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGER J., STAFFORD R. S. EVIDENCE FOR TWO MECHANISMS OF PHOTOREACTIVATION IN ESCHERICHIA COLI B. Biophys J. 1965 Jan;5:75–88. doi: 10.1016/s0006-3495(65)86703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern I. E., van Winden M. P., Rörsch A. The range of action of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):111–131. doi: 10.1016/0027-5107(65)90042-4. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. Macromolecular radiosensitivity. Brookhaven Symp Biol. 1961 Nov;14:1–17. [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M. Chromosome transfer from F-lac+ strains of Escherichia coli K-12 mutant at recA, recB, or recC. J Bacteriol. 1969 May;98(2):599–604. doi: 10.1128/jb.98.2.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M., Howard-Flanders P. The genetic properties of DNA transferred from ultraviolet-irradiated Hfr cells of Escherichia coli K-12 during mating. Genetics. 1968 Oct;60(2):243–255. doi: 10.1093/genetics/60.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]



