Abstract
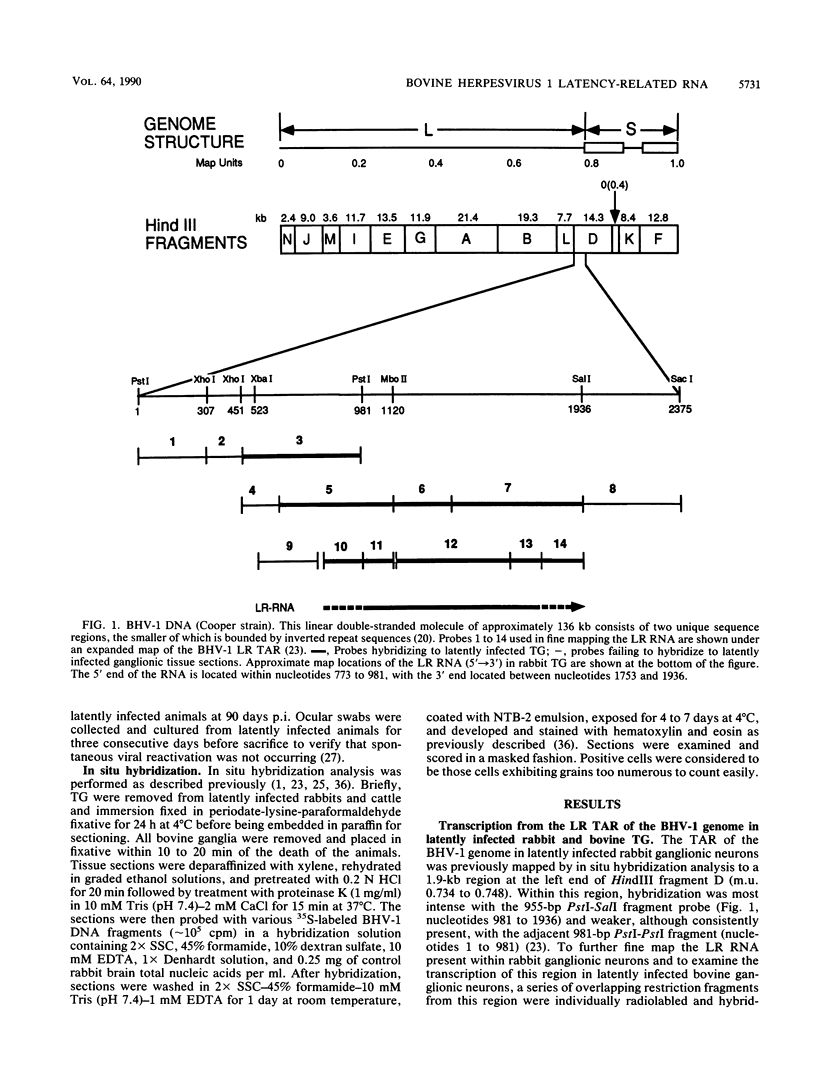
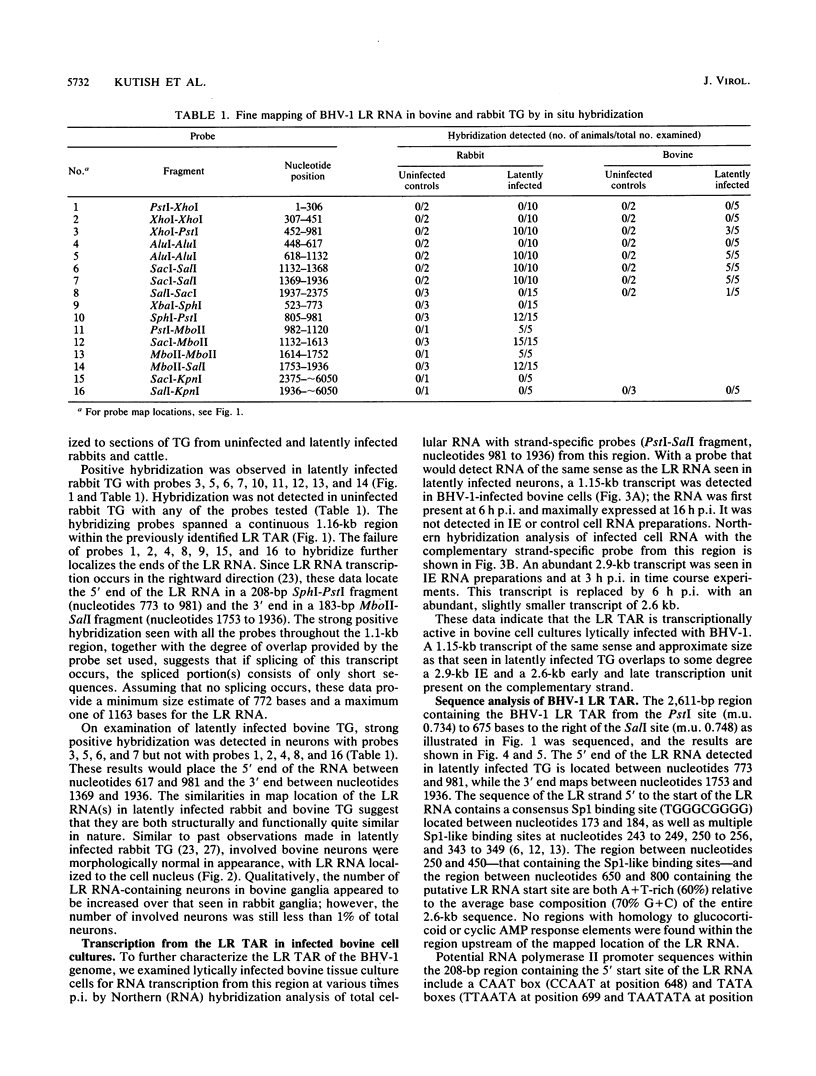
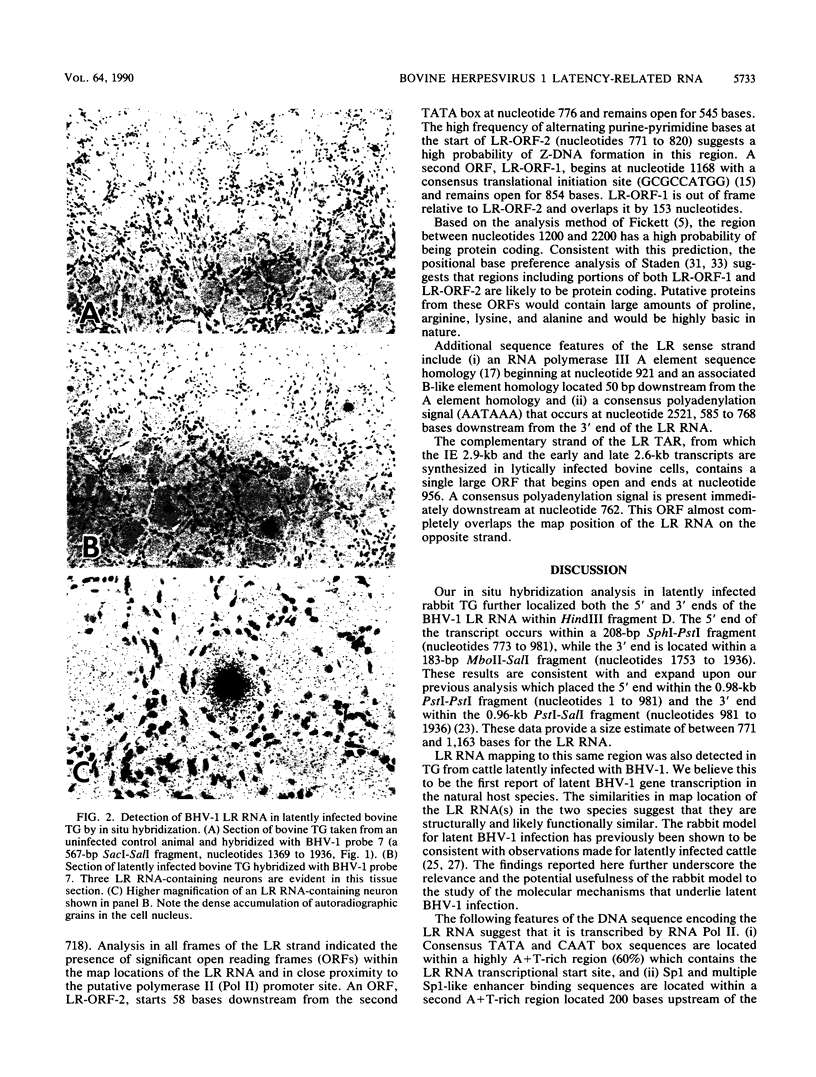
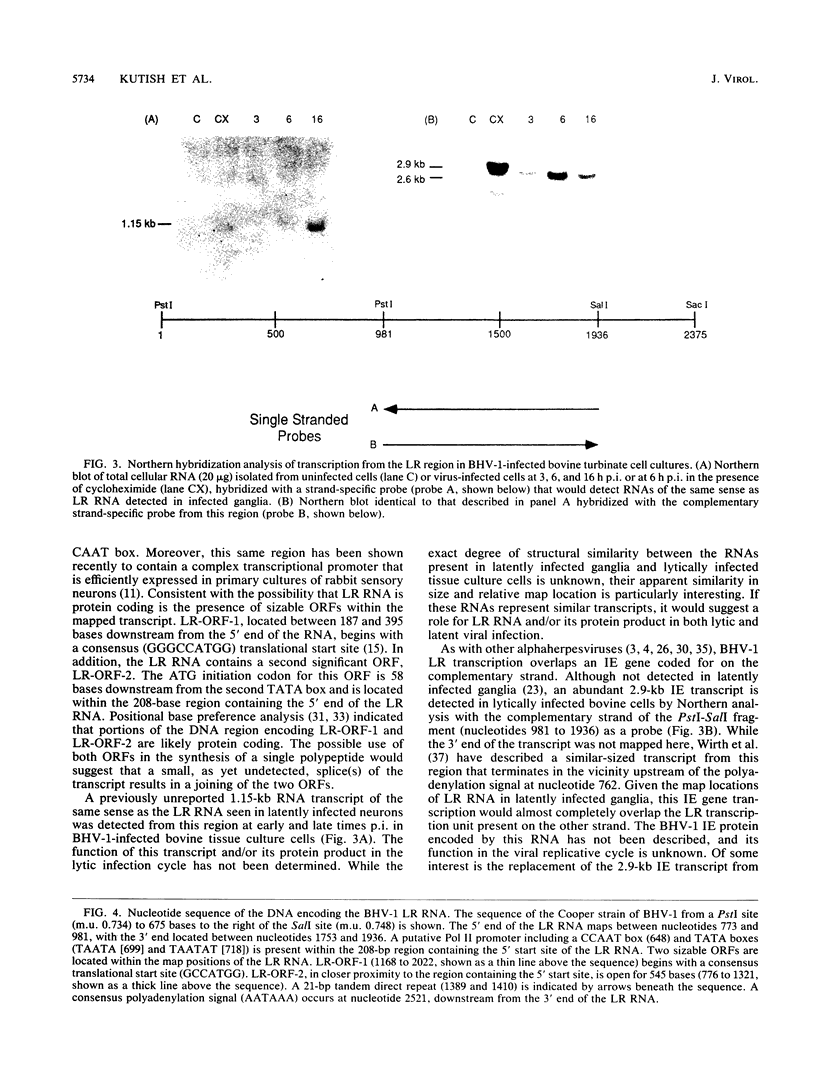
Approximate 5' and 3' ends of the bovine herpesvirus 1 (BHV-1) latency-related RNA (LR RNA) were mapped in rabbit trigeminal ganglia (TG) by in situ hybridization. The data provide a size estimate of 0.77 to 1.16 kb for the LR RNA. An LR RNA mapping to a similar location was also detected in TG of cattle latently infected with BHV-1. The BHV-1 LR region is transcriptionally active in bovine cell cultures lytically infected with BHV-1. A 1.15-kb transcript, present at early and late times postinfection, of the same sense and approximate size that seen in latently infected TG overlaps a 2.9-kb immediate-early and a 2.6-kb early and late transcription unit present on the complementary strand. Sequence analysis of the LR RNA sense strand indicates the presence of a potential polymerase II promoter in close proximity to the 5' terminus of the LR RNA and two open reading frames within its map positions. The complementary strand contains the 3' portion of a large open reading frame that almost completely overlaps the map position of the LR RNA present on the opposite strand.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh J. A., Middendorf L. R., Grone D. L., Ruth J. L. Continuous, on-line DNA sequencing using oligodeoxynucleotide primers with multiple fluorophores. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5610–5614. doi: 10.1073/pnas.85.15.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. K. Detection of pseudorabies virus transcripts in trigeminal ganglia of latently infected swine. J Virol. 1989 Jul;63(7):2908–2913. doi: 10.1128/jvi.63.7.2908-2913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. K. Fine mapping of the immediate-early gene of the Indiana-Funkhauser strain of pseudorabies virus. J Virol. 1988 Dec;62(12):4763–4766. doi: 10.1128/jvi.62.12.4763-4766.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Homan E. J., Easterday B. C. Experimental latent and recrudescent bovine herpesvirus-1 infections in calves. Am J Vet Res. 1983 Feb;44(2):309–313. [PubMed] [Google Scholar]
- Homan E. J., Easterday B. C. Isolation of bovine herpesvirus-1 from trigeminal ganglia of clinically normal cattle. Am J Vet Res. 1980 Aug;41(8):1212–1213. [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Jones C., Delhon G., Bratanich A., Kutish G., Rock D. Analysis of the transcriptional promoter which regulates the latency-related transcript of bovine herpesvirus 1. J Virol. 1990 Mar;64(3):1164–1170. doi: 10.1128/jvi.64.3.1164-1170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. E., Good P. J., VanOort H. J., Campbell A. R., Reed D. E. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (Cooper strain). J Virol. 1983 Jul;47(1):259–264. doi: 10.1128/jvi.47.1.259-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Inui S., Namba K., Shimizu Y. Neural changes in recurrent infection of infectious bovine rhinotracheitis virus in calves treated with dexamethasone. Am J Vet Res. 1978 Sep;39(9):1399–1403. [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Hagemoser W. A., Osorio F. A., McAllister H. A. Transcription from the pseudorabies virus genome during latent infection. Brief report. Arch Virol. 1988;98(1-2):99–106. doi: 10.1007/BF01321010. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Hagemoser W. A., Osorio F. A., Reed D. E. Detection of bovine herpesvirus type 1 RNA in trigeminal ganglia of latently infected rabbits by in situ hybridization. J Gen Virol. 1986 Nov;67(Pt 11):2515–2520. doi: 10.1099/0022-1317-67-11-2515. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Reed D. E. Persistent infection with bovine herpesvirus type 1: rabbit model. Infect Immun. 1982 Jan;35(1):371–373. doi: 10.1128/iai.35.1.371-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffy B. E., Davies D. H. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc Soc Exp Biol Med. 1972 Jul;140(3):974–976. doi: 10.3181/00379727-140-36592. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Wirth U. V., Gunkel K., Engels M., Schwyzer M. Spatial and temporal distribution of bovine herpesvirus 1 transcripts. J Virol. 1989 Nov;63(11):4882–4889. doi: 10.1128/jvi.63.11.4882-4889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]