Abstract
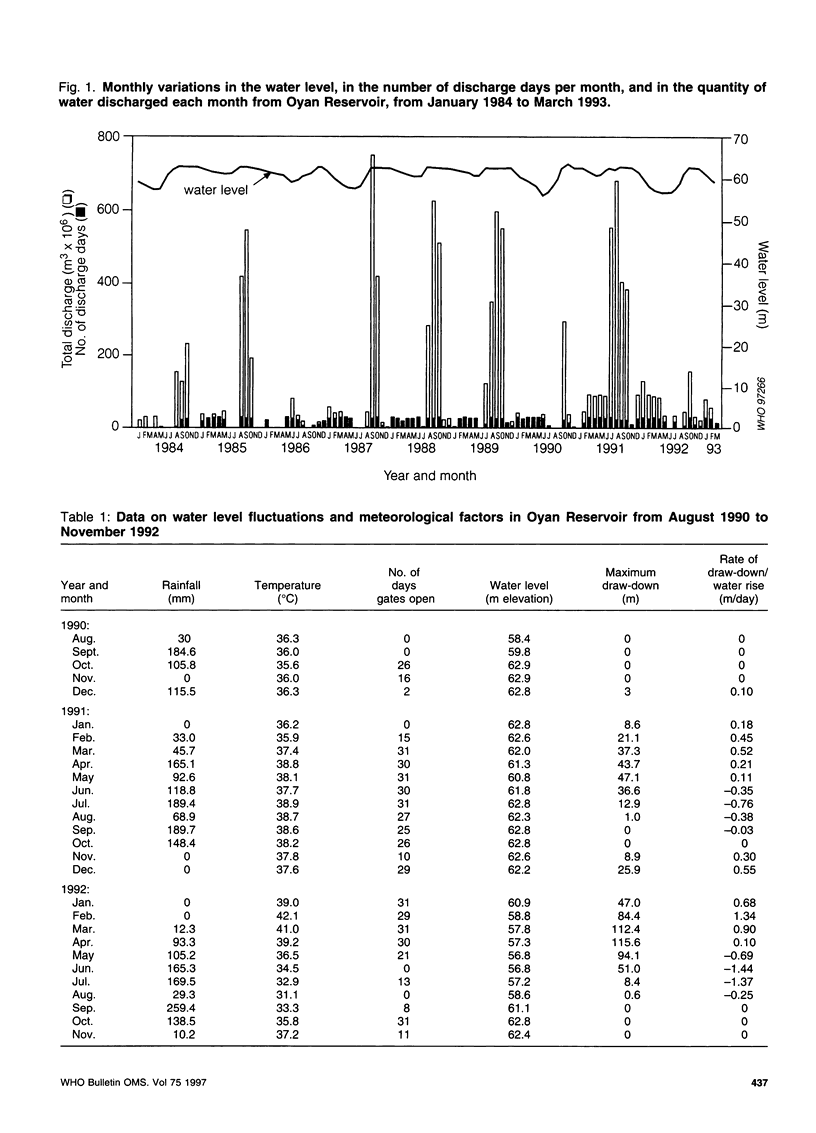
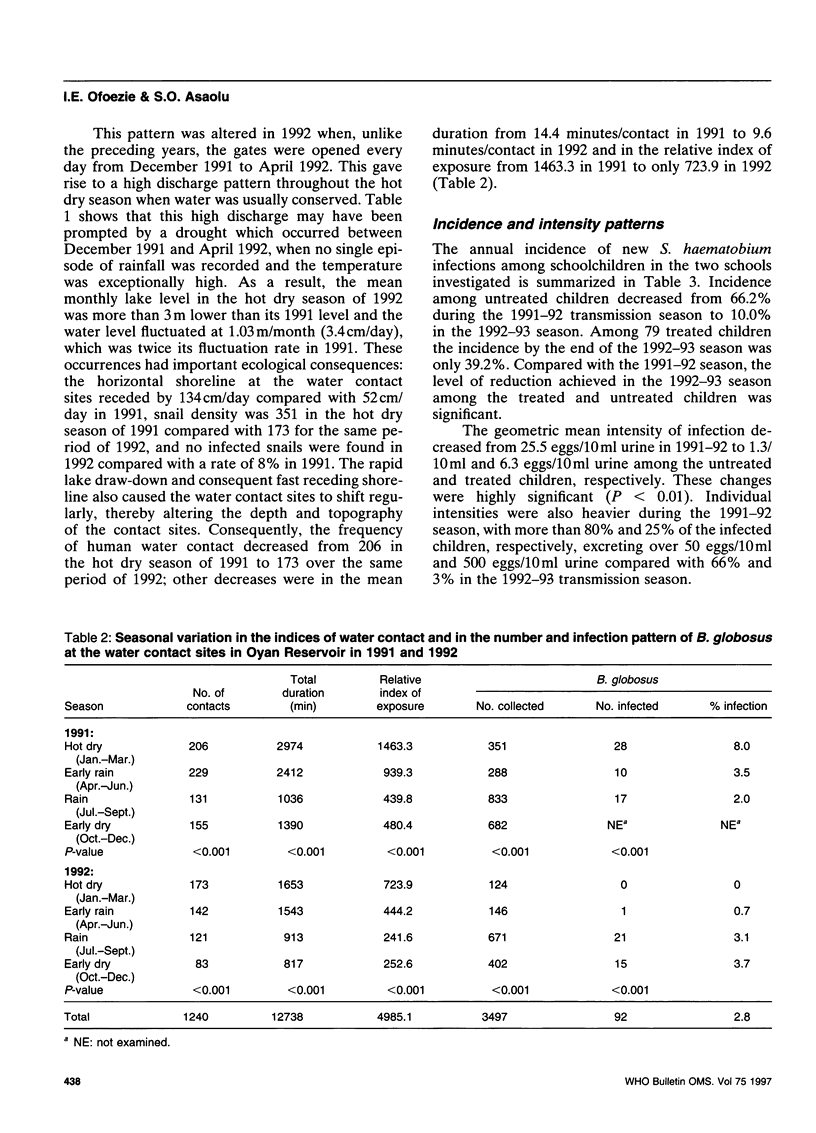
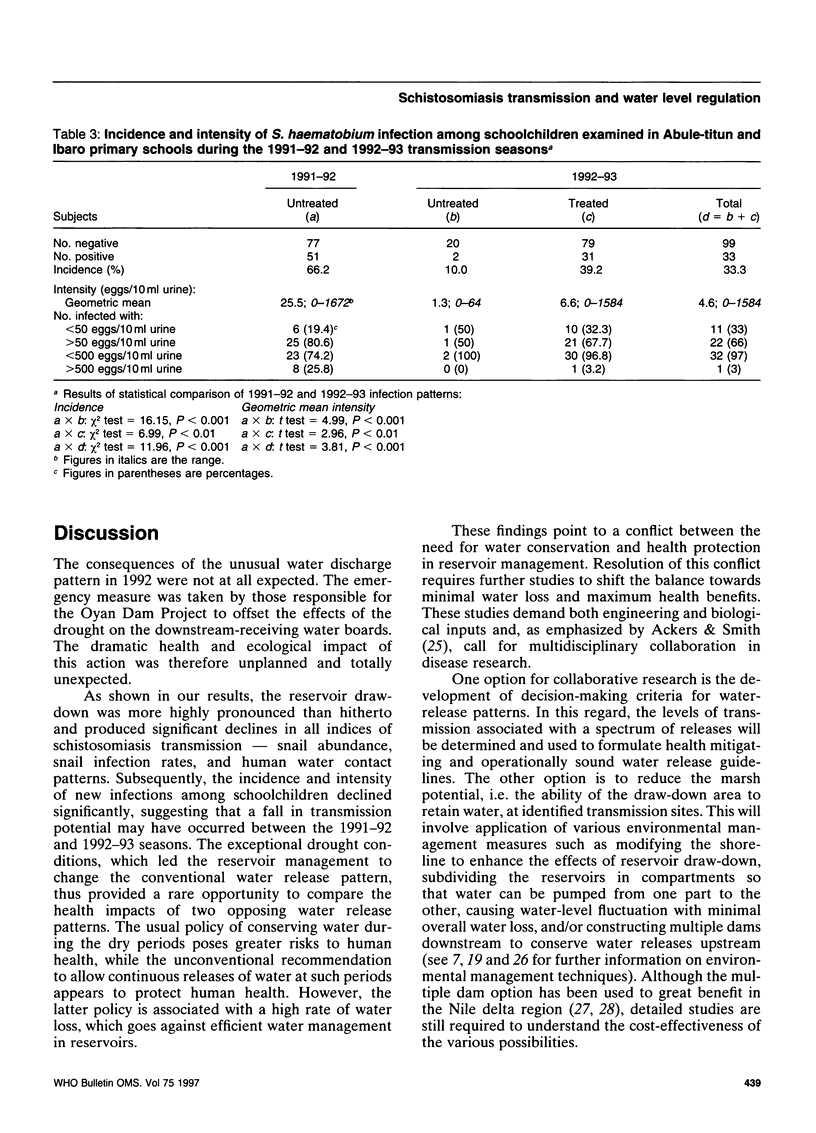
The effect of different water discharge patterns from the artificial Oyan Reservoir in Ogun State, Nigeria, on water level fluctuations and on schistosomiasis transmission was investigated between August 1990 and March 1993 to determine the impact of water level regulation on schistosomiasis transmission and control. The results show that transmission was greatly influenced by the pattern of water discharge during the hot dry season (January-April). A high discharge during this period of no rainfall, high temperatures, and intense sunshine stimulated rapid water level fluctuations and lake draw-down, which led to significant reductions in all indices of schistosomiasis transmission, i.e. snail density, snail infection rates, human water contact patterns, and incidence of infection. Although these results support continuous water discharges from the reservoir during the hot dry season, this may run counter to current water management policies. Further investigation is therefore required to harmonize the potential benefits in this type of discharge pattern with the objectives of efficient water management in artificial reservoirs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Wahab M. F., Strickland G. T., El-Sahly A., El-Kady N., Zakaria S., Ahmed L. Changing pattern of schistosomiasis in Egypt 1935--79. Lancet. 1979 Aug 4;2(8136):242–244. doi: 10.1016/s0140-6736(79)90249-6. [DOI] [PubMed] [Google Scholar]
- Ackers G. L., Smith D. H. Design and management of development projects to avoid health hazards. J Trop Med Hyg. 1988 Jun;91(3):115–129. [PubMed] [Google Scholar]
- Chandiwana S. K. Community water-contact patterns and the transmission of Schistosoma haematobium in the highveld region of Zimbabwe. Soc Sci Med. 1987;25(5):495–505. doi: 10.1016/0277-9536(87)90173-0. [DOI] [PubMed] [Google Scholar]
- Frandsen F., Christensen N. O. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984 Jun;41(2):181–202. [PubMed] [Google Scholar]
- Gryseels B., Stelma F. F., Talla I., van Dam G. J., Polman K., Sow S., Diaw M., Sturrock R. F., Doehring-Schwerdtfeger E., Kardorff R. Epidemiology, immunology and chemotherapy of Schistosoma mansoni infections in a recently exposed community in Senegal. Trop Geogr Med. 1994;46(4 Spec No):209–219. [PubMed] [Google Scholar]
- Jenkins D. W. Bilharzia defeats development. Lakartidningen. 1972 May 31;69(23):2822–2827. [PubMed] [Google Scholar]
- Jewsbury J. M., Imevbore A. M. Small dam health studies. Parasitol Today. 1988 Feb;4(2):57–59. doi: 10.1016/0169-4758(88)90069-5. [DOI] [PubMed] [Google Scholar]
- Jobin W. R. Control of Biomphalaria glabrata in a small reservoir by fluctuation of the water level. Am J Trop Med Hyg. 1970 Nov;19(6):1049–1054. doi: 10.4269/ajtmh.1970.19.1049. [DOI] [PubMed] [Google Scholar]
- Klumpp R. K., Chu K. Y. Ecological studies of Bulinus rohlfsi, the intermediate host of Schistosoma haematobium in the Volta Lake. Bull World Health Organ. 1977;55(6):715–730. [PMC free article] [PubMed] [Google Scholar]
- Michelson M. K., Azziz F. A., Gamil F. M., Wahid A. A., Richards F. O., Juranek D. D., Habib M. A., Spencer H. C. Recent trends in the prevalence and distribution of schistosomiasis in the Nile delta region. Am J Trop Med Hyg. 1993 Jul;49(1):76–87. doi: 10.4269/ajtmh.1993.49.76. [DOI] [PubMed] [Google Scholar]
- Ofoezie I. E., Imevbore A. M., Balogun M. O., Ogunkoya O. O., Asaolu S. O. A study of an outbreak of schistosomiasis in two resettlement villages near Abeokuta, Ogun State, Nigeria. J Helminthol. 1991 Jun;65(2):95–102. doi: 10.1017/s0022149x00010531. [DOI] [PubMed] [Google Scholar]
- Ouma J. H., Wijers D. J., Arap Siongok T. K. The effect of repeated targeted mass treatment on the prevalence of schistosomiasis mansoni and the intensity of infection in Machakos, Kenya. Ann Trop Med Parasitol. 1985 Aug;79(4):431–438. doi: 10.1080/00034983.1985.11811941. [DOI] [PubMed] [Google Scholar]
- Richards F. O., Jr, Hassan F., Cline B. L., El Alamy M. A. An evaluation of quantitative techniques for Schistosoma haematobium eggs in urine preserved with carbolfuchsin. Am J Trop Med Hyg. 1984 Sep;33(5):857–861. doi: 10.4269/ajtmh.1984.33.857. [DOI] [PubMed] [Google Scholar]
- Waddy B. B. Research into the health problems of manmade lakes, with special reference to Africa. Trans R Soc Trop Med Hyg. 1975;69(1):39–50. doi: 10.1016/0035-9203(75)90009-7. [DOI] [PubMed] [Google Scholar]
- Wegner D. H. The profile of the trematodicidal compound praziquantel. Arzneimittelforschung. 1984;34(9B):1132–1136. [PubMed] [Google Scholar]
- Wilkins H. A. Reinfection after treatment of schistosome infections. Parasitol Today. 1989 Mar;5(3):83–88. doi: 10.1016/0169-4758(89)90008-2. [DOI] [PubMed] [Google Scholar]


