Abstract
An estimated 400,000 deaths occur annually from neonatal tetanus (NT). In 1989 WHO adopted the goal of eliminating NT as a public health problem worldwide. To achieve this, and to control non-neonatal tetanus (non-NT), WHO recommends that newborns be passively protected at birth by the antepartum administration of at least two doses of tetanus toxoid (TT) to their mothers and that all children subsequently receive at least three doses of diphtheria-tetanus-pertussis (DTP) vaccine. For this strategy to be effective, the TT used must be immunogenic. Potential factors that may affect TT immunogenicity need to be evaluated if NT is to be eliminated and if non-NT is to be controlled. Although data are conflicting, concurrent malarial infection may decrease the immune response to TT; however, malarial chemoprophylaxis may enhance the immune response. Malnutrition does not appear to affect immunogenicity; nevertheless, one study suggests that vitamin A deficiency is associated with an impaired immune response. Although it has been postulated that placental transfer of tetanus antibody is impaired in African women, a survey of the published literature suggests that this is not the case. Freezing TT has been shown to decrease its potency, but its impact on immunogenicity needs more evaluation.
Full text
PDF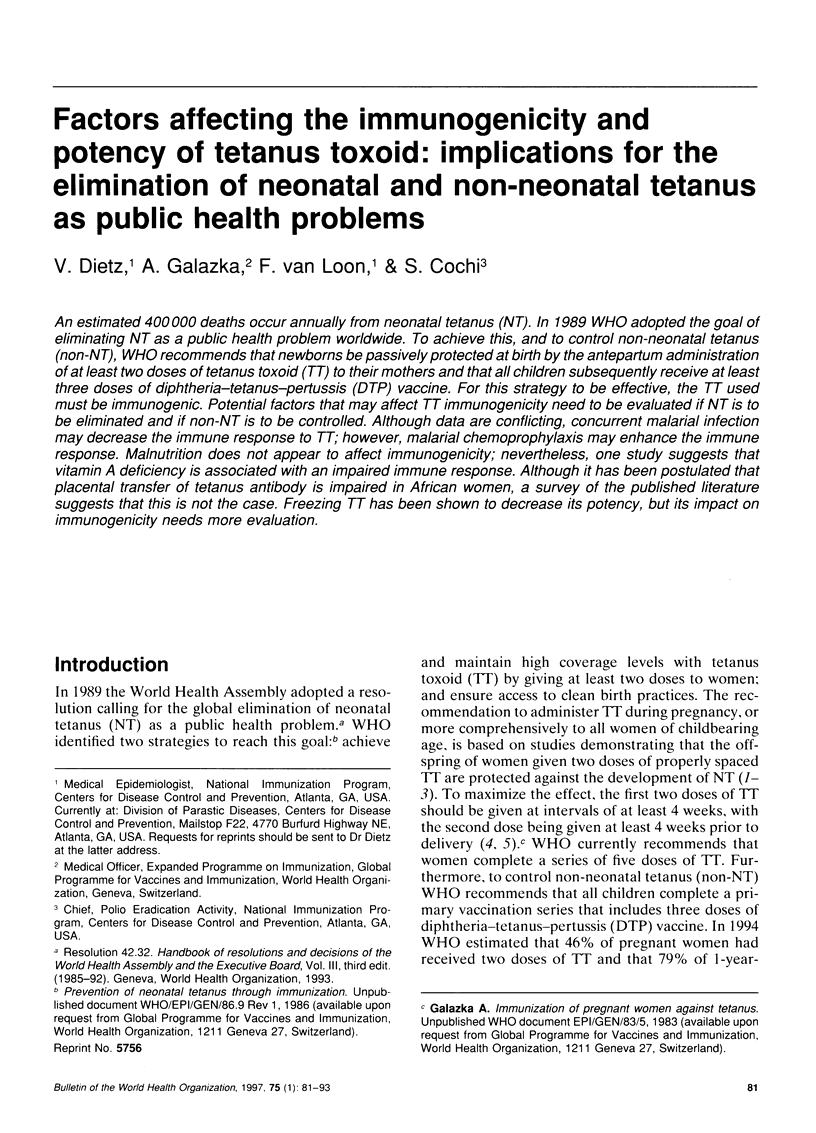









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afonja A. O., Jaiyeola B. O., Tunwashe O. L. Tetanus in Lagos: a review of 228 adult Nigerian patients. J Trop Med Hyg. 1973 Jul;76(7):171–174. [PubMed] [Google Scholar]
- Brabin B. J., Nagel J., Hagenaars A. M., Ruitenberg E., van Tilborgh A. M. The influence of malaria and gestation on the immune response to one and two doses of adsorbed tetanus toxoid in pregnancy. Bull World Health Organ. 1984;62(6):919–930. [PMC free article] [PubMed] [Google Scholar]
- Bradley-Moore A. M., Greenwood B. M., Bradley A. K., Bartlett A., Bidwell D. E., Voller A., Craske J., Kirkwood B. R., Gilles H. M. Malaria chemoprophylaxis with chloroquine in young Nigerian children. II. Effect on the immune response to vaccination. Ann Trop Med Parasitol. 1985 Dec;79(6):563–573. doi: 10.1080/00034983.1985.11811963. [DOI] [PubMed] [Google Scholar]
- Brair M. E., Brabin B. J., Milligan P., Maxwell S., Hart C. A. Reduced transfer of tetanus antibodies with placental malaria. Lancet. 1994 Jan 22;343(8891):208–209. doi: 10.1016/s0140-6736(94)90991-1. [DOI] [PubMed] [Google Scholar]
- Brown K. H., Rajan M. M., Chakraborty J., Aziz K. M. Failure of a large dose of vitamin A to enhance the antibody response to tetanus toxoid in children. Am J Clin Nutr. 1980 Feb;33(2):212–217. doi: 10.1093/ajcn/33.2.212. [DOI] [PubMed] [Google Scholar]
- Chandra R. K., Chandra S., Gupta S. Antibody affinity and immune complexes after immunization with tetanus toxoid in protein-energy malnutrition. Am J Clin Nutr. 1984 Jul;40(1):131–134. doi: 10.1093/ajcn/40.1.131. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Immunocompetence in undernutrition. J Pediatr. 1972 Dec;81(6):1194–1200. doi: 10.1016/s0022-3476(72)80262-2. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Immunocompetence in undernutrition. J Pediatr. 1972 Dec;81(6):1194–1200. doi: 10.1016/s0022-3476(72)80262-2. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Edsall G., Peel M. M., Sinnathuray T. A. Timing of antenatal tetanus immunization for effective protection of the neonate. Bull World Health Organ. 1983;61(1):159–165. [PMC free article] [PubMed] [Google Scholar]
- Corrigall R. J. Asymptomatic malaria parasitaemia and the antibody response to tetanus toxoid vaccination. Trans R Soc Trop Med Hyg. 1988;82(4):540–541. doi: 10.1016/0035-9203(88)90497-x. [DOI] [PubMed] [Google Scholar]
- GITLIN D., KUMATE J., URRUSTI J., MORALES C. THE SELECTIVITY OF THE HUMAN PLACENTA IN THE TRANSFER OF PLASMA PROTEINS FROM MOTHER TO FETUS. J Clin Invest. 1964 Oct;43:1938–1951. doi: 10.1172/JCI105068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geefhuysen J., Rosen E. U., Katz J., Ipp T., Metz J. Impaired cellular immunity in kwashiorkor with improvement after therapy. Br Med J. 1971 Nov 27;4(5786):527–529. doi: 10.1136/bmj.4.5786.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel D., Richard-Lenoble D., Massamba M. B., Picaud A., Moreno J. L., Gendrel C., Baziomo J. M., Francoual C., Blot P. Transfert placentaire des anticorps antitétaniques et protection du nouveau-né. Arch Fr Pediatr. 1990 Dec;47(10):725–729. [PubMed] [Google Scholar]
- Ghosh J. B. Prevention of tetanus neonatorum. Indian Pediatr. 1990 Feb;27(2):210–210. [PubMed] [Google Scholar]
- Ghosh S., Mohan M., Khoche S., Ray S. N., Ray K. DPT immunisation and malnutrition. Indian Pediatr. 1980 Feb;17(2):123–125. [PubMed] [Google Scholar]
- Gilles H. M., Greenwood B. M., Greenwood A. M., Bradley A. K., Blakebrough I., Pugh R. N., Musa B., Shehu U., Tayo M., Jewsbury J. The Malumfashi project--an epidemiological, clinical and laboratory study. Trans R Soc Trop Med Hyg. 1983;77(1):24–31. doi: 10.1016/0035-9203(83)90005-6. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Bradley-Moore A. M., Bryceson A. D., Palit A. Immunosuppression in children with malaria. Lancet. 1972 Jan 22;1(7743):169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- Hood N., Chan M. C., Maxwell S. M., Familusi J. B., Hart C. A. Placental transfer of tetanus toxoid antibodies in Nigerian mothers. Ann Trop Paediatr. 1994;14(3):179–182. doi: 10.1080/02724936.1994.11747714. [DOI] [PubMed] [Google Scholar]
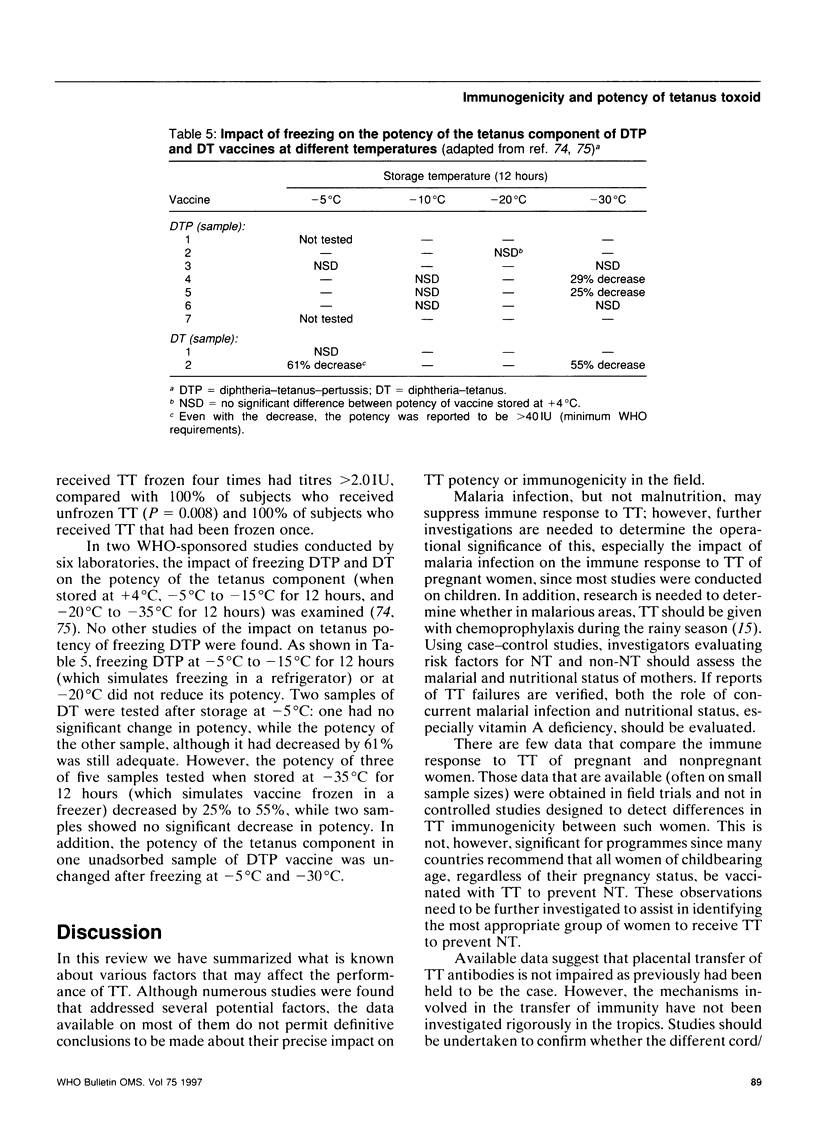
- Kindt H., Milcke L., Engelhardt H., Reber G., Pranter W., Blackkolb F. Stability of DTP vaccine. J Biol Stand. 1974 Jul;2(3):183–187. doi: 10.1016/0092-1157(74)90014-6. [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Pasatiempo A. M., Taylor C. E., Ross A. C. Immunological memory to tetanus toxoid is established and maintained in the vitamin A-depleted rat. FASEB J. 1991 Jul;5(10):2473–2481. doi: 10.1096/fasebj.5.10.2065894. [DOI] [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Mitsuya H., Fujiwara H., Tsuda H. Age-related decline in the in vitro and in vivo syntheses of anti-tetanus toxoid antibody in humans. J Immunol. 1980 Nov;125(5):2347–2352. [PubMed] [Google Scholar]
- Kohler P. F., Farr R. S. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature. 1966 Jun 4;210(5040):1070–1071. doi: 10.1038/2101070a0. [DOI] [PubMed] [Google Scholar]
- Krishnan S., Bhuyan U. N., Talwar G. P., Ramalingaswami V. Effect of vitamin A and protein-calorie undernutrition on immune responses. Immunology. 1974 Sep;27(3):383–392. [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Sahai G., Gupta P., Kumar A. Studies on the stability of tetanus and pertussis components of DPT vaccine on exposure to different temperatures. Indian J Pathol Microbiol. 1982 Jan;25(1):50–54. [PubMed] [Google Scholar]
- Kutty P. M., Mohanram M., Vinodini R. Humoral immune response in vitamin A deficient children. Acta Vitaminol Enzymol. 1981;3(4):231–235. [PubMed] [Google Scholar]
- Logie D. E., McGregor I. A., Rowe D. S., Billewicz W. Z. Plasma immunoglobulin concentrations in mothers and newborn children with special reference to placental malaria: Studies in the Gambia, Nigeria, and Switzerland. Bull World Health Organ. 1973;49(6):547–554. [PMC free article] [PubMed] [Google Scholar]
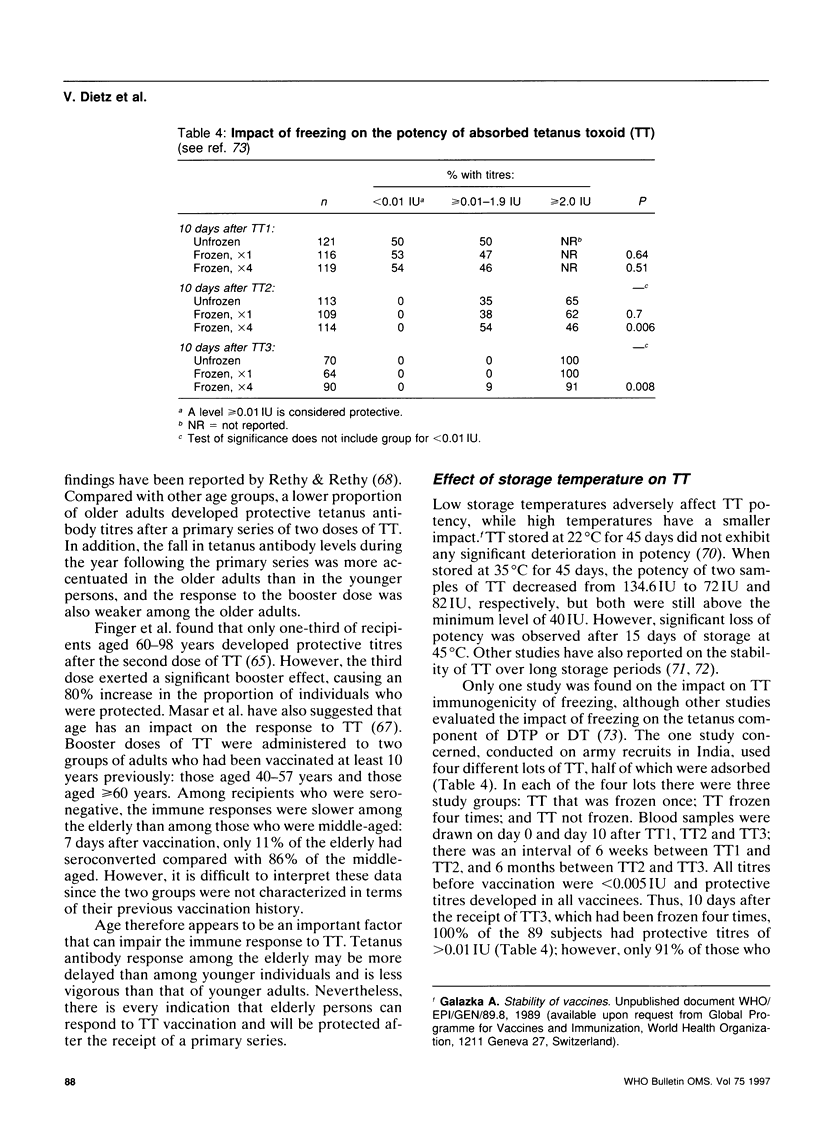
- Menon P. S., Sahai G., Joshi V. B., Murthy R. G., Boparai M. S., Thomas A. K. Field trial on frozen and thawed tetanus toxoid. Indian J Med Res. 1976 Jan;64(1):25–32. [PubMed] [Google Scholar]
- Michaux J. L., Heremans J. F., Hitzig W. H. Immunoglobulin levels in cord-blood serum of negroes and Caucasians. Trop Geogr Med. 1966 Mar;18(1):10–14. [PubMed] [Google Scholar]
- Monjour L., Bourdillon F., Schlumberger M., Fayet M. T., Michon C., Ballet J. J., Gouba E., Gentilini M. Etude de l'immunité humorale et cellulaire après vaccination antitétanique chez l'enfant africain malnutri et paludéen. 1. Etude de la réponse en anticorps antitétaniques. Bull World Health Organ. 1982;60(4):589–596. [PMC free article] [PubMed] [Google Scholar]
- Owa J. A., Makinde O. O. Neonatal tetanus in babies of immunized mothers. J Trop Pediatr. 1990 Jun;36(3):143–144. doi: 10.1093/tropej/36.3.143. [DOI] [PubMed] [Google Scholar]
- Owa J. A., Makinde O. O. Neonatal tetanus in babies of women immunized with tetanus toxoid during pregnancy. Trop Doct. 1990 Oct;20(4):156–157. doi: 10.1177/004947559002000404. [DOI] [PubMed] [Google Scholar]
- Pitcher-Wilmott R. W., Hindocha P., Wood C. B. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980 Aug;41(2):303–308. [PMC free article] [PubMed] [Google Scholar]
- Price P., Bell R. G. The effects of nutritional rehabilitation on antibody production in protein-deficient mice. Immunology. 1976 Dec;31(6):953–960. [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Chen L. C., Chakraborty J., Yunus M., Chowdhury A. I., Sarder A. M., Bhatia S., Curlin G. T. Use of tetanus toxoid for the prevention of neonatal tetanus. 1. Reduction of neonatal mortality by immunization of non-pregnant and pregnant women in rural Bangladesh. Bull World Health Organ. 1982;60(2):261–267. [PMC free article] [PubMed] [Google Scholar]
- Reddy V., Bhaskaram C., Raghuramulu N. Immunological responses in malnourished children. Indian Pediatr. 1977 Apr;14(4):255–258. [PubMed] [Google Scholar]
- Rowe D. S., McGregor I. A., Smith S. J., Hall P., Williams K. Plasma immunoglobulin concentrations in a West African (Gambian) community and in a group of healthy British adults. Clin Exp Immunol. 1968 Jan;3(1):63–79. [PMC free article] [PubMed] [Google Scholar]
- Ruben F. L., Nagel J., Fireman P. Antitoxin responses in the elderly to tetanus-diphtheria (TD) immunization. Am J Epidemiol. 1978 Aug;108(2):145–149. doi: 10.1093/oxfordjournals.aje.a112598. [DOI] [PubMed] [Google Scholar]
- SCHOFIELD F. D., TUCKER V. M., WESTBROOK G. R. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. Br Med J. 1961 Sep 23;2(5255):785–789. doi: 10.1136/bmj.2.5255.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangpetchsong V., Vichaikummart S., Vichitnant A., Podhipak A. Transfer rate of transplacental immunity to tetanus from non-immunized and immunized mothers. Southeast Asian J Trop Med Public Health. 1984 Sep;15(3):275–280. [PubMed] [Google Scholar]
- Semba R. D., Muhilal, Scott A. L., Natadisastra G., Wirasasmita S., Mele L., Ridwan E., West K. P., Jr, Sommer A. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr. 1992 Jan;122(1):101–107. doi: 10.1093/jn/122.1.101. [DOI] [PubMed] [Google Scholar]
- Simonsen O., Schou C., Heron I. Modification of the ELISA for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1987 Apr;15(2):143–157. doi: 10.1016/0092-1157(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Solomonova K., Vizev S. Immunological reactivity of senescent and old people actively immunized with tetanus toxoid. Z Immunitatsforsch Exp Klin Immunol. 1973 Sep;146(1):81–90. [PubMed] [Google Scholar]
- Solomonova K., Vizev S. Secondary response to boostering by purified aluminium-hydroxide-adsorbed tetanus anatoxin in aging and in aged adults. Immunobiology. 1981;158(4):312–319. doi: 10.1016/S0171-2985(81)80002-2. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Hart F. E. The stability of bacterial vaccines at elevated temperatures. Dev Biol Stand. 1978;41:249–253. [PubMed] [Google Scholar]
- Sundar S., Wagle N., Varudkar B. L., Vaidya A., Merchant S. M. Study of anti tetanus IgG and IgM levels in maternal and cord sera by enzyme linked immunosorbent assay (ELISA). Indian J Pediatr. 1990 Nov-Dec;57(6):763–770. doi: 10.1007/BF02722271. [DOI] [PubMed] [Google Scholar]
- Tarzaali A., Viens P., Quevillon M. Inhibition of the immune response to whooping cough and tetanus vaccines by malaria infection, and the effect of pertussis adjuvant. Am J Trop Med Hyg. 1977 May;26(3):520–524. doi: 10.4269/ajtmh.1977.26.520. [DOI] [PubMed] [Google Scholar]
- Zuber P. L., Schierz A., Aréstegui G., Steffen R. Tetanus in Switzerland 1980-1989. Eur J Epidemiol. 1993 Nov;9(6):617–624. doi: 10.1007/BF00211435. [DOI] [PubMed] [Google Scholar]


