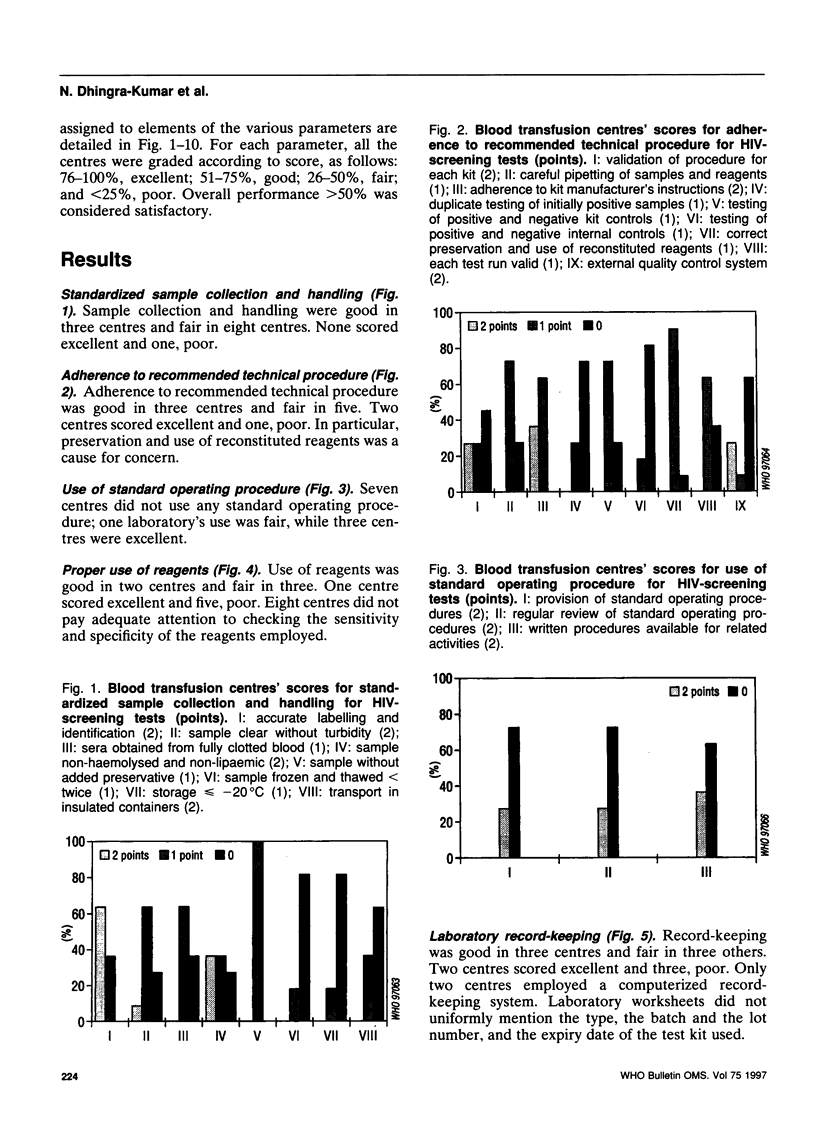
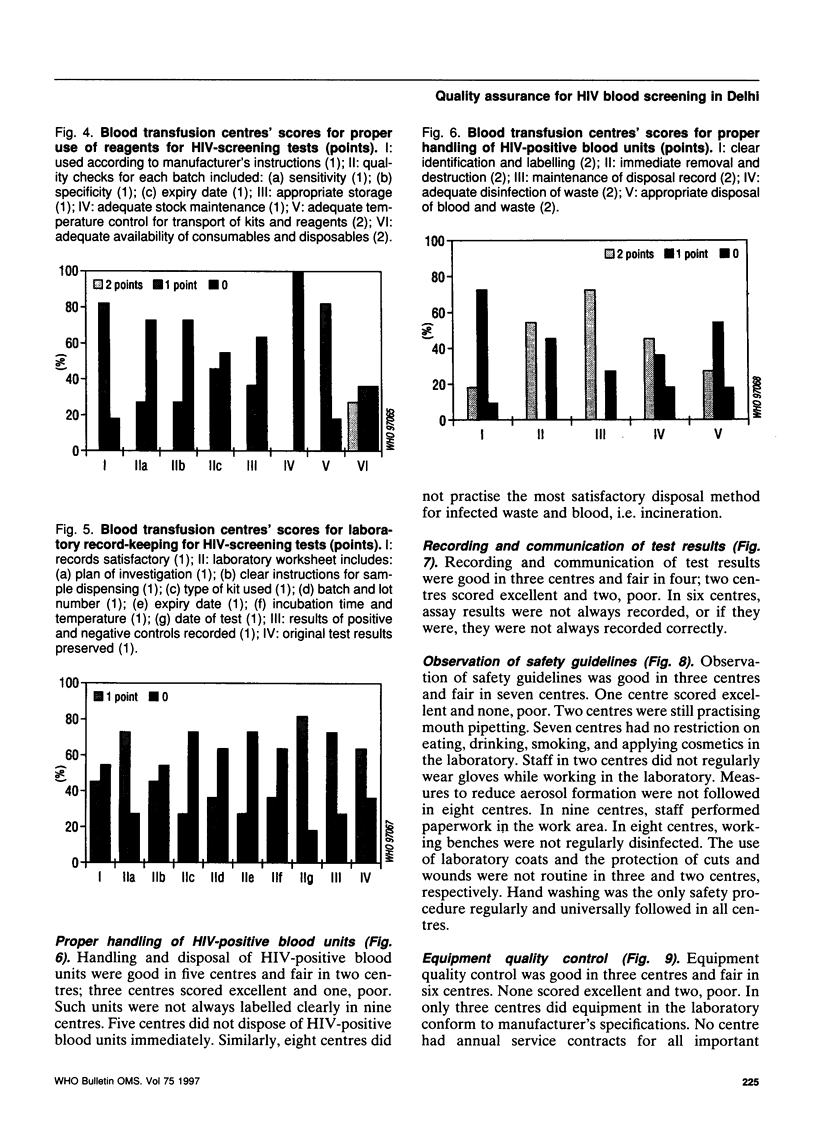
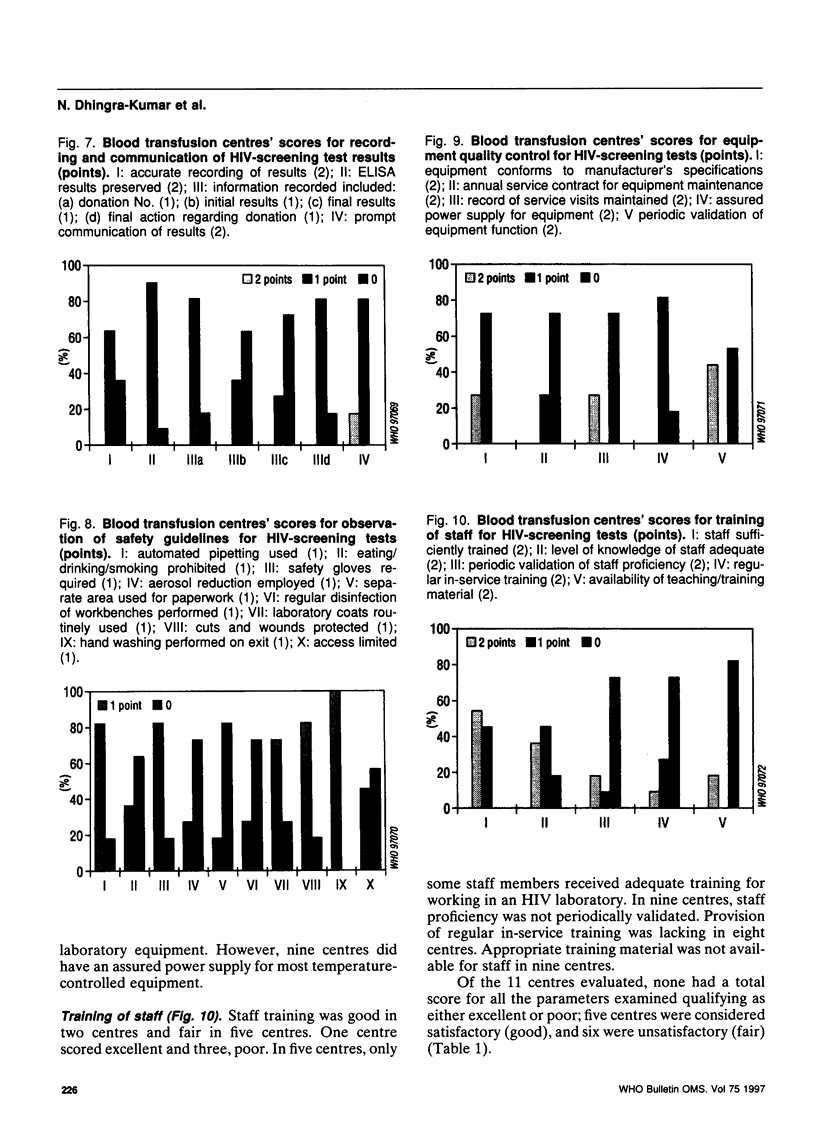
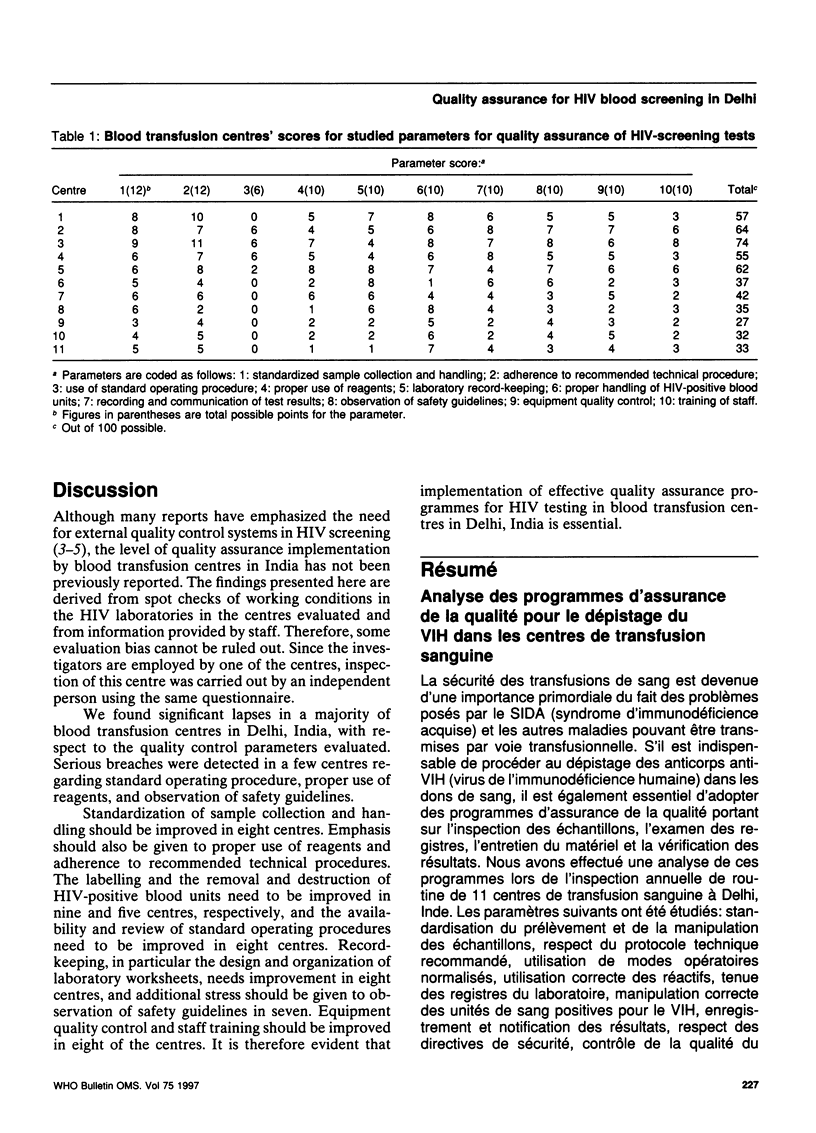
Abstract
The safety of blood transfusion has attained tremendous importance because of the problems posed by acquired immunodeficiency syndrome (AIDS) and other transfusion-transmissible diseases. While performing screening tests for human immunodeficiency virus (HIV) antibodies in donated blood is indispensable, it is also essential to introduce an effective quality assurance programme covering inspection of specimens, review of record-keeping, maintenance of equipment, and verification of results. We carried out an analysis of such quality assurance programmes during routine annual inspection of 11 blood transfusion centres in Delhi, India. The following parameters were studied: standardization of sample collection and handling; adherence to the recommended technical procedure; use of standard operating procedure; proper use of test reagents; laboratory record-keeping; proper handling of HIV-positive blood units; recording and communication of results; observation of safety guidelines; equipment quality control; and training of staff. A pretested closed-type questionnaire with a weighted scoring system was used for evaluation. Performance for each parameter was graded as follows: 76-100%, excellent; 51-75%, good; 26-50%, fair; and < 25%, poor. Centres were categorized according to the total score obtained for all parameters. Overall performance > 50% was considered satisfactory. Of the 11 centres, none was excellent overall, five were considered satisfactory, and six were unsatisfactory.
Full text
PDF