Abstract
Typhoid fever remains an underestimated important health problem in many developing countries, causing more than 600,000 deaths annually in the world. Because of the reactogenicity of the parenteral, killed whole-cell vaccine, research has been oriented towards vaccination orally using live organisms and purified antigen. Live vaccine Ty21a, given by the oral route, has been extensively tested in several studies in developing countries. Its liquid formulation was the most effective, providing more than 60% protection after 7 years of follow-up. A Vi polysaccharide vaccine has been elaborated and provided more than 65% protection; after 3 years of follow-up the Vi antibody level was still at a high level. These two vaccines are therefore candidates for use in public health control programmes. Before such use, however, they need further evaluation for safety and protective efficacy when administered to the EPI-targeted age groups. The question of whether typhoid fever vaccines interfere with the response to simultaneously administered measles vaccine must also be studied. New live vaccines, given by the oral route in one dose, have been constructed through genetic engineering. The first results are promising, but they must be improved before use in a large-scale study. These strains could be used as live vector to deliver foreign antigens to the intestinal mucosa.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya I. L., Lowe C. U., Thapa R., Gurubacharya V. L., Shrestha M. B., Cadoz M., Schulz D., Armand J., Bryla D. A., Trollfors B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987 Oct 29;317(18):1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Baron L. S., Noon K. F., Rubin F. A., Kopecko D. J. Construction and characterization of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a. Infect Immun. 1989 Dec;57(12):3863–3868. doi: 10.1128/iai.57.12.3863-3868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Vanprapar N., Thisyakorn U., Olanratmanee T., Losonsky G., Levine M. M., Chearskul S. Safety and immunogenicity of Salmonella typhi Ty21a vaccine in young Thai children. Infect Immun. 1993 Mar;61(3):1149–1151. doi: 10.1128/iai.61.3.1149-1151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Levine M. M. Summary of an international workshop on typhoid fever. Rev Infect Dis. 1986 May-Jun;8(3):329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
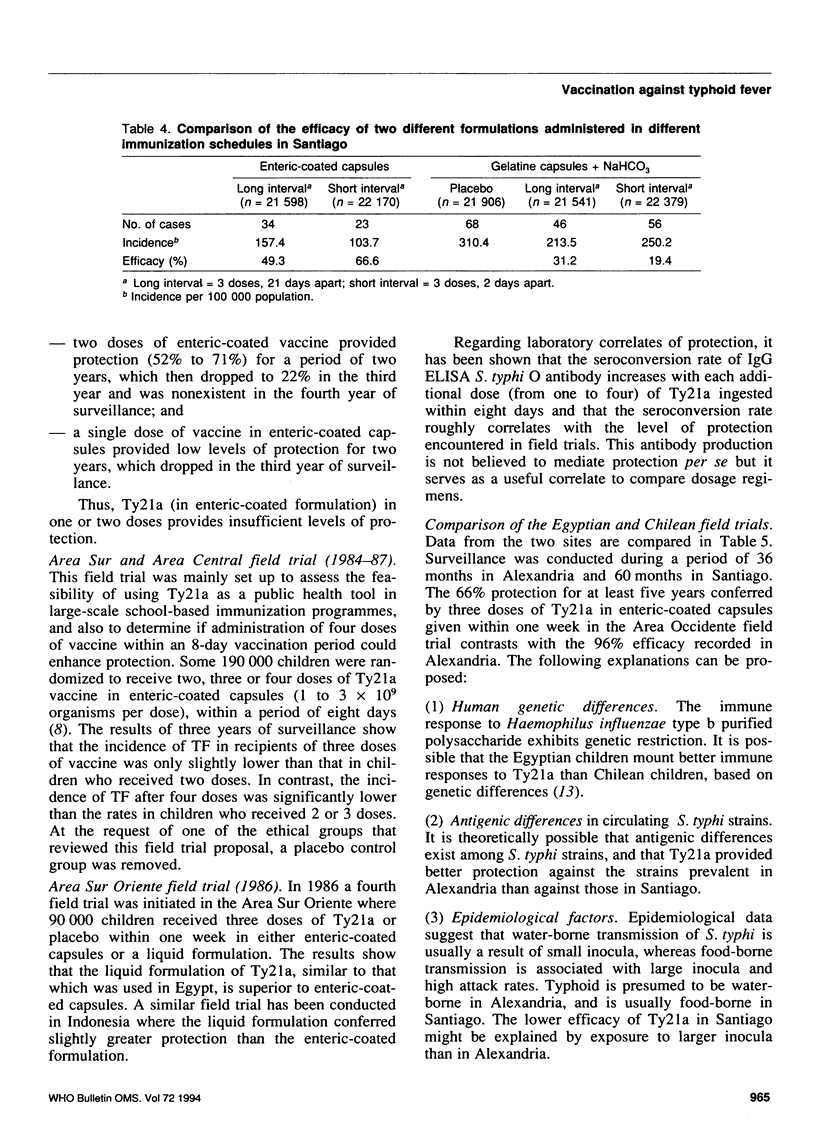
- Ferreccio C., Levine M. M., Rodriguez H., Contreras R. Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J Infect Dis. 1989 Apr;159(4):766–769. doi: 10.1093/infdis/159.4.766. [DOI] [PubMed] [Google Scholar]
- Forrest B. D., LaBrooy J. T. Effect of parenteral immunization on the intestinal immune response to Salmonella typhi Ty21a as measured using peripheral blood lymphocytes. Vaccine. 1993;11(2):136–139. doi: 10.1016/0264-410x(93)90008-l. [DOI] [PubMed] [Google Scholar]
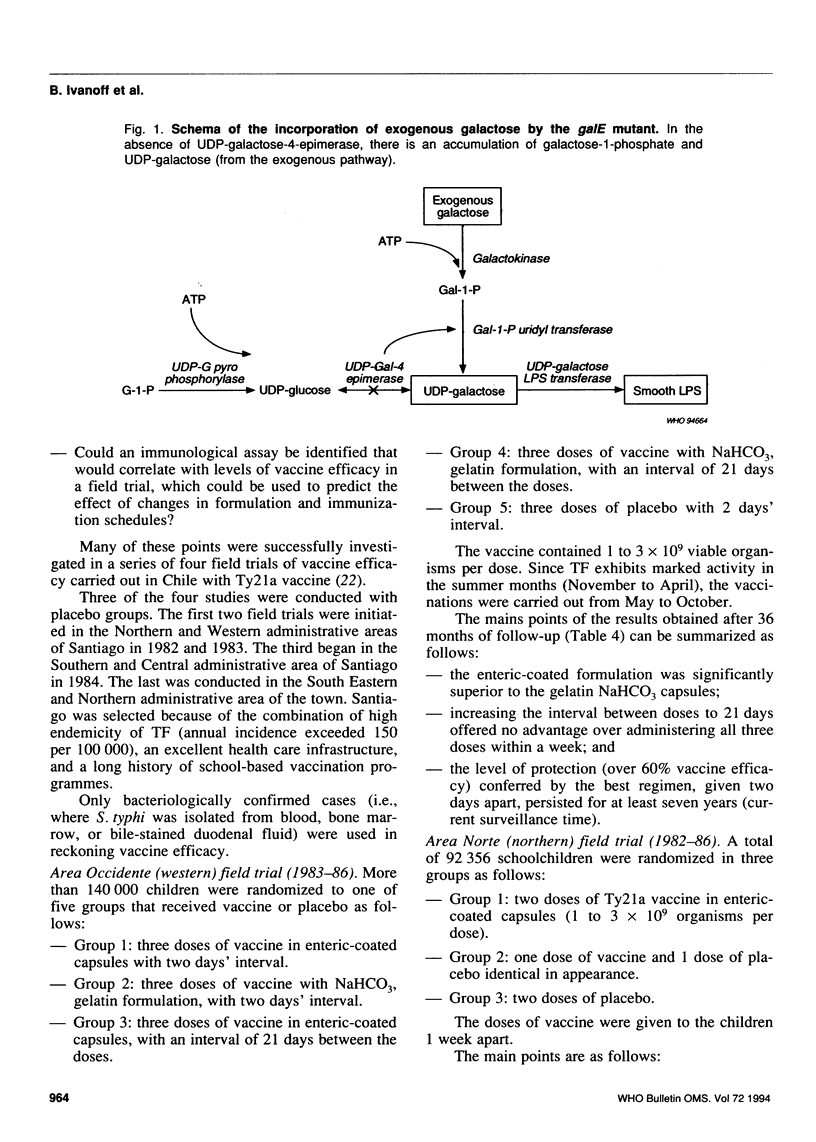
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Gotuzzo E., Frisancho O., Sanchez J., Liendo G., Carrillo C., Black R. E., Morris J. G., Jr Association between the acquired immunodeficiency syndrome and infection with Salmonella typhi or Salmonella paratyphi in an endemic typhoid area. Arch Intern Med. 1991 Feb;151(2):381–382. [PubMed] [Google Scholar]
- Herrera P., Valenzuela C. Factores genéticos en fiebre tifoidea. Rev Chil Pediatr. 1989 Sep-Oct;60(5):297–303. [PubMed] [Google Scholar]
- Holmgren J., Czerkinsky C., Lycke N., Svennerholm A. M. Mucosal immunity: implications for vaccine development. Immunobiology. 1992 Feb;184(2-3):157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- Hone D. M., Tacket C. O., Harris A. M., Kay B., Losonsky G., Levine M. M. Evaluation in volunteers of a candidate live oral attenuated Salmonella typhi vector vaccine. J Clin Invest. 1992 Aug;90(2):412–420. doi: 10.1172/JCI115876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantele A., Mäkelä P. H. Different profiles of the human immune response to primary and secondary immunization with an oral Salmonella typhi Ty21a vaccine. Vaccine. 1991 Jun;9(6):423–427. doi: 10.1016/0264-410x(91)90129-t. [DOI] [PubMed] [Google Scholar]
- Klugman K. P., Gilbertson I. T., Koornhof H. J., Robbins J. B., Schneerson R., Schulz D., Cadoz M., Armand J. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987 Nov 21;2(8569):1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- LANDY M. Studies on Vi antigen. VI. Immunization of human beings with purified Vi antigen. Am J Hyg. 1954 Jul;60(1):52–62. doi: 10.1093/oxfordjournals.aje.a119703. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982 Dec;146(6):724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Taylor D. N., Ferreccio C. Typhoid vaccines come of age. Pediatr Infect Dis J. 1989 Jun;8(6):374–381. doi: 10.1097/00006454-198906000-00010. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Loomis W. P., Alpuche-Aranda C., Behlau I., Hohmann E. The PhoP virulence regulon and live oral Salmonella vaccines. Vaccine. 1993;11(2):122–125. doi: 10.1016/0264-410x(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Baqar S., Muñoz C., Schlesinger L., Ferreccio C., Lindberg A. A., Svenson S., Losonsky G., Koster F., Levine M. M. Characteristics of humoral and cellular immunity to Salmonella typhi in residents of typhoid-endemic and typhoid-free regions. J Infect Dis. 1987 Dec;156(6):1005–1009. doi: 10.1093/infdis/156.6.1005. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Grez L., Schlesinger L., Ferreccio C., Baqar S., Muñoz C., Wasserman S. S., Losonsky G., Olson J. G., Levine M. M. Immunogenicity of Salmonella typhi Ty21a vaccine for young children. Infect Immun. 1991 Nov;59(11):4291–4293. doi: 10.1128/iai.59.11.4291-4293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisini R., Biselli R., Matricardi P. M., Fattorossi A., D'Amelio R. Clinical and immunological response to typhoid vaccination with parenteral or oral vaccines in two groups of 30 recruits. Vaccine. 1993;11(5):582–586. doi: 10.1016/0264-410x(93)90237-r. [DOI] [PubMed] [Google Scholar]
- Olanratmanee T., Levine M., Losonsky G., Thisyakorn V., Cryz S. J., Jr Safety and immunogenicity of Salmonella typhi Ty21a liquid formulation vaccine in 4- to 6-year-old Thai children. J Infect Dis. 1992 Aug;166(2):451–452. doi: 10.1093/infdis/166.2.451. [DOI] [PubMed] [Google Scholar]
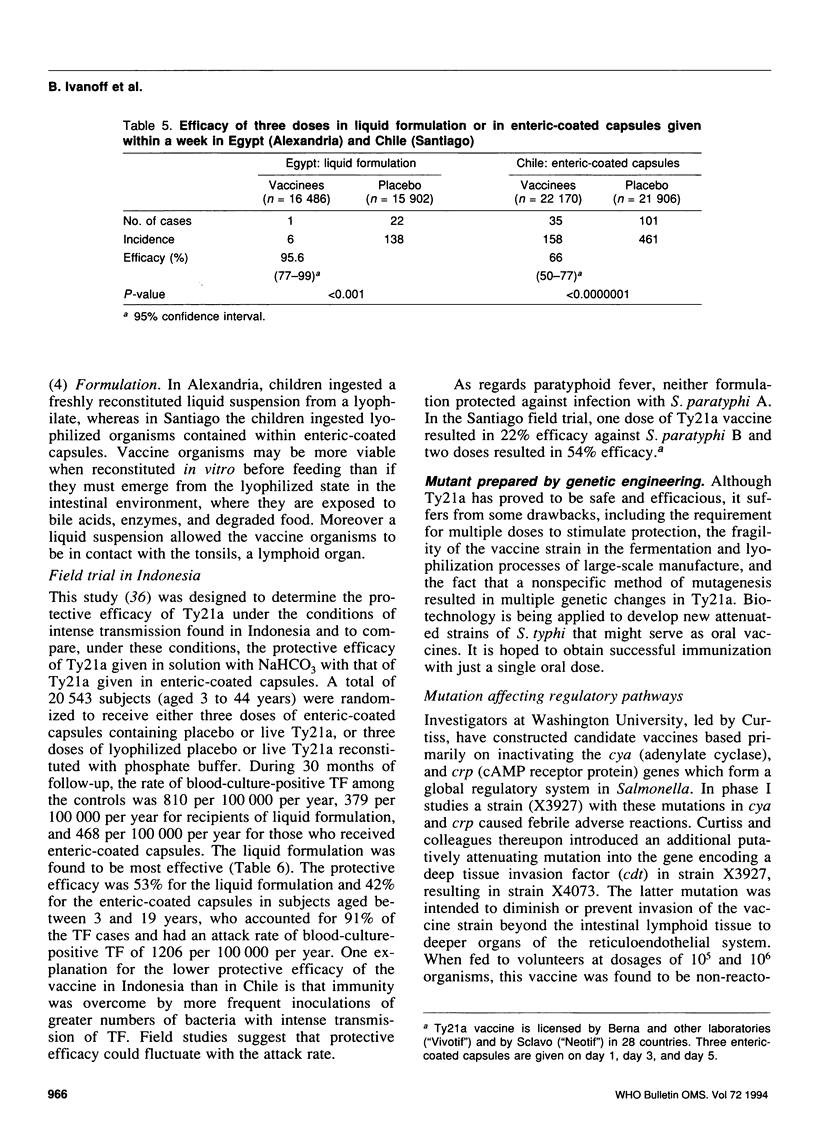
- Simanjuntak C. H., Paleologo F. P., Punjabi N. H., Darmowigoto R., Soeprawoto, Totosudirjo H., Haryanto P., Suprijanto E., Witham N. D., Hoffman S. L. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991 Oct 26;338(8774):1055–1059. doi: 10.1016/0140-6736(91)91910-m. [DOI] [PubMed] [Google Scholar]
- Spread of multiresistant Salmonella typhi. Lancet. 1990 Oct 27;336(8722):1065–1066. [PubMed] [Google Scholar]
- Szu S. C., Li X. R., Schneerson R., Vickers J. H., Bryla D., Robbins J. B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989 Dec;57(12):3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Stone A. L., Robbins J. D., Schneerson R., Robbins J. B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Ferreccio C., Robbins J. B., Tsai C. M., Schulz D., Cadoz M., Goudeau A., Levine M. M. Safety and immunogenicity of two Salmonella typhi Vi capsular polysaccharide vaccines. J Infect Dis. 1986 Aug;154(2):342–345. doi: 10.1093/infdis/154.2.342. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Hone D. M., Losonsky G. A., Guers L., Edelman R., Levine M. M. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine. 1992;10(7):443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Levine M. M., Robbins J. B. Persistence of antibody titres three years after vaccination with Vi polysaccharide vaccine against typhoid fever. Vaccine. 1988 Aug;6(4):307–308. doi: 10.1016/0264-410x(88)90175-2. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Losonsky G., Taylor D. N., Baron L. S., Kopecko D., Cryz S., Levine M. M. Lack of immune response to the Vi component of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a in human studies. J Infect Dis. 1991 Apr;163(4):901–904. doi: 10.1093/infdis/163.4.901. [DOI] [PubMed] [Google Scholar]
- Tagliabue A. Immune response to oral Salmonella vaccines. Curr Top Microbiol Immunol. 1989;146:225–231. doi: 10.1007/978-3-642-74529-4_24. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Nencioni L., Caffarena A., Villa L., Boraschi D., Cazzola G., Cavalieri S. Cellular immunity against Salmonella typhi after live oral vaccine. Clin Exp Immunol. 1985 Nov;62(2):242–247. [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C. Isolation of Vi antigen and a simple method for its measurement. Appl Microbiol. 1972 Oct;24(4):628–633. doi: 10.1128/am.24.4.628-633.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C., Pittman M. Effect of a Vi-degrading enzyme on potency of typhoid vaccines in mice. J Infect Dis. 1972 Apr;125(4):360–366. doi: 10.1093/infdis/125.4.360. [DOI] [PubMed] [Google Scholar]


