Abstract
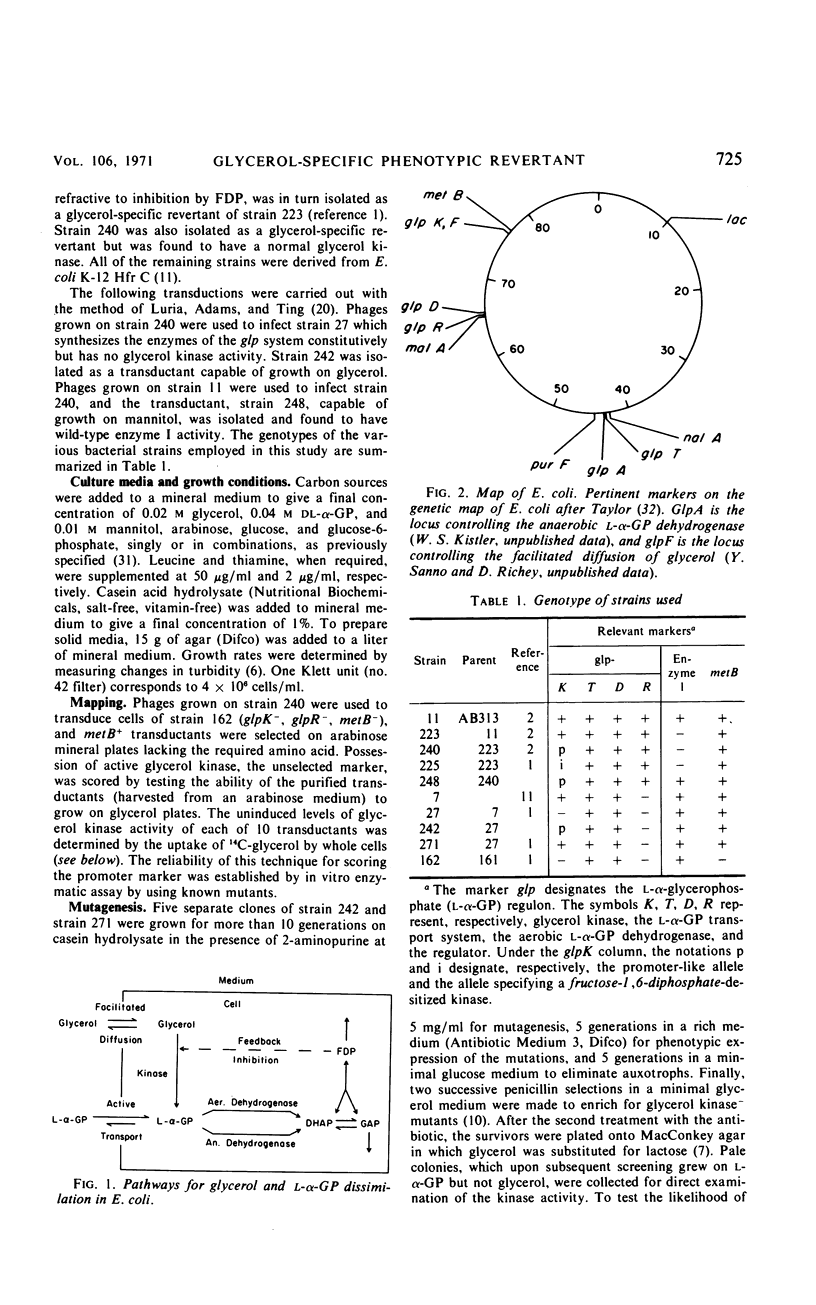
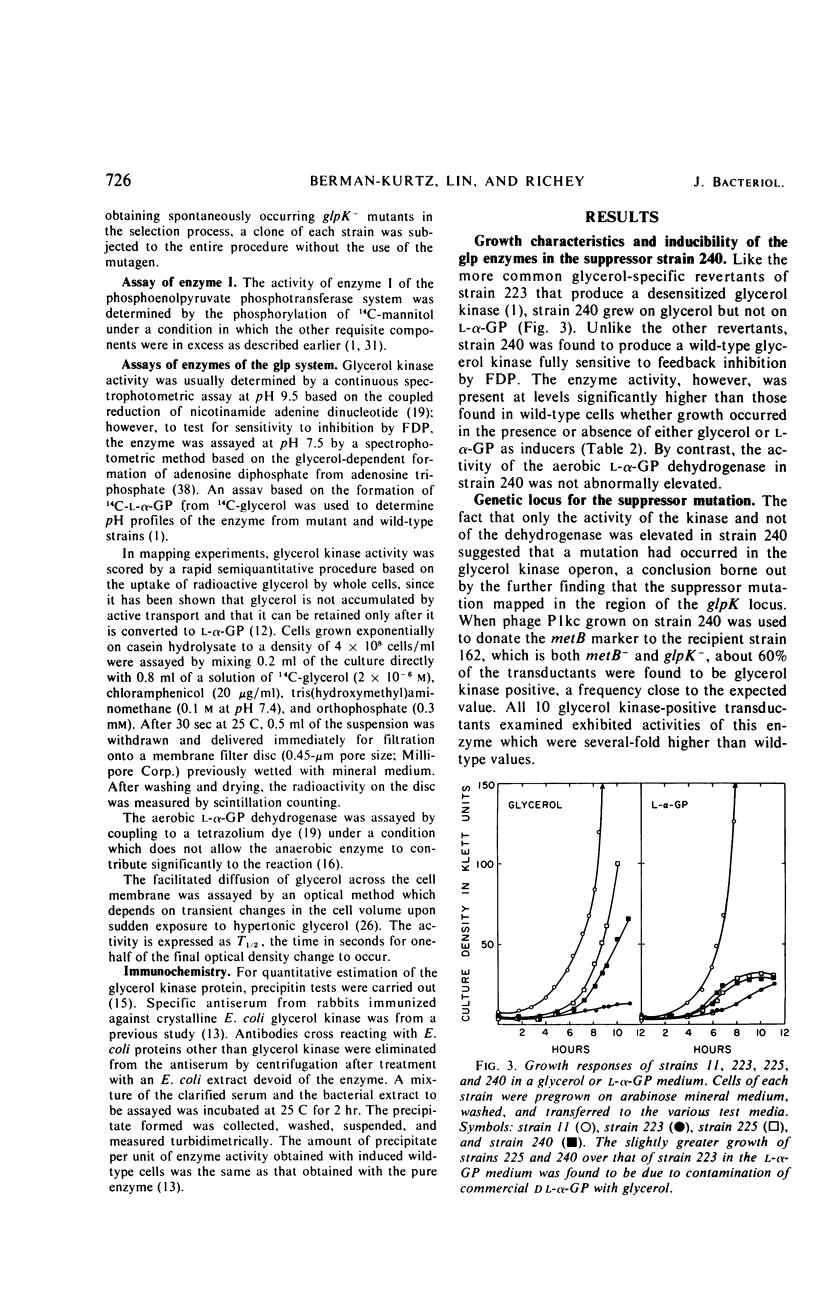
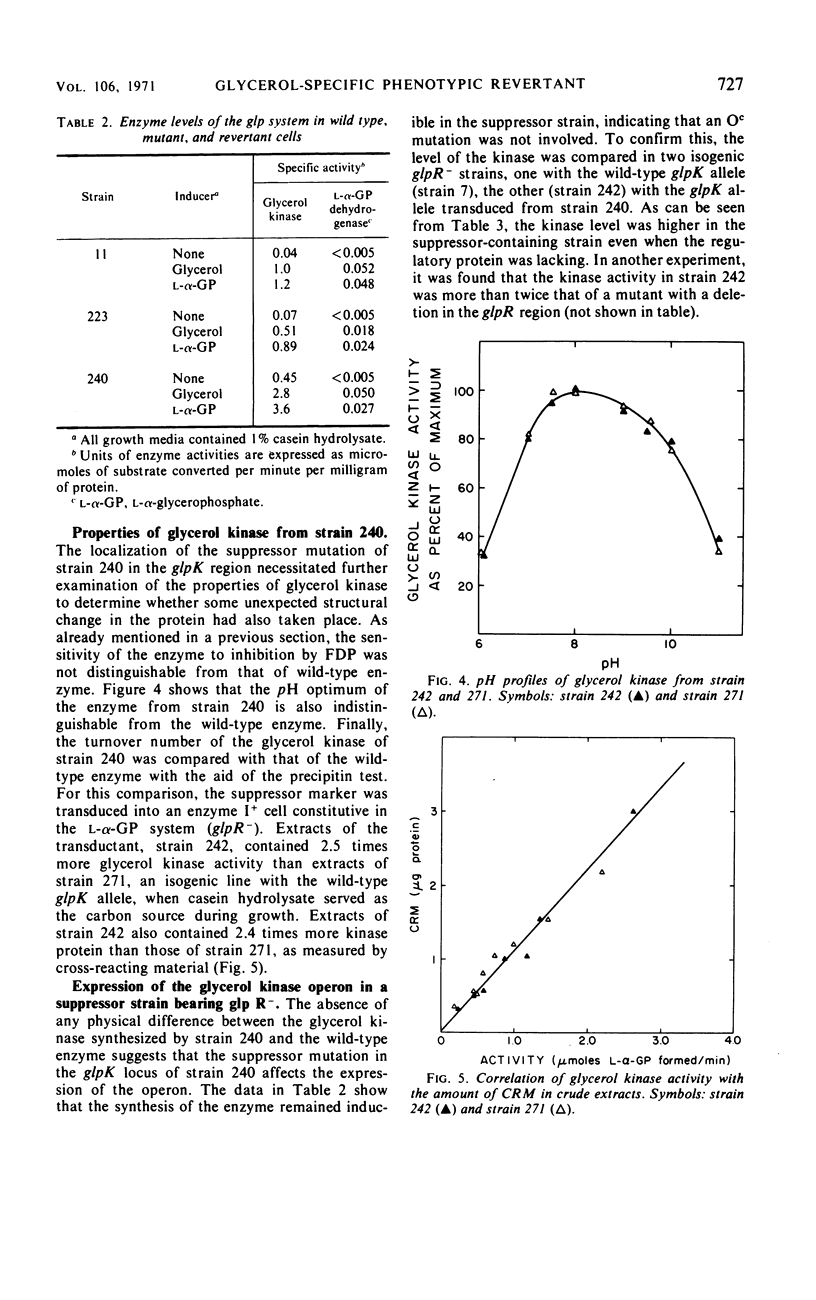
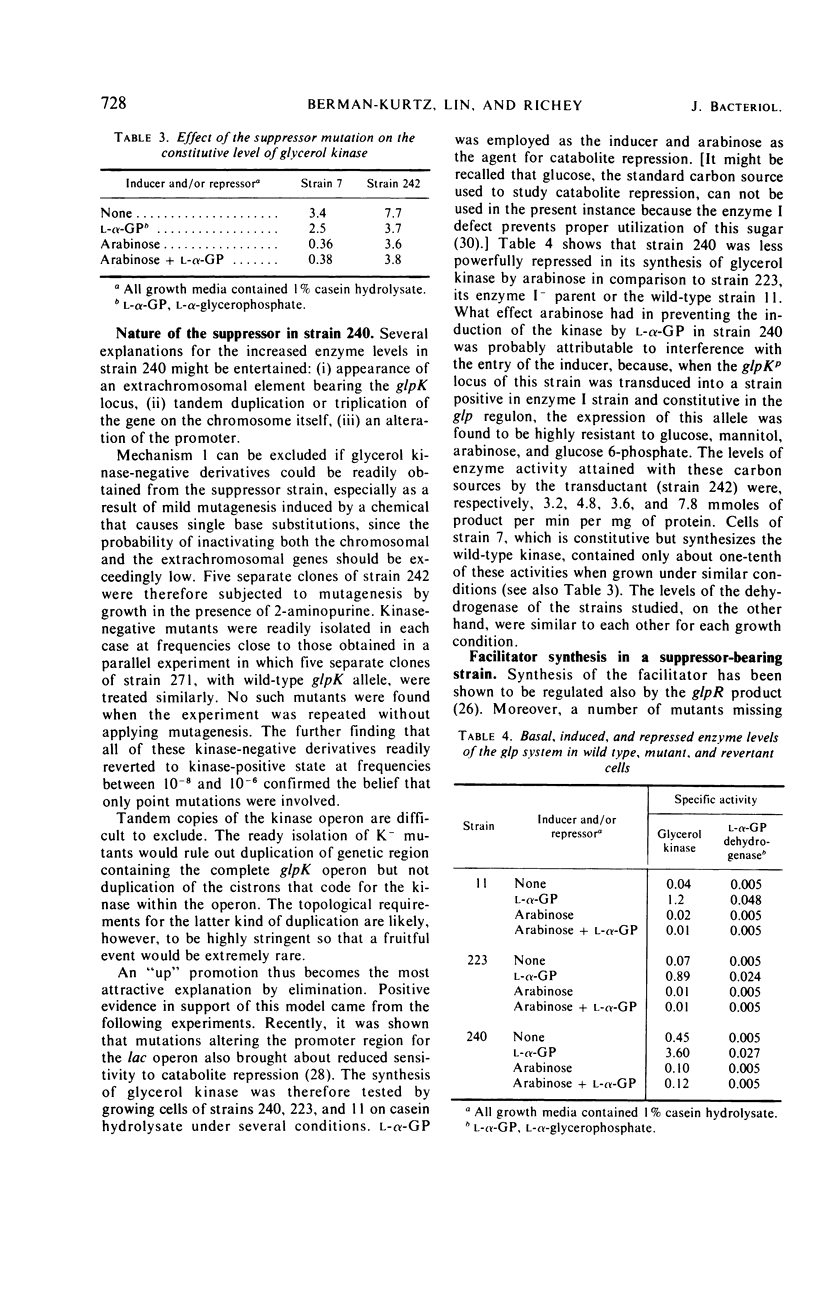
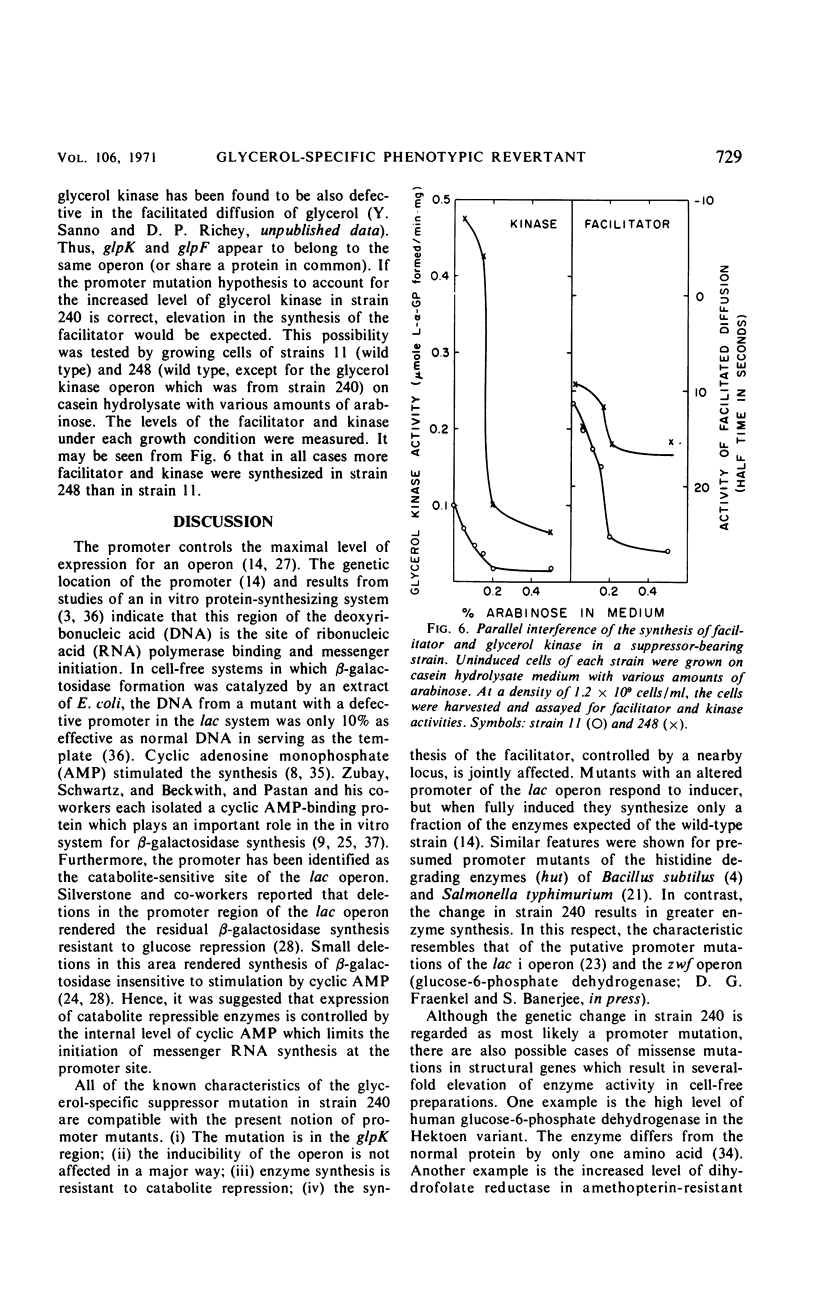
A glycerol-specific phenotypic revertant isolated from a mutant of Escherichia coli missing enzyme I of the phosphoenolpyruvate phosphotransferase system was studied. This revertant is capable of producing higher levels of glycerol kinase and the protein mediating the facilitated diffusion of glycerol (facilitator) than wild-type cells. The kinase of the revertant is indistinguishable from the wild-type enzyme with respect to its sensitivity to feedback inhibition by fructose-1,6-diphosphate, its pH optimum, and its turnover number. The synthesis of glycerol kinase in strains bearing the suppressor locus is resistant to catabolite repression. The suppressor mutation mapped at the known glpK locus. Thus, it is suggested that the mutation occurred in the promoter of the operon specifying the kinase and the facilitator.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman M., Lin E. C. Glycerol-specific revertants of a phosphoenolpyruvate phosphotransferase mutant: suppression by the desensitization of glycerol kinase to feedback inhibition. J Bacteriol. 1971 Jan;105(1):113–120. doi: 10.1128/jb.105.1.113-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M., Zwaig N., Lin E. C. Suppression of a pleiotropic mutant affecting glycerol dissimilation. Biochem Biophys Res Commun. 1970 Jan 23;38(2):272–278. doi: 10.1016/0006-291x(70)90708-4. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- Ippen K., Miller J. H., Scaife J., Beckwith J. New controlling element in the Lac operon of E. coli. Nature. 1968 Mar 2;217(5131):825–827. doi: 10.1038/217825a0. [DOI] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Monard D., Janecek J., Rickenberg H. V. The enzymic degradation of 3',5' cyclic AMP in strains of E. Coli sensitive and resistant to catobolite repression. Biochem Biophys Res Commun. 1969 May 22;35(4):584–591. doi: 10.1016/0006-291x(69)90388-x. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. The role of the lac promotor locus in the regulation of beta-galactosidase synthesis by cyclic 3',5'-adenosine monophosphate. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1336–1342. doi: 10.1073/pnas.61.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Scaife J., Beckwith J. R. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. doi: 10.1101/sqb.1966.031.01.052. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Magasanik B., Reznikoff W. S., Miller J. H., Beckwith J. R. Catabolite sensitive site of the lac operon. Nature. 1969 Mar 15;221(5185):1012–1014. doi: 10.1038/2211012b0. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Hachtel S. L., Williams W. A. Increased dihydrofolate reductase synthesis in Diplococcus pneumoniae following translatable alteration of the structural gene. II. Individual and dual effects on the properties and rate of synthesis of the enzyme. Genetics. 1969 Feb;61(2):313–326. doi: 10.1093/genetics/61.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate Accumulation and Metabolism in Escherichia coli: Characteristics of the Reversions of ctr Mutations. J Bacteriol. 1970 Dec;104(3):1318–1324. doi: 10.1128/jb.104.3.1318-1324.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A. Amino acid substitution (histidine to tyrosine) in a glucose-6-phosphate dehydrogenase variant (G6PD Hektoen) associated with over-production. J Mol Biol. 1970 Sep 28;52(3):483–490. doi: 10.1016/0022-2836(70)90414-6. [DOI] [PubMed] [Google Scholar]
- Zubay G., Chambers D. A. A DNA-directed cell-free system for beta-galactosidase synthesis; characterization of the de novo synthesized enzyme and some aspects of the regulation of synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:753–761. doi: 10.1101/sqb.1969.034.01.085. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Varmus H. E., Perlman R. L., Pastan I. H. Stimulation of lac mRNA synthesis by cyclic AMP in cell free extracts of Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 12;38(5):894–901. doi: 10.1016/0006-291x(70)90805-3. [DOI] [PubMed] [Google Scholar]



