Abstract
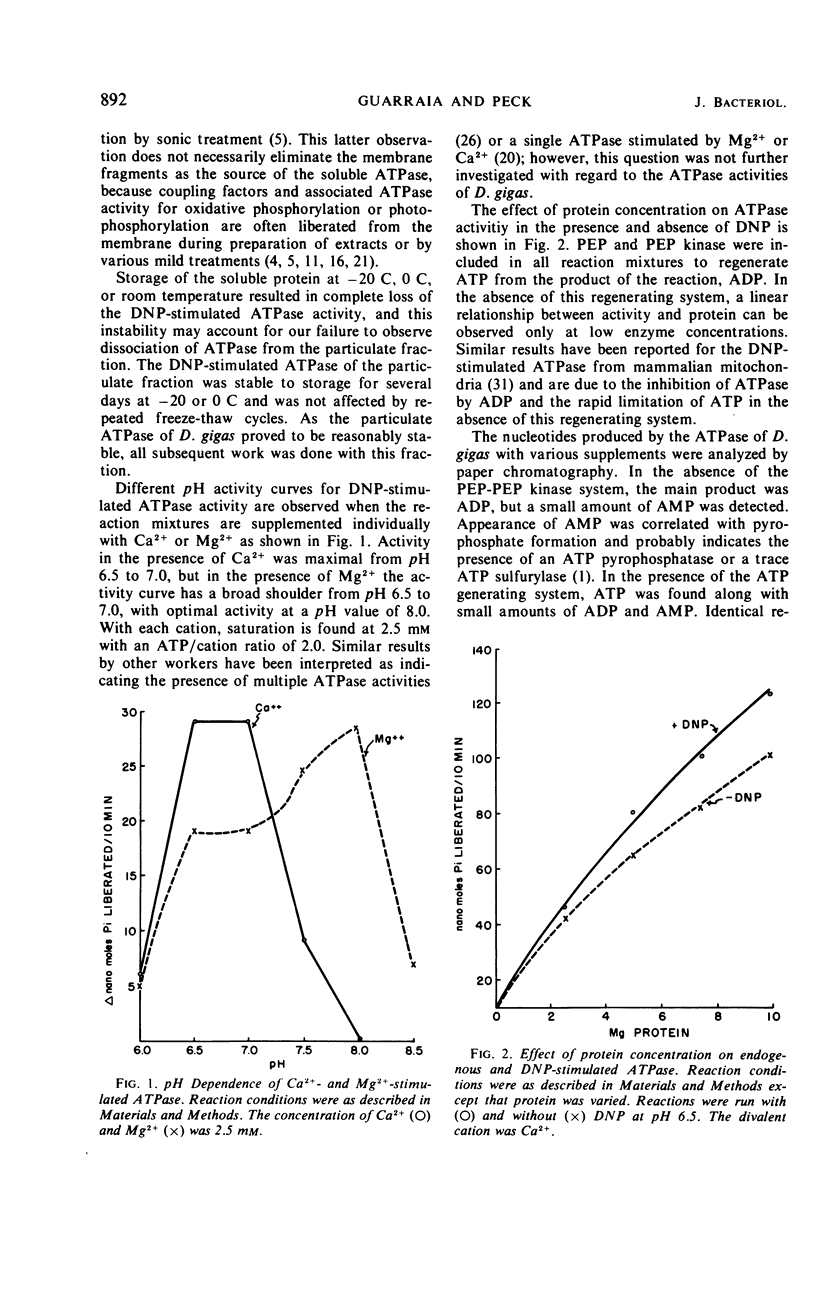
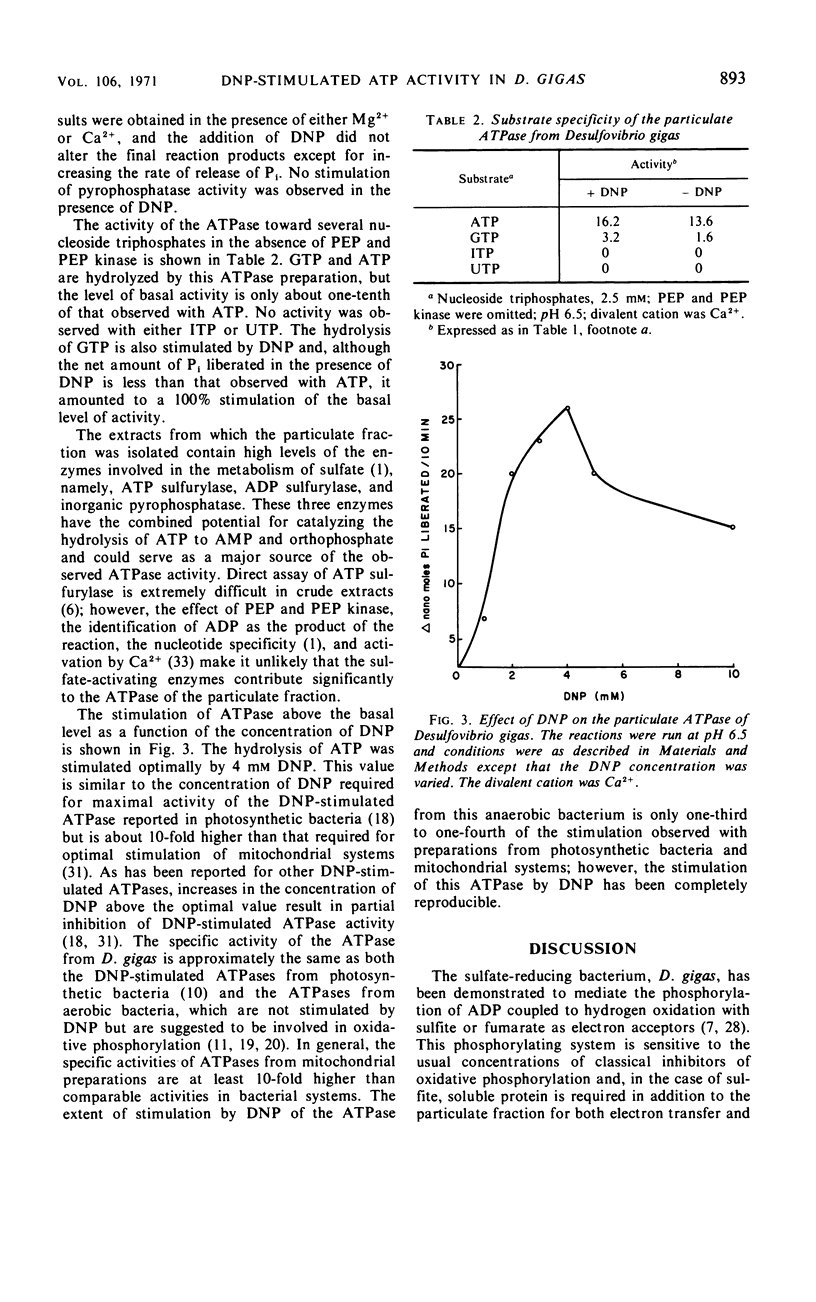
A dinitrophenol (DNP)-stimulated adenosine triphosphatase (ATPase) has been found in both the soluble and particulate fractions of the anaerobic sulfate-reducing bacterium, Desulfovibrio gigas. As the soluble ATPase was labile to storage, only the particulate enzyme was studied in detail. It was optimally stimulated by DNP at 4 mm, and activity was insensitive to inhibition by ouabain. The ATPase was stimulated by both Ca2+ and Mg2+, but the magnitude of the stimulation was dependent upon pH. In the presence of Ca2+ the optimum pH was 6.5, whereas, in the presence of Mg2+ the pH optimum was 8.0. However, under optimal conditions the activity was the same with either Mg2+ or Ca2+. Both adenosine triphosphate and guanosine triphosphate were hydrolyzed, but activity toward guanosine triphosphate was only one-tenth that observed with adenosine triphosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi J. M., Campbell L. L. STUDIES ON THERMOPHILIC SULFATE-REDUCING BACTERIA III. : Adenosine Triphosphate-sulfurylase of Clostridium nigrificans and Desulfovibrio desulfuricans. J Bacteriol. 1962 Dec;84(6):1194–1201. doi: 10.1128/jb.84.6.1194-1201.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amons R., van den Bergh S. G., Slater E. C. The effect of monovalent cations on the dinitrophenol-induced ATPase of rat-liver mitochondria. Biochim Biophys Acta. 1968 Oct 1;162(3):452–454. doi: 10.1016/0005-2728(68)90131-x. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L., HAWKINS N. M. Studies on sodium-potassium-activated adenosinetriphosphatase. IV. Correlation with cation transport sensitive to cardiac glycosides. Arch Biochem Biophys. 1962 Sep;98:413–419. doi: 10.1016/0003-9861(62)90206-0. [DOI] [PubMed] [Google Scholar]
- BOSE S. K., GEST H. PROPERTIES OF ADENOSINE TRIPHOSPHATASE IN A PHOTOSYNTHETIC BACTERIUM. Biochim Biophys Acta. 1965 Jan;96:159–162. doi: 10.1016/0005-2787(65)90621-0. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. I. Role of soluble factors. J Biol Chem. 1959 Feb;234(2):398–404. [PubMed] [Google Scholar]
- Baccarini-Melandri A., Gest H., Pietro A. S. A coupling factor in bacterial photophosphorylation. J Biol Chem. 1970 Mar 10;245(5):1224–1226. [PubMed] [Google Scholar]
- Barton L. L., Le Gall J., Peck H. D., Jr Phosphorylation coupled to oxidation of hydrogen with fumarate in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1036–1042. doi: 10.1016/0006-291x(70)90189-0. [DOI] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. Interchangeability of coupling factors from bacterial and mammalian origin. Biochem Biophys Res Commun. 1970 Feb 6;38(3):478–483. doi: 10.1016/0006-291x(70)90738-2. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Oxidative phosphorylation in Escherichia coli. Can J Biochem. 1968 Jul;46(7):631–641. doi: 10.1139/o68-099. [DOI] [PubMed] [Google Scholar]
- Farron F., Racker E. Studies on the mechanism of the conversion of coupling factor 1 from chloroplasts to an active adenosine triphosphatase. Biochemistry. 1970 Sep 15;9(19):3829–3836. doi: 10.1021/bi00821a024. [DOI] [PubMed] [Google Scholar]
- Gross R., Coles N. W. Adenosine triphosphatase in isolated membranes of Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1322–1326. doi: 10.1128/jb.95.4.1322-1326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., NISHIKAWA K., KATSUMATA M., YAMASHITA J. POSSIBLE PARTIAL REACTIONS OF THE PHOTOPHOSPHORYLATION PROCESS IN CHROMATOPHORES FROM RHODOSPIRILLUM RUBRUM. Biochim Biophys Acta. 1965 Mar 29;94:371–382. doi: 10.1016/0926-6585(65)90045-2. [DOI] [PubMed] [Google Scholar]
- Higashi T., Bogin E., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XLII. The effect of coupling factors on urea-treated particles from M. phlei. Arch Biochem Biophys. 1970 Feb;136(2):331–336. doi: 10.1016/0003-9861(70)90203-1. [DOI] [PubMed] [Google Scholar]
- Horiuti Y., Nishikawa K., Horio T. Oxidation-reduction potential-dependent adenosine triphosphatase activity of chromatophores from Rhodospirillum rubrum. J Biochem. 1968 Nov;64(5):577–587. doi: 10.1093/oxfordjournals.jbchem.a128934. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. I. Preparation and properties of phosphorylating membrane fragments. Biochim Biophys Acta. 1967;143(3):462–476. doi: 10.1016/0005-2728(67)90052-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Properties of an oxidative phosphorylation system reconstituted from coupling factors in Micrococcus lysodeikticus. J Biochem. 1970 Feb;67(2):297–312. doi: 10.1093/oxfordjournals.jbchem.a129253. [DOI] [PubMed] [Google Scholar]
- LEGALL J. A NEW SPECIES OF DESULFOVIBRIO. J Bacteriol. 1963 Nov;86:1120–1120. doi: 10.1128/jb.86.5.1120-1120.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- LEVIN R., BRAUER R. W. The biuret reaction for the determination of proteins; an improved reagent and its application. J Lab Clin Med. 1951 Sep;38(3):474–480. [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER L. Regulation of respiration in heart sarcosomes. Exp Cell Res. 1958 Dec;15(3):551–559. doi: 10.1016/0014-4827(58)90103-4. [DOI] [PubMed] [Google Scholar]
- PENEFSKY H. S., PULLMAN M. E., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. II. Participation of a soluble adenosine tolphosphatase in oxidative phosphorylation. J Biol Chem. 1960 Nov;235:3330–3336. [PubMed] [Google Scholar]
- PINCHOT G. B. Phosphorylation coupled to electron transport in cell-free extracts of Alcaligenes faecalis. J Biol Chem. 1953 Nov;205(1):65–74. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Peck H. D., Jr Phosphorylation coupled with electron transfer in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Jan 4;22(1):112–118. doi: 10.1016/0006-291x(66)90611-5. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- Schatz G., Penefsky H. S., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XIV. J Biol Chem. 1967 May 25;242(10):2552–2560. [PubMed] [Google Scholar]
- TISSIERES A., SLATER E. C. Respiratory chain phosphorylation in extracts of Azotobacter vinelandii. Nature. 1955 Oct 15;176(4485):736–737. doi: 10.1038/176736a0. [DOI] [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]


