Abstract
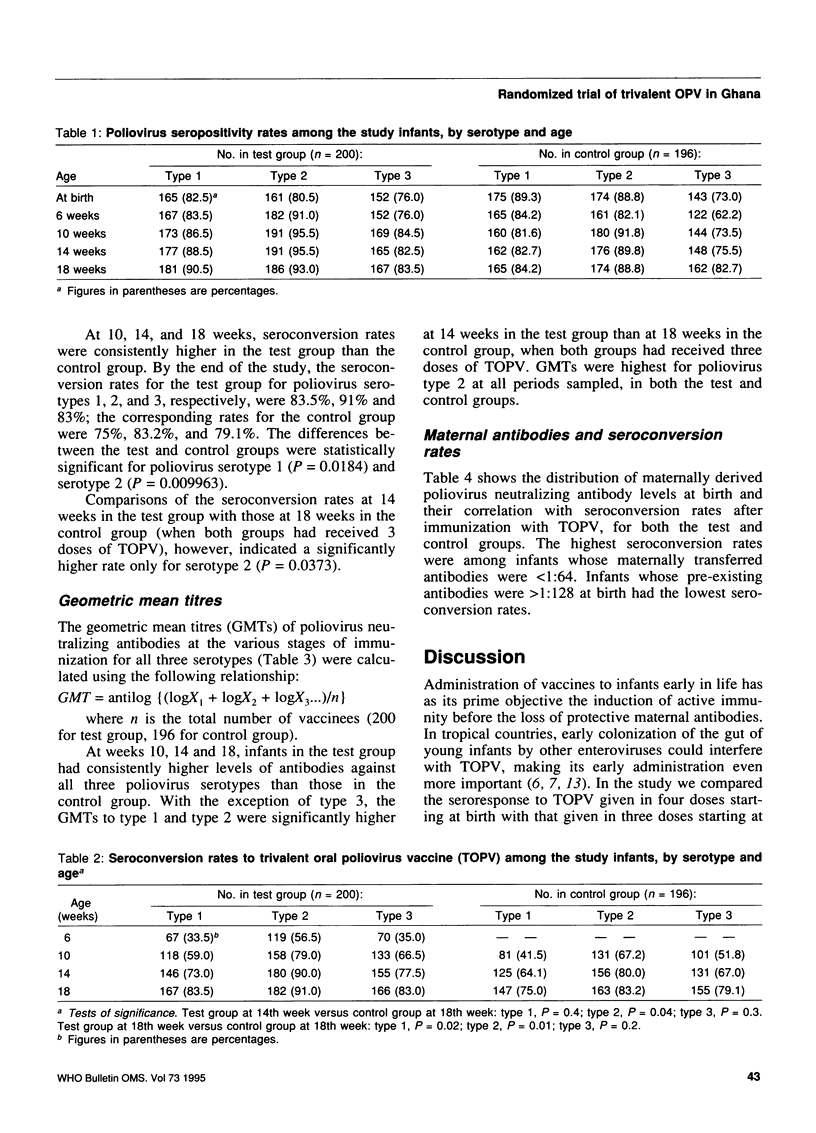
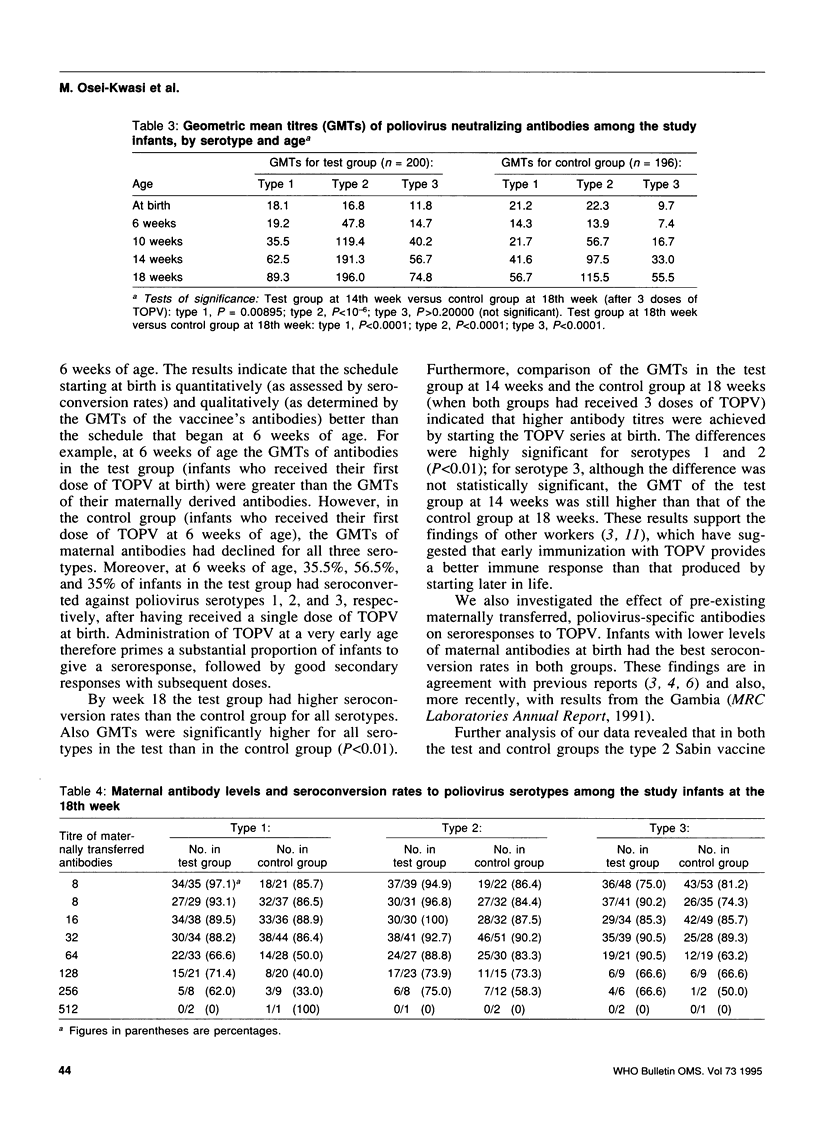
To evaluate the efficacy of the schedule currently recommended for immunization with trivalent oral poliovirus vaccine (TOPV) (i.e., at birth, 6 weeks, 10 weeks, and 14 weeks after birth), we randomly assigned 452 infants into test (231 infants) and control (221 infants) groups. The test group received TOPV as currently recommended, and the dose at birth was omitted for the control group. At 10, 14, and 18 weeks of age, the levels of poliovirus neutralizing antibodies as well as seroconversion rates were consistently higher for the test group than for the control group. The final seroconversion rates against poliovirus types 1, 2, and 3 were 83.5%, 91% and 83%, respectively, for the test group and 75%, 83.2%, and 79.1%, respectively, for the control group. The TOPV immunization schedule starting at birth therefore produced better results. Seroconversion rates as well as antibody levels were highest in infants with low maternal antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dömök I., Balayan M. S., Fayinka O. A., Skrtić N., Soneji A. D., Harland P. S. Factors affecting the efficacy of live poliovirus vaccine in warm climates. Efficacy of type 1 Sabin vaccine administered together with antihuman gamma-globulin horse serum to breast-fed and artificially fed infants in Uganda. Bull World Health Organ. 1974;51(4):333–347. [PMC free article] [PubMed] [Google Scholar]
- Ehrengut W., AgRhaly A., Koch I., Koumaré B., Simaga S. Y., Diallo D. Serokonversionsrate nach Polioschluckimpfung in Mali/Afrika 1982. Monatsschr Kinderheilkd. 1984 Jan;132(1):29–31. [PubMed] [Google Scholar]
- Halsey N., Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ. 1985;63(6):1151–1169. [PMC free article] [PubMed] [Google Scholar]
- John T. J. Antibody response of infants in tropics to five doses of oral polio vaccine. Br Med J. 1976 Apr 3;1(6013):812–812. doi: 10.1136/bmj.1.6013.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T. J., Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972 Oct;96(4):263–269. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- Kim-Farley R. J., Rutherford G., Lichfield P., Hsu S. T., Orenstein W. A., Schonberger L. B., Bart K. J., Lui K. J., Lin C. C. Outbreak of paralytic poliomyelitis, Taiwan. Lancet. 1984 Dec 8;2(8415):1322–1324. doi: 10.1016/s0140-6736(84)90831-6. [DOI] [PubMed] [Google Scholar]
- Kok P. W., Leeuwenburg J., Tukei P., van Wezel A. L., Kapsenberg J. G., van Steenis G., Galazka A., Robertson S. E., Robinson D. Serological and virological assessment of oral and inactivated poliovirus vaccines in a rural population in Kenya. Bull World Health Organ. 1992;70(1):93–103. [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L. Combined use of live and killed vaccines to control poliomyelitis in tropical areas. Dev Biol Stand. 1981;47:265–273. [PubMed] [Google Scholar]
- Pangi N. S., Master J. M., Dave K. H. Efficacy of oral poliovaccine in infancy. Indian Pediatr. 1977 Jul;14(7):523–528. [PubMed] [Google Scholar]
- Patriarca P. A., Wright P. F., John T. J. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991 Sep-Oct;13(5):926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A., Katz M., Brown R. E., Pagano J. S. Oral poliovirus vaccination in newborn African infants. The inhibitory effect of breast feeding. Am J Dis Child. 1966 Jan;111(1):27–30. doi: 10.1001/archpedi.1966.02090040063004. [DOI] [PubMed] [Google Scholar]
- Sutter R. W., Patriarca P. A., Brogan S., Malankar P. G., Pallansch M. A., Kew O. M., Bass A. G., Cochi S. L., Alexander J. P., Hall D. B. Outbreak of paralytic poliomyelitis in Oman: evidence for widespread transmission among fully vaccinated children. Lancet. 1991 Sep 21;338(8769):715–720. doi: 10.1016/0140-6736(91)91442-w. [DOI] [PubMed] [Google Scholar]


