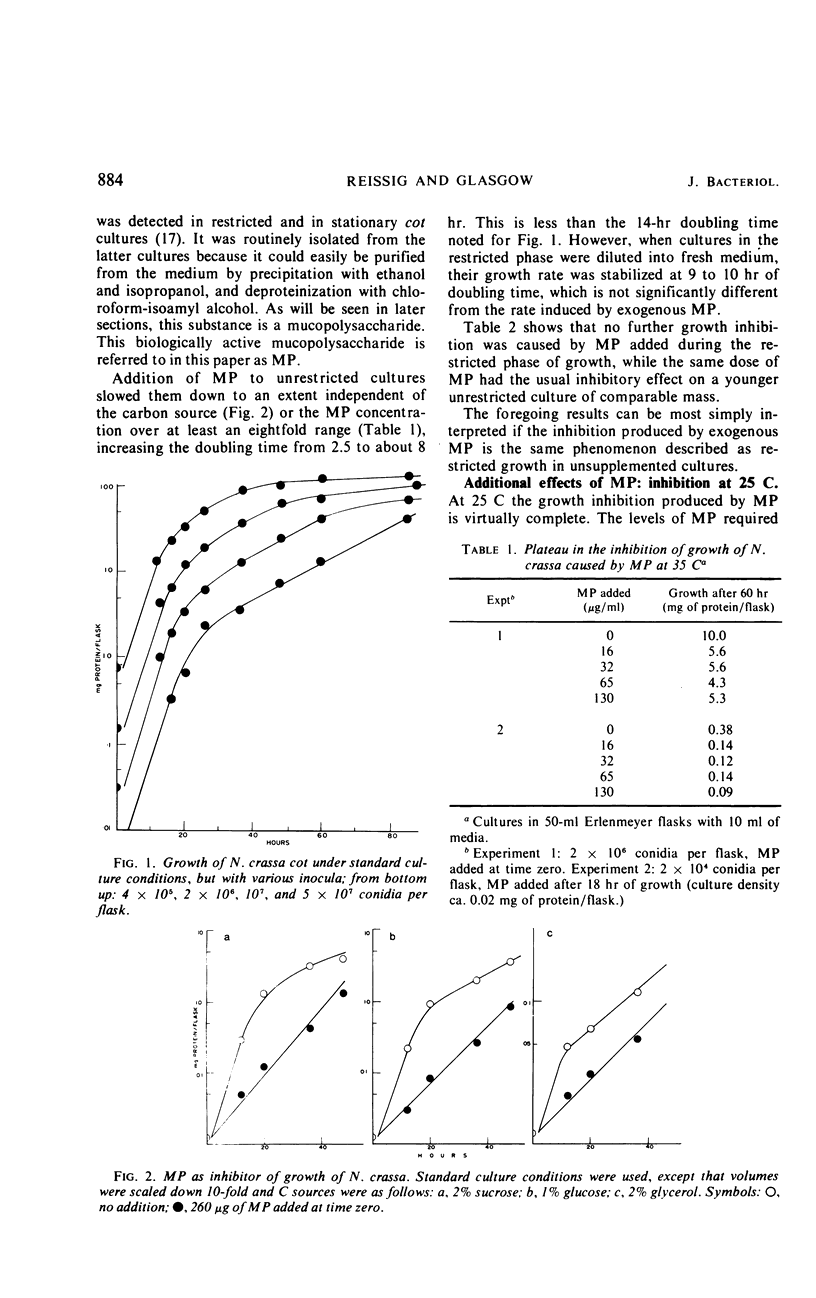
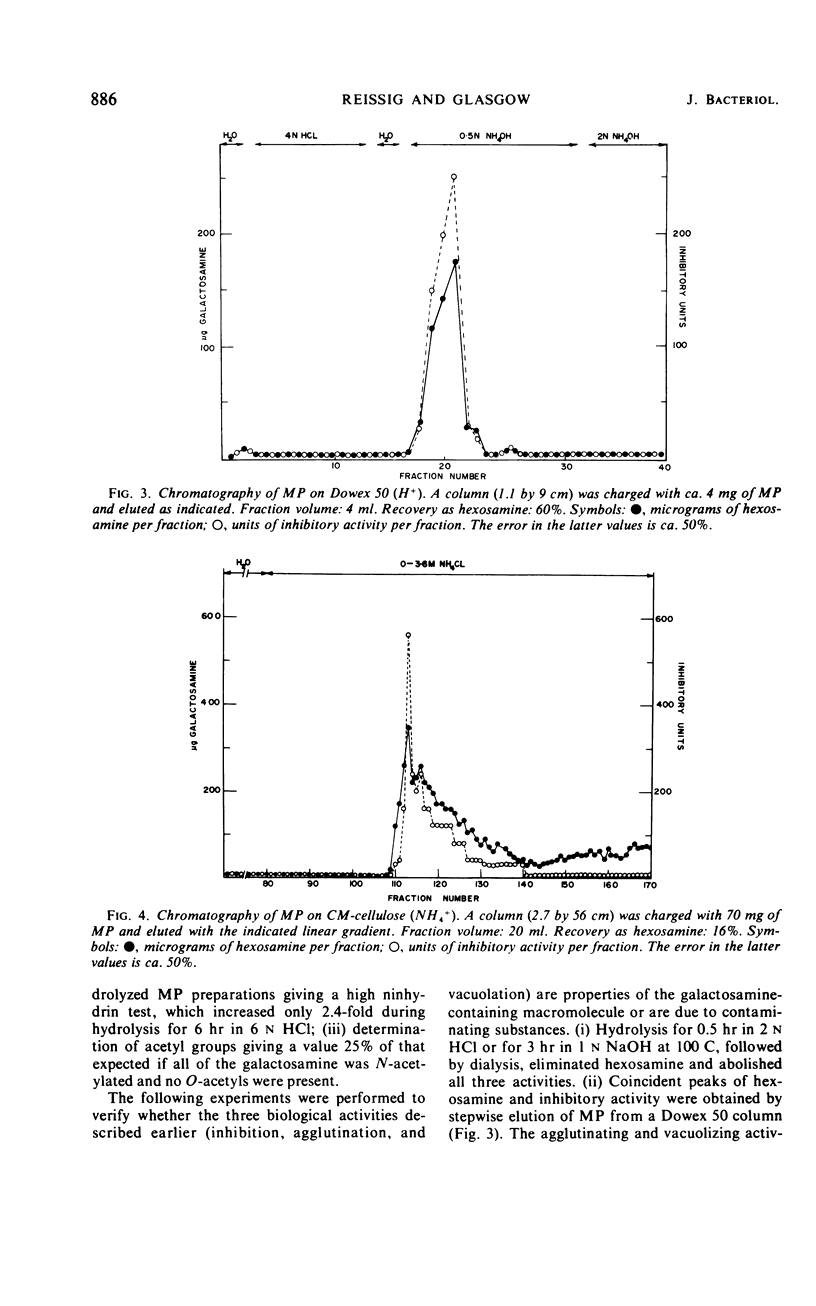
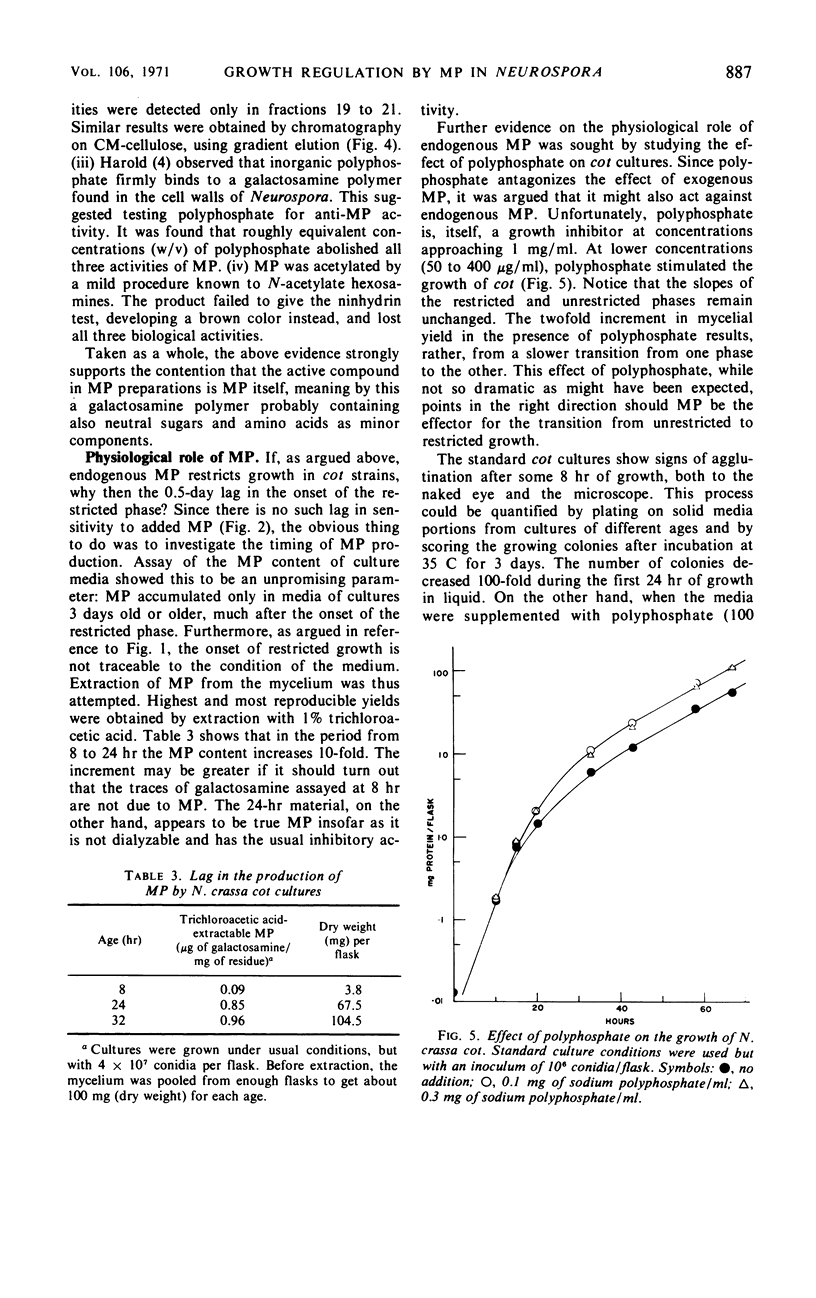
Abstract
Neurospora produces a mucopolysaccharide (called MP) which inhibits its growth, causes vacuolation and agglutination of its cells, and precipitates its purified membrane protein. Cultures of a colonial strain display a phase of slow growth; the induction of this phase is traced to the production of MP by the mold. Stationary-phase cultures of wild type also produce MP. MP is a polymer of galactosamine, its amino groups only partially acetylated, probably containing other minor components. MP molecular weight is approximately 106. Complete acetylation abolishes the biological activities of MP. It is suggested that the regulatory effect of MP is mediated by its interaction with the protoplasmic membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Changeux J. P., Thiéry J. On the mode of action of colicins: a model of regulation at the membrane level. J Theor Biol. 1967 Nov;17(2):315–318. doi: 10.1016/0022-5193(67)90175-0. [DOI] [PubMed] [Google Scholar]
- DISTLER J. J., ROSEMAN S. Galactosamine polymers produced by Aspergillus parasiticus. J Biol Chem. 1960 Sep;235:2538–2541. [PubMed] [Google Scholar]
- HAROLD F. M. Binding of inorganic polyphosphate to the cell wall of Neurospora crassa. Biochim Biophys Acta. 1962 Feb 12;57:59–66. doi: 10.1016/0006-3002(62)91077-6. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966 Dec;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Ludowieg J., Benmaman J. D. Colorimetric differentiation of hexosamines. Anal Biochem. 1967 Apr;19(1):80–88. doi: 10.1016/0003-2697(67)90136-4. [DOI] [PubMed] [Google Scholar]
- Luria S. E. Phage, colicins, and macroregulatory phenomena. Science. 1970 Jun 5;168(3936):1166–1170. doi: 10.1126/science.168.3936.1166. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Localization of structural polymers in the cell wall of Neurospora crassa. J Cell Biol. 1967 Nov;35(2):295–302. doi: 10.1083/jcb.35.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K. A PARTIAL MAP OF LINKAGE GROUP D IN NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1954 Jun;40(6):436–440. doi: 10.1073/pnas.40.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Terenzi H. F., Reissig J. L. Modifiers of the cot gene in Neurospora: the gulliver mutants. Genetics. 1967 Jun;56(2):321–329. doi: 10.1093/genetics/56.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F. Cellular membranes and tumor behavior: a new hypothesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):868–874. doi: 10.1073/pnas.61.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]



