Abstract
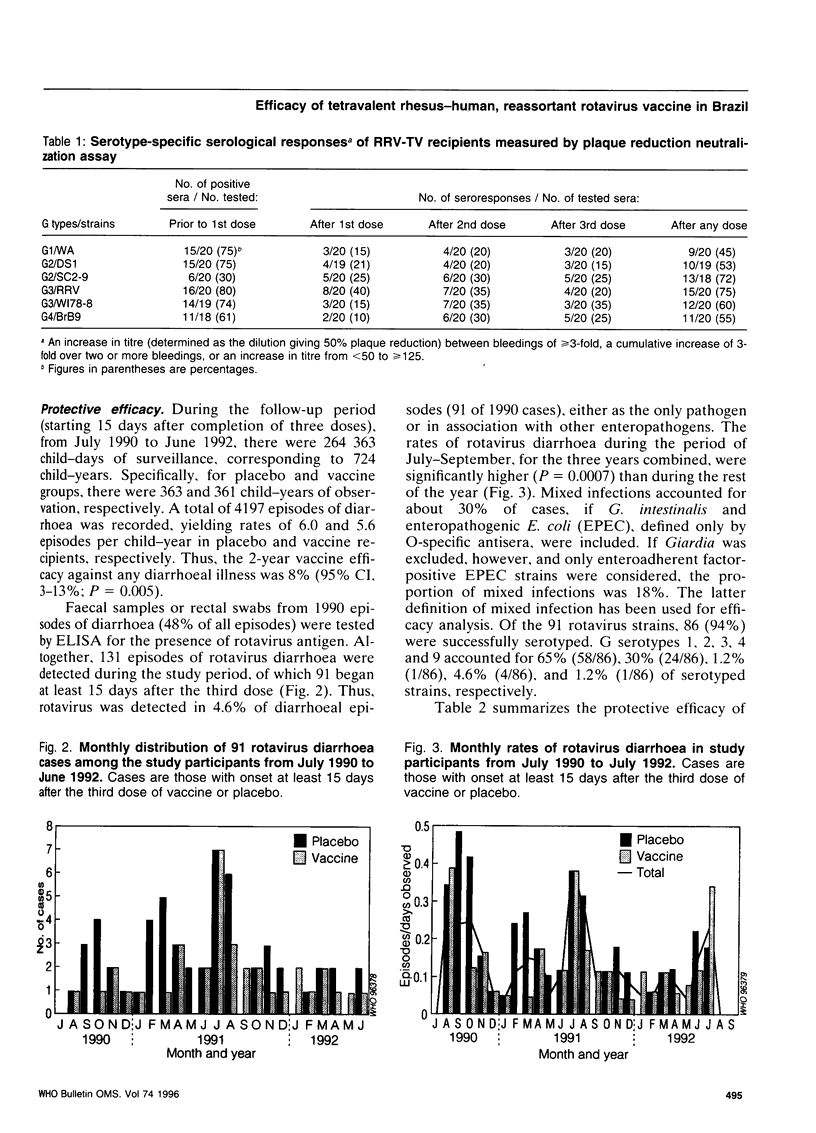
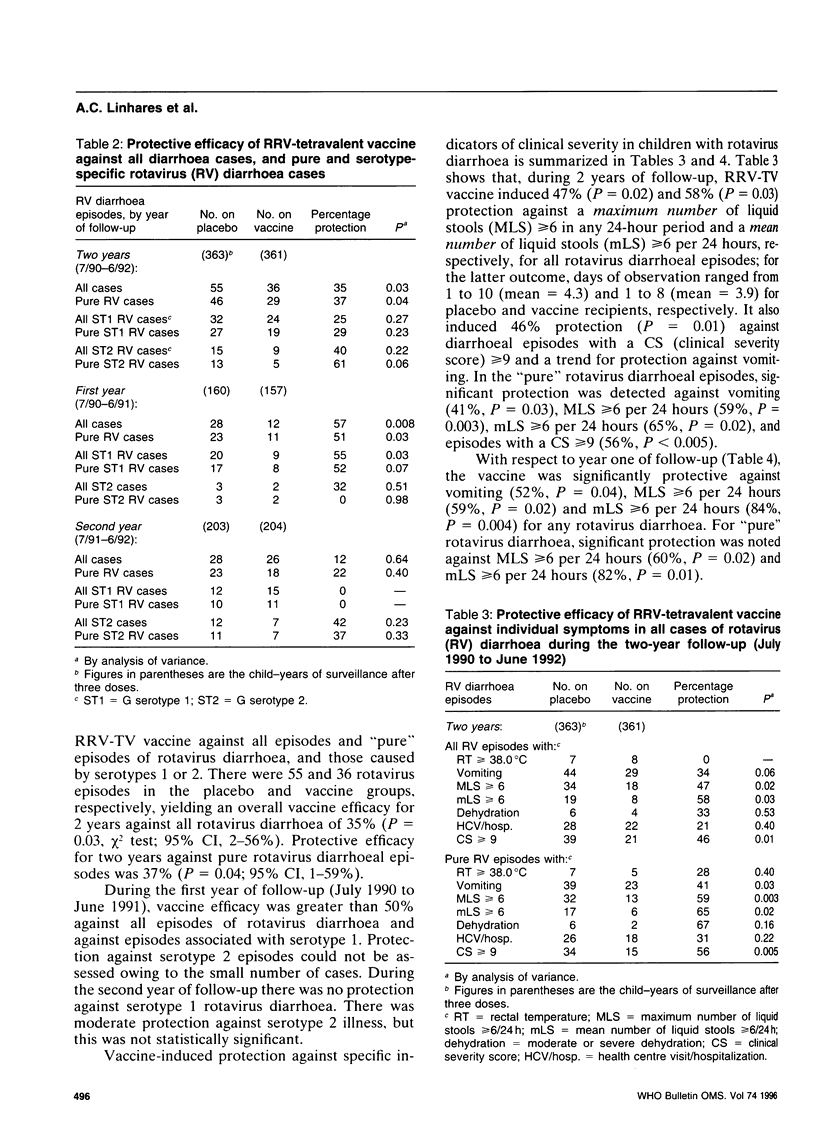
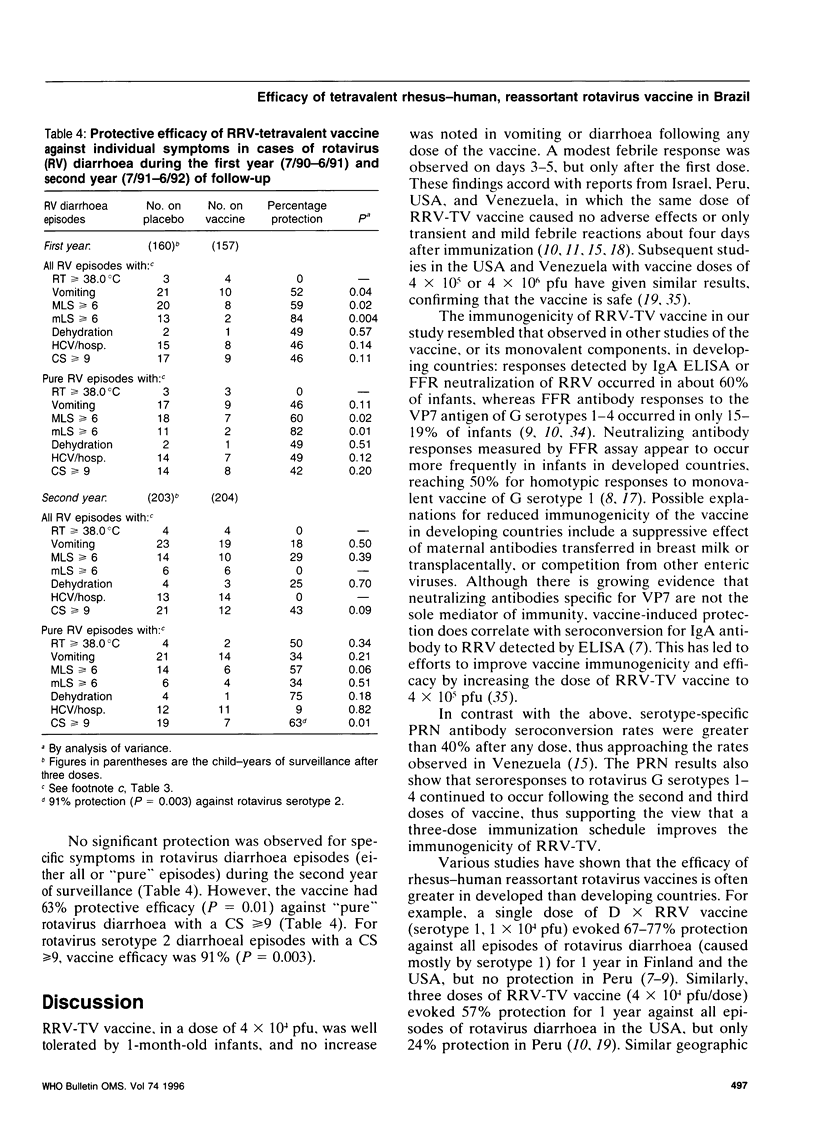
A tetravalent rhesus-human reassortant rotavirus (RRV-TV) vaccine (4 x 10(4) plaque-forming units/dose) was evaluated for safety, immunogenicity and efficacy in a prospective, randomized, double-blind, placebo-controlled trial involving 540 Brazilian infants. Doses of vaccine or placebo were given at ages 1, 3 and 5 months. No significant differences were noted in the occurrence of diarrhoea or vomiting in vaccine and placebo recipients following each dose. Low-grade fever occurred on days 3-5 in 2-3% of vaccinees after the first dose, but not after the second or third doses of vaccine. An IgA antibody response to rhesus rotavirus (RRV) occurred in 58% of vaccinees and 33% of placebo recipients. Neutralizing antibody responses to individual serotypes did not exceed 20% when measured by fluorescent focus reduction, but exceeded 40% when assayed by plaque reduction neutralization. There were 91 cases of rotavirus diarrhoea among the 3-dose (vaccine or placebo) recipients during two years of follow-up, 36 of them among children given the vaccine. Overall vaccine efficacy was 8% (P = 0.005) against any diarrhoea and 35% (P = 0.03) against any rotavirus diarrhoea. Protection during the first year of follow-up, when G serotype 1 rotavirus predominated, was 57% (P = 0.008), but fell to 12% in the second year. Similar results were obtained when analysis was restricted to episodes in which rotavirus was the only identified pathogen. There was a tendency for enhanced protection by vaccine against illness associated with an average of 6 or more stools per day. These results are sufficiently encouraging to warrant further studies of this vaccine in developing countries using a higher dosage in an attempt to improve its immunogenicity and efficacy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. I., Glass R. I., Rodgers G., Davidson B. L., Sack D. A. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus Vaccine Efficacy Group. JAMA. 1995 Apr 19;273(15):1191–1196. [PubMed] [Google Scholar]
- Bernstein D. I., Kacica M. A., McNeal M. M., Schiff G. M., Ward R. L. Local and systemic antibody response to rotavirus WC3 vaccine in adult volunteers. Antiviral Res. 1989 Dec;12(5-6):293–300. doi: 10.1016/0166-3542(89)90056-9. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Cipriani E., Lund J. S., Barnes G. L., Hosking C. S. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol. 1984 Apr;19(4):447–452. doi: 10.1128/jcm.19.4.447-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F. Development of candidate rotavirus vaccines. Vaccine. 1993;11(2):247–254. doi: 10.1016/0264-410x(93)90025-s. [DOI] [PubMed] [Google Scholar]
- Dagan R., Kassis I., Sarov B., Midthun K., Davidson B. L., Vesikari T., Sarov I. Safety and immunogenicity of oral tetravalent human-rhesus reassortant rotavirus vaccine in neonates. Pediatr Infect Dis J. 1992 Dec;11(12):991–996. doi: 10.1097/00006454-199211120-00001. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Arias C. F., Avendano L. F., Ghafoor A., Mathan M. M., Mendis L., Moe K., Bishop R. F. Comparative evaluation of the WHO and DAKOPATTS enzyme-linked immunoassay kits for rotavirus detection. Bull World Health Organ. 1989;67(4):369–374. [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Garcia L. S., Bruckner D. A., Brewer T. C., Shimizu R. Y. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol. 1983 Jul;18(1):185–190. doi: 10.1128/jcm.18.1.185-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Passarani N., Sarasini A., Battaglia M. Characterization of serotypes of human rotavirus strains by solid-phase immune electron microscopy. J Infect Dis. 1985 Dec;152(6):1143–1151. doi: 10.1093/infdis/152.6.1143. [DOI] [PubMed] [Google Scholar]
- Halsey N. A., Anderson E. L., Sears S. D., Steinhoff M., Wilson M., Belshe R. B., Midthun K., Kapikian A. Z., Chanock R. M., Samorodin R. Human-rhesus reassortant rotavirus vaccines: safety and immunogenicity in adults, infants, and children. J Infect Dis. 1988 Dec;158(6):1261–1267. doi: 10.1093/infdis/158.6.1261. [DOI] [PubMed] [Google Scholar]
- Ing D. J., Glass R. I., Woods P. A., Simonetti M., Pallansch M. A., Wilcox W. D., Davidson B. L., Sievert A. J. Immunogenicity of tetravalent rhesus rotavirus vaccine administered with buffer and oral polio vaccine. Am J Dis Child. 1991 Aug;145(8):892–897. doi: 10.1001/archpedi.1991.02160080070023. [DOI] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., Flores J., Lazo F., Butron B., Linares A., Huapaya A., Ventura G., Gil A., Kapikian A. Z. Immunogenicity, safety and protective efficacy of one dose of the rhesus rotavirus vaccine and serotype 1 and 2 human-rhesus rotavirus reassortants in children from Lima, Peru. Vaccine. 1996 Feb;14(3):237–243. doi: 10.1016/0264-410x(95)00132-k. [DOI] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., del Aguila R., Gil A., Verastegui H., Gerna G., Flores J., Kapikian A. Z., Andre F. E. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989 Mar;159(3):452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Prado V., Robins-Browne R., Lior H., Kaper J. B., Moseley S. L., Gicquelais K., Nataro J. P., Vial P., Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988 Jul;158(1):224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Gabbay Y. B., Mascarenhas J. D., Freitas R. B., Flewett T. H., Beards G. M. Epidemiology of rotavirus subgroups and serotypes in Belem, Brazil: a three-year study. Ann Inst Pasteur Virol. 1988 Jan-Mar;139(1):89–99. doi: 10.1016/s0769-2617(88)80009-1. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Monço H. C., Gabbay Y. B., de Araújo V. L., Serruya A. C., Loureiro E. C. Acute diarrhoea associated with rotavirus among children living in Belém, Brazil. Trans R Soc Trop Med Hyg. 1983;77(3):384–390. doi: 10.1016/0035-9203(83)90170-0. [DOI] [PubMed] [Google Scholar]
- Madore H. P., Christy C., Pichichero M., Long C., Pincus P., Vosefsky D., Kapikian A. Z., Dolin R. Field trial of rhesus rotavirus or human-rhesus rotavirus reassortant vaccine of VP7 serotype 3 or 1 specificity in infants. The Elmwood, Panorama, and Westfall Pediatric Groups. J Infect Dis. 1992 Aug;166(2):235–243. doi: 10.1093/infdis/166.2.235. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Blanco M., Garcia D., White L., Alfonzo E., Crespo I., Cunto W., Pittman A. L., Kapikian A. Z., Flores J. Evaluation of the antigenicity and reactogenicity of varying formulations of the rhesus rotavirus-based quadrivalent and the M37 rotavirus vaccine candidates. J Med Virol. 1994 Apr;42(4):330–337. doi: 10.1002/jmv.1890420403. [DOI] [PubMed] [Google Scholar]
- Perez-Schael I., Blanco M., Vilar M., Garcia D., White L., Gonzalez R., Kapikian A. Z., Flores J. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. J Clin Microbiol. 1990 Mar;28(3):553–558. doi: 10.1128/jcm.28.3.553-558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E., Marsocci S. M., Francis A. B., Green J. L., Disney F. A., Rennels M. B., Lewis E. D., Sugarman L., Losonsky G. A., Zito E. A comparative evaluation of the safety and immunogenicity of a single dose of unbuffered oral rhesus rotavirus serotype 3, rhesus/human reassortant serotypes 1, 2 and 4 and combined (tetravalent) vaccines in healthy infants. Vaccine. 1993;11(7):747–753. doi: 10.1016/0264-410x(93)90260-5. [DOI] [PubMed] [Google Scholar]
- Raymond C. A. Experimental rotavirus vaccine passes first test; eventual goal: immunize newborns against most prevalent cause of life-threatening diarrhea. JAMA. 1987 Jul 3;258(1):12–13. [PubMed] [Google Scholar]
- Tajima T., Thompson J., Wright P. F., Kondo Y., Tollefson S. J., King J., Kapikian A. Z. Evaluation of a reassortant rhesus rotavirus vaccine in young children. Vaccine. 1990 Feb;8(1):70–74. doi: 10.1016/0264-410x(90)90181-k. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Ruuska T., Green K. Y., Flores J., Kapikian A. Z. Protective efficacy against serotype 1 rotavirus diarrhea by live oral rhesus-human reassortant rotavirus vaccines with human rotavirus VP7 serotype 1 or 2 specificity. Pediatr Infect Dis J. 1992 Jul;11(7):535–542. doi: 10.1097/00006454-199207000-00006. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Varis T., Green K., Flores J., Kapikian A. Z. Immunogenicity and safety of rhesus-human rotavirus reassortant vaccines with serotype 1 or 2 VP7 specificity. Vaccine. 1991 May;9(5):334–339. doi: 10.1016/0264-410x(91)90060-j. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Bernstein D. I., Shukla R., Young E. C., Sherwood J. R., McNeal M. M., Walker M. C., Schiff G. M. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989 Jan;159(1):79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- de Zoysa I., Feachem R. G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull World Health Organ. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]


