Abstract
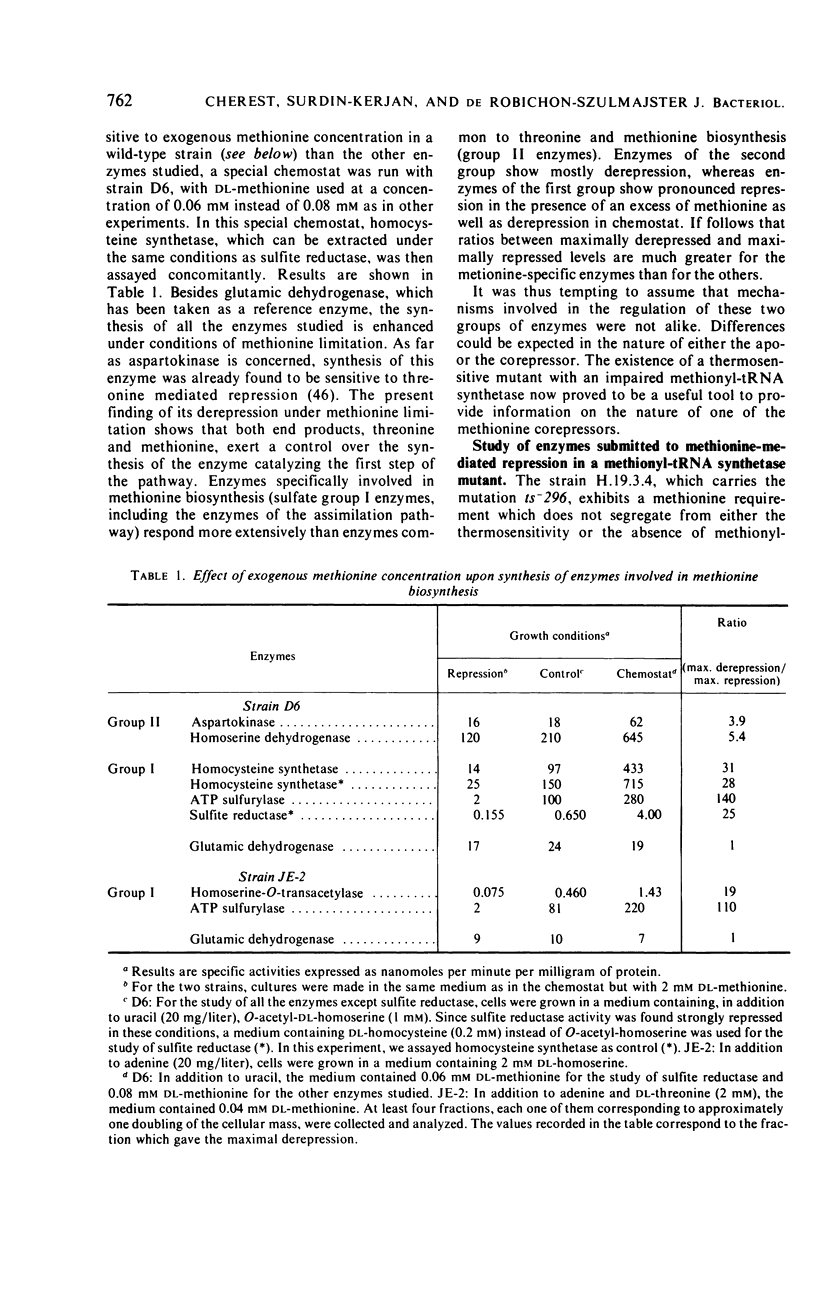
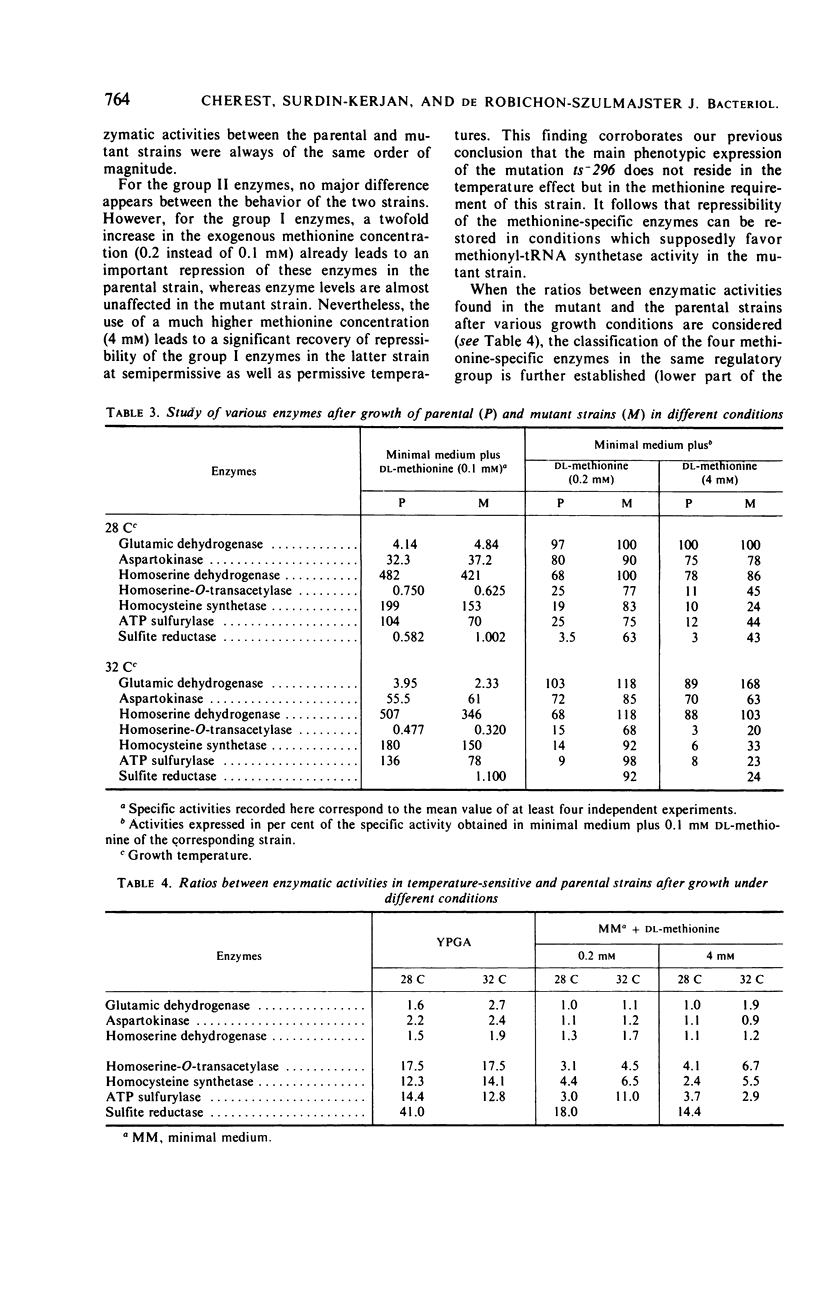
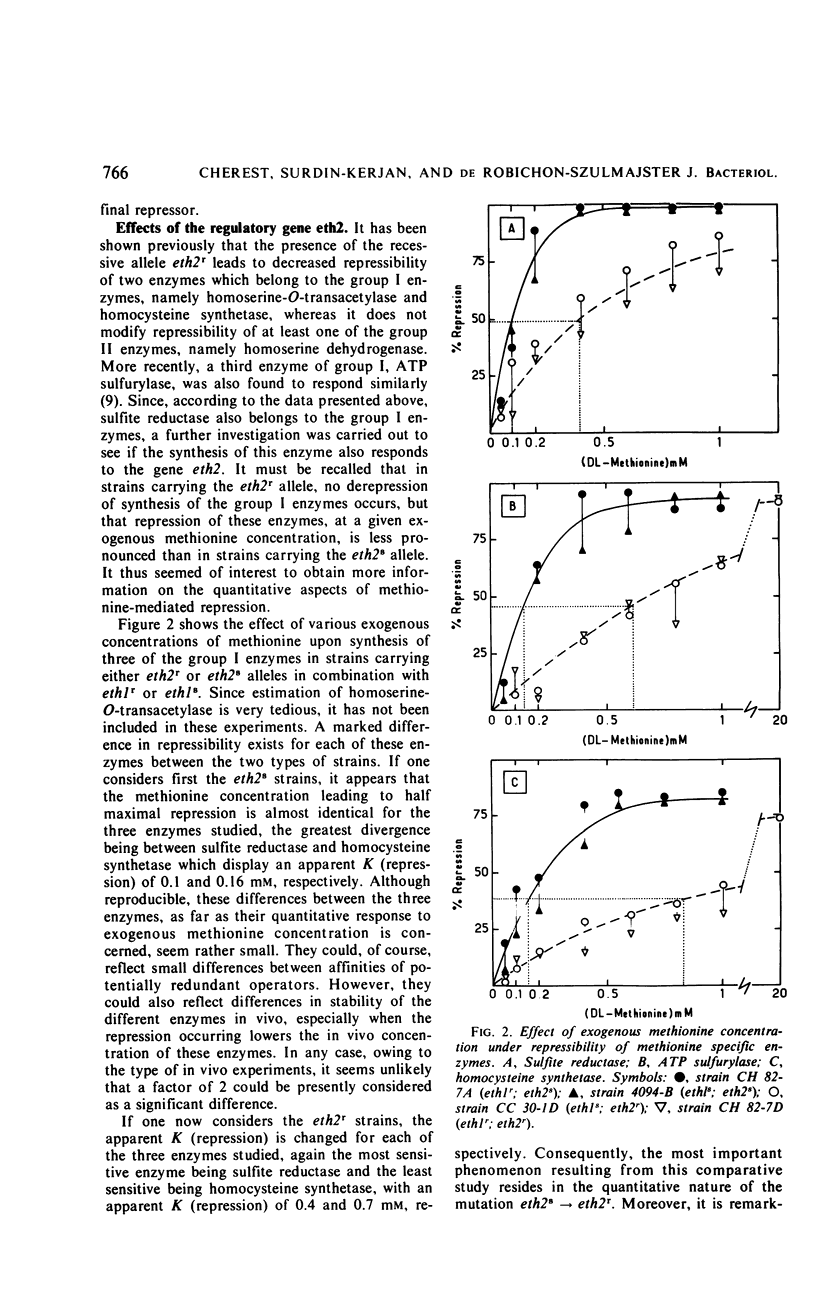
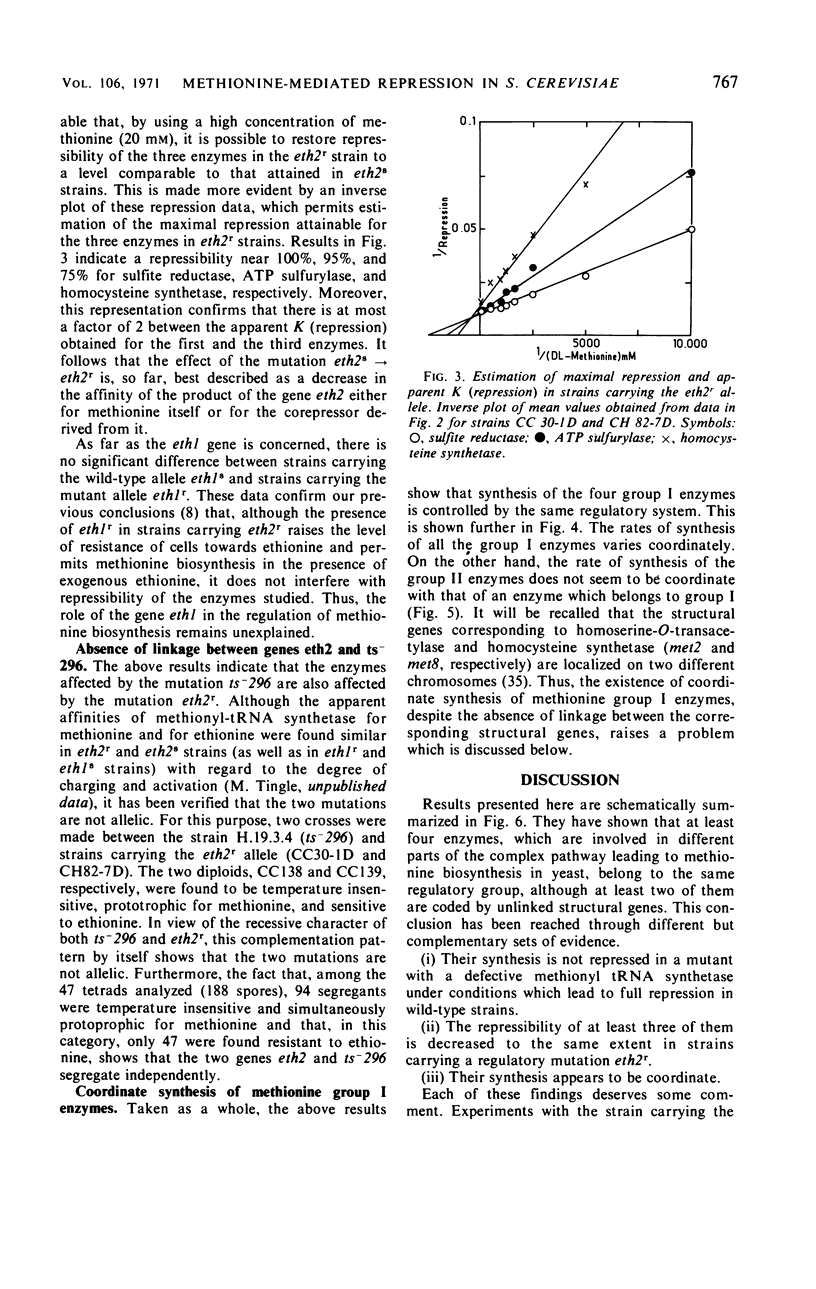
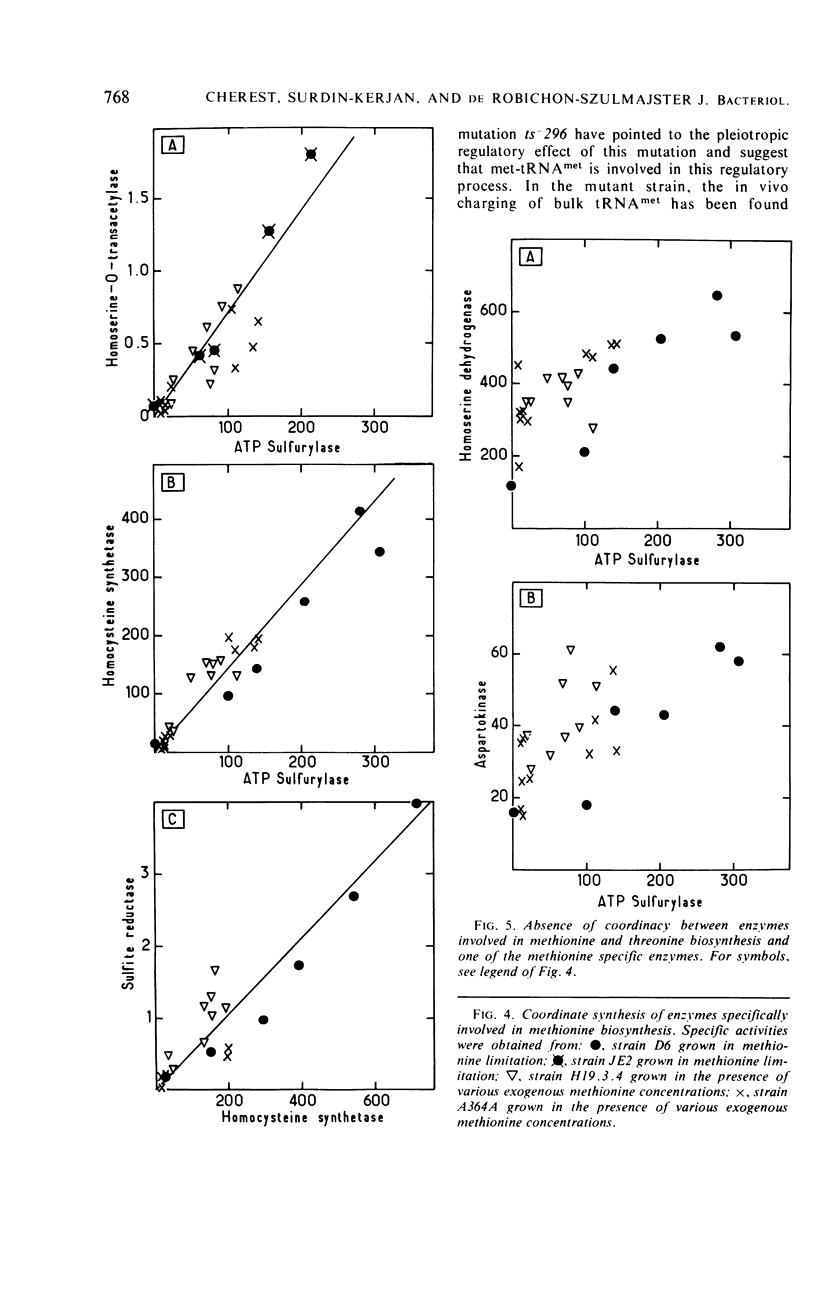
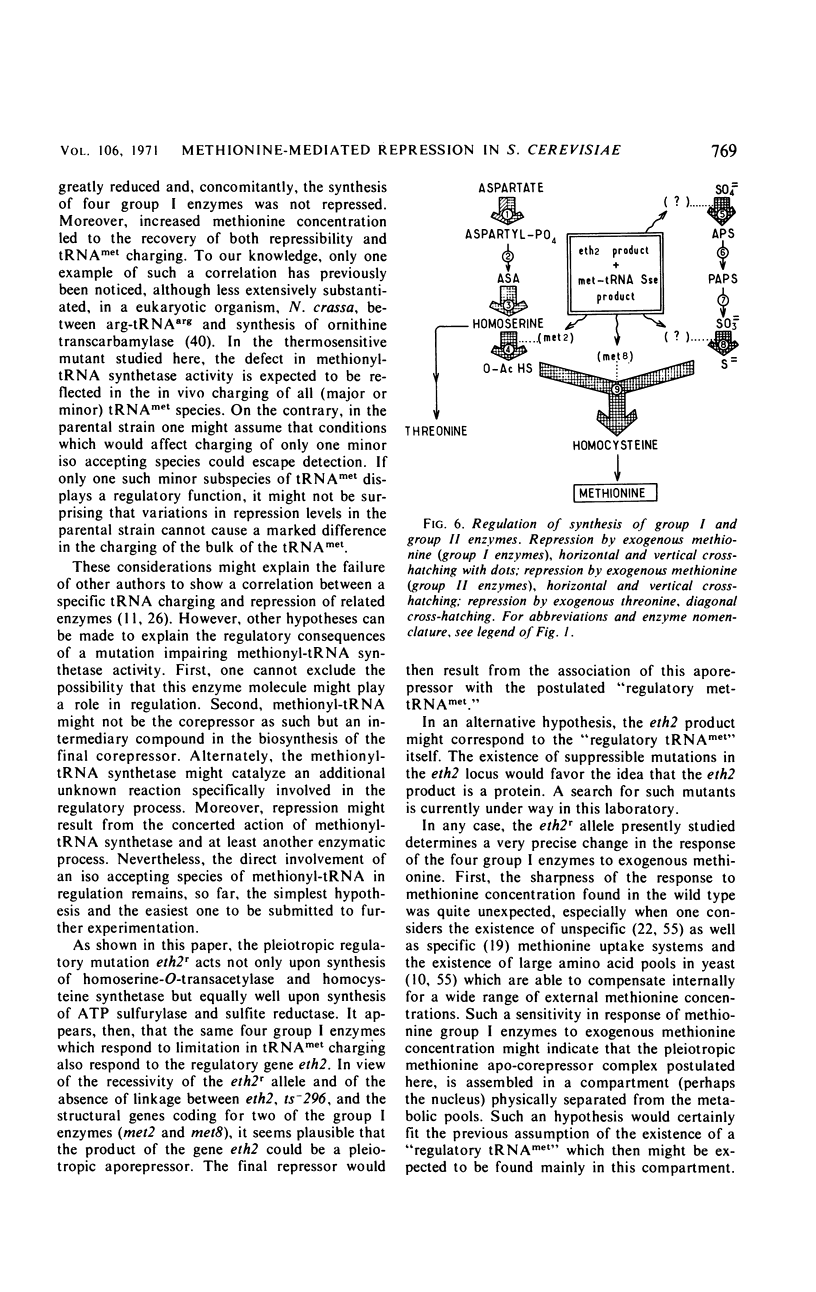
Detailed study of methionine-mediated repression of enzymes involved in methionine biosynthesis in Saccharomyces cerevisiae led to classification of these enzymes into two distinct regulatory groups. Group I comprises four enzymes specifically involved in different parts of methionine biosynthesis, namely, homoserine-O-transacetylase, homocysteine synthetase, adenosine triphosphate sulfurylase, and sulfite reductase. Repressibility of these enzymes is greatly decreased in strains carrying a genetically impaired methionyl-transfer ribonucleic acid (tRNA) synthetase (mutation ts− 296). Conditions leading to absence of repression in the mutant strain have been correlated with a sharp decrease in bulk tRNAmet charging, whereas conditions which restore repressibility of group I enzymes also restore tRNAmet charging. These findings implicate methionyl-tRNA in the regulatory process. However, the absence of a correlation in the wild type between methionyl-tRNA charging and the levels of methionine group I enzymes suggests that only a minor iso accepting species of tRNAmet may be devoted with a regulatory function. Repressibility of the same four enzymes (group I) was also decreased in strains carrying the regulatory mutation eth2r. Although structural genes coding for two of these enzymes, as well as mutations ts− 296 and eth2r segregate independently to each other, synthesis of group I enzymes is coordinated. The pleiotropic regulatory system involved seems then to comprise beside a “regulatory methionyl tRNAmet,” another element, product of gene eth2, which might correspond either to an aporepressor protein or to the “regulatory tRNAmet” itself. Regulation of group II enzymes is defined by response to exogenous methionine, absence of response to either mutations ts− 296 and eth2r, and absence of coordinacy with group I enzymes. However, the two enzymes which belong to this group and are both involved in threonine and methionine biosynthesis undergo distinct regulatory patterns. One, aspartokinase, is subject to a bivalent repression exerted by threonine and methionine, and the other, homoserine dehydrogenase, is subject only to methionine-mediated repression. Participation of at least another aporepressor and another corepressor, different from the ones involved in regulation of group I enzymes, is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- BLACK S., WRIGHT N. G. Homoserine dehydrogenase. J Biol Chem. 1955 Mar;213(1):51–60. [PubMed] [Google Scholar]
- Böck A., Faiman L. E., Neidhardt F. C. Biochemical and genetic characterization of a mutant of Escherichia coli with a temperature-sensitive valyl ribonucleic acid synthetase. J Bacteriol. 1966 Oct;92(4):1076–1082. doi: 10.1128/jb.92.4.1076-1082.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo R. A., Calvo J. M. Lack of end-product inhibition and repression of leucine synthesis in a strain of Salmonella typhimurium. Science. 1967 May 26;156(3778):1107–1109. doi: 10.1126/science.156.3778.1107. [DOI] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Lacy A. M., Cleary T. J., Fankhauser D. B. Histidine-mediated control of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1970 Oct;104(1):98–106. doi: 10.1128/jb.104.1.98-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassio D., Lemoine F., Waller J. P., Sandrin E., Boissonnas R. A. Selective inhibition of aminoacyl ribonucleic acid synthetases by aminoalkyl adenylates. Biochemistry. 1967 Mar;6(3):827–836. doi: 10.1021/bi00855a024. [DOI] [PubMed] [Google Scholar]
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich M., Trela J. M. Control of isoleucine, valine, and leucine biosynthesis. VI. Effect of 5',5',5'-trifluoroleucine on repression in Salmonella typhimurium. J Bacteriol. 1969 Jul;99(1):101–106. doi: 10.1128/jb.99.1.101-106.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich M. Valyl-Transfer RNA: Role in Repression of the Isoleucine-Valine Enzymes in Escherichia coli. Science. 1967 Aug 18;157(3790):823–825. doi: 10.1126/science.157.3790.823-a. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Gits J. J., Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. 3. Evidence for a specific methionine-transporting system. Biochim Biophys Acta. 1967 Jul 3;135(3):507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Methionyl transfer RNA synthetase mutants of Salmonella typhimurium which have normal control of the methionine biosynthetic enzymes. Biochim Biophys Acta. 1969 Jun 17;184(1):233–236. doi: 10.1016/0304-4165(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Horn P. C., Hopwood D. A., Maas W. K., DeDeken R. Studies on the mechanism of repression of arginine biosynthesis in Escherichia coli. 3. Repression of enzymes of arginine biosynthesis in arginyl-tRNA synthetase mutants. J Mol Biol. 1968 Jul 14;35(1):83–93. doi: 10.1016/s0022-2836(68)80038-5. [DOI] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Temperature-sensitive repression of the tryptophan operon in Escherichia coli. J Bacteriol. 1969 Jul;99(1):279–286. doi: 10.1128/jb.99.1.279-286.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- McClary D. O., Nulty W. L., Miller G. R. EFFECT OF POTASSIUM VERSUS SODIUM IN THE SPORULATION OF SACCHAROMYCES. J Bacteriol. 1959 Sep;78(3):362–368. doi: 10.1128/jb.78.3.362-368.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Hartwell L. H. A mutant of yeast with a defective methionyl-tRNA synthetase. Genetics. 1969 Mar;61(3):557–566. doi: 10.1093/genetics/61.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Magee P. T., Hartwell L. H. Role of isoleucyl-transfer ribonucleic acid synthetase in ribonucleic acid synthesis and enzyme repression in yeast. J Bacteriol. 1969 Nov;100(2):579–584. doi: 10.1128/jb.100.2.579-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Experiments with the Chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci U S A. 1950 Dec;36(12):708–719. doi: 10.1073/pnas.36.12.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Flavin M. Acetylhomoserine. An intermediate in the fungal biosynthesis of methionine. J Biol Chem. 1967 Sep 10;242(17):3884–3895. [PubMed] [Google Scholar]
- Nass G., Hasenbank R. Effect of Borrelidin on the threonyl-tRNA-synthetase activity and the regulation of threonine-biosynthetic enzymes in Saccharomyces cerivisiae. Mol Gen Genet. 1970;108(1):28–32. doi: 10.1007/BF00343181. [DOI] [PubMed] [Google Scholar]
- Nass G., Poralla K., Zähner H. Effect of the antibiotic Borrelidin on the regulation of threonine biosynthetic enzymes in E. coli. Biochem Biophys Res Commun. 1969 Jan 6;34(1):84–91. doi: 10.1016/0006-291x(69)90532-4. [DOI] [PubMed] [Google Scholar]
- Nass G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Mol Gen Genet. 1967;100(2):216–224. doi: 10.1007/BF00333608. [DOI] [PubMed] [Google Scholar]
- Nazario M. The accumulation of argininosuccinate in Neurospora crassa. II. Inhibition of arginyl-tRNA synthesis by argininosuccinate. Biochim Biophys Acta. 1967 Aug 22;145(1):146–152. doi: 10.1016/0005-2787(67)90663-6. [DOI] [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Ravel J. M., White M. N., Shive W. Activation of tyrosine analogs in relation to enzyme repression. Biochem Biophys Res Commun. 1965 Jul 26;20(3):352–359. doi: 10.1016/0006-291x(65)90372-4. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- SIEGEL L. M. A DIRECT MICRODETERMINATION FOR SULFIDE. Anal Biochem. 1965 Apr;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Fink G. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium 3. A class of regulatory mutants deficient in tRNA for histidine. J Mol Biol. 1966 Dec 28;22(2):335–347. doi: 10.1016/0022-2836(66)90136-7. [DOI] [PubMed] [Google Scholar]
- Surdin Y., Sly W., Sire J., Bordes A. M., Robichon-Szulmajster H. Propriétés et contrôle génétique du système d'accumulation des acides aminés chez Saccharomyces cerevisiae. Biochim Biophys Acta. 1965 Oct 18;107(3):546–566. [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi K., Ukita T., Nishimura S. Characterization of two species of methionine transfer ribonucleic acid from bakers' yeast. J Biol Chem. 1968 Nov 10;243(21):5761–5768. [PubMed] [Google Scholar]
- Tsuzukida Y., Isemura S., Kitano T., Matsui K. Partial purification and some properties of methionine transfer RNA of baker's yeast. J Biochem. 1969 Nov;66(5):573–579. [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]
- de ROBICHON-SZULMAJSTER, CORRIVAUX D. [Metabolic regulation of the biosynthesis of methionine and threonine in Saccharomyces cerevisiae. I. Repression and retroinhibition of aspartokinase]. Biochim Biophys Acta. 1963 Jun 11;73:248–256. doi: 10.1016/0006-3002(63)90309-3. [DOI] [PubMed] [Google Scholar]



