Abstract
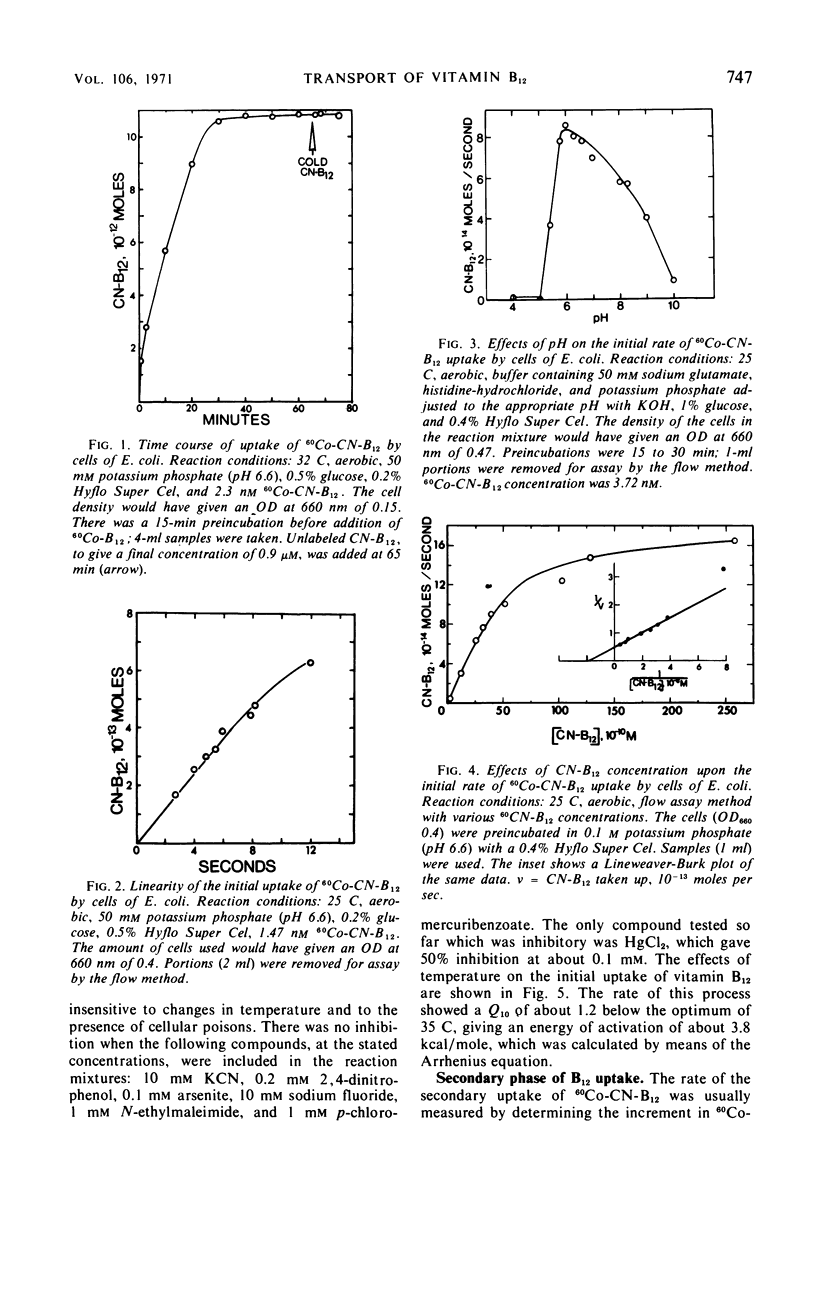
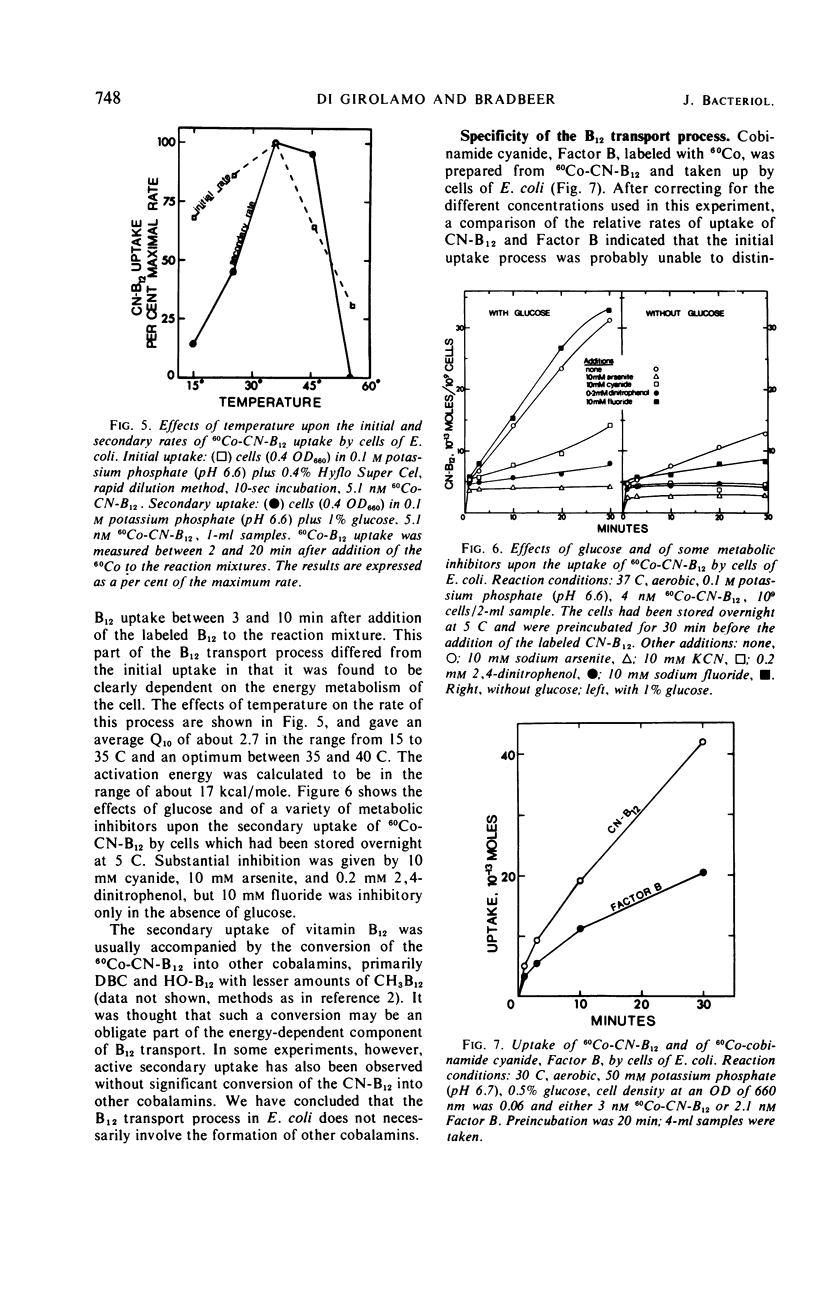
The uptake of 60Co-labeled cyanocobalamin (vitamin B12) by cells of Escherichia coli K-12λ was shown to consist of an initial rapid phase (complete in <1 min), followed by a slower secondary phase. Methods enabling the measurement of 60Co-B12 uptake after incubation times of 1 to 2 sec were used in studies on the initial rate of B12 uptake. This initial process showed saturation kinetics, with a Vmax of 56 molecules per sec per cell and a Km of 5 nm, and was essentially independent of cellular energy metabolism. No inhibition was obtained with cyanide, fluoride, arsenite, or 2, 4-dinitrophenol, and an energy of activation of 3.8 kcal/mole for this initial phase of uptake was calculated from its response to temperature changes between 15 and 35 C. The inhibition by HgCl2 (50% at 0.1 mm) but not by 1 mmN-ethylmaleimide or 1 mmp-chloromercuribenzoate was consistent with a role for a relatively inaccessible sulfhydryl residue at the initial B12 binding site. The secondary phase of B12 uptake was clearly dependent on the energy metabolism of the cell, and, from its response to temperature, an energy of activation of about 17 kcal/mole was calculated. Cyanide (10 mm), arsenite (10 mm), and 2, 4-dinitrophenol (0.1 mm) gave at least 70% inhibition of the rate of the secondary phase. The formation of other cobalamins, such as 5′-deoxyadenosyl cobalamin, was not an obligate part of B12 transport. The cells were also able to take up 60Co-labeled cobinamide cyanide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo P. M., Kadner R. J., Bradbeer C. Isolation of vitamin B 12 transport mutants of Escherichia coli. J Bacteriol. 1971 Jun;106(3):751–757. doi: 10.1128/jb.106.3.751-757.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET S., KAUFMAN J. T., BECK W. S. The metabolic functions of vitamin B12. III. Vitamin B12 binding in Lactobacillus leichmannii and other lactobacilli. Biochim Biophys Acta. 1962 Nov 5;64:447–457. doi: 10.1016/0006-3002(62)90302-5. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- OGINSKY E. L. Uptake of vitamin B12 by Escherichia coli. Arch Biochem Biophys. 1952 Mar;36(1):71–79. doi: 10.1016/0003-9861(52)90378-0. [DOI] [PubMed] [Google Scholar]
- PARANCHYCH W., COOPER B. A. Factors influencing the uptake of cyanocobalamin (vitamin B12) by Ehrlich ascites carcinoma cells. Biochim Biophys Acta. 1962 Jul 2;60:393–403. doi: 10.1016/0006-3002(62)90415-8. [DOI] [PubMed] [Google Scholar]
- Reeves R. B., Fay F. S. Cyanocobalamin (vitamin B12) uptake by Ochromonas malhamensis. Am J Physiol. 1966 Jun;210(6):1273–1278. doi: 10.1152/ajplegacy.1966.210.6.1273. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]



