Abstract
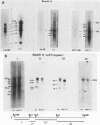
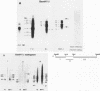
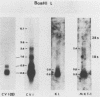
Marek's disease herpesvirus (MDV) induces tumors in chickens, and lymphoblastoid cells derived from such tumors contain the viral genome in a latent state and do not produce infectious virus. Poly(A)+ RNAs extracted from MDV-induced kidney lymphoma cells and from MKT-1 cells, a nonproducing lymphoblastoid cell line derived from a tumor induced by MDV, were electrophoretically separated under denaturing conditions, transferred to a solid substrate, and hybridized with labeled DNA probes representing approximately 95% of the virus genome. These analyses revealed 29 viral RNA transcripts in the kidney lymphoma and 32 viral RNAs in MKT-1 cells. In both instances, the transcripts hybridized to a restricted region comprising approximately 20% of the MDV genome located in the repeats flanking the long and short unique sequences and in the adjacent unique sequences. The sizes of the transcripts derived from kidney lymphoma and MKT-1 cells and the distributions of the homologous regions in the viral genome were very similar. The most abundant transcripts were homologous to the BamHI I2 fragment. The results suggest that expression of the MDV genome in MKT-1 cells closely reflects the expression of the MDV genome in tumors. The gene expression over extended regions of the genome is in concordance with that observed in latent gammaherpesviruses rather than that observed in latent alphaherpesviruses.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Lancz G., Tanaka A., Nonoyama M. Loss of Marek's disease virus tumorigenicity is associated with truncation of RNAs transcribed within BamHI-H. J Virol. 1989 Oct;63(10):4129–4135. doi: 10.1128/jvi.63.10.4129-4135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian J., Kaschka-Dierich C., Berthelot N., Sheldrick P. Inverted repeat nucleotide sequences in the genomes of Marek disease virus and the herpesvirus of the turkey. Proc Natl Acad Sci U S A. 1982 Jan;79(2):555–558. doi: 10.1073/pnas.79.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka A., Schierman L. W., Witter R. L., Nonoyama M. The structure of Marek disease virus DNA: the presence of unique expansion in nonpathogenic viral DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(3):751–754. doi: 10.1073/pnas.82.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Nonoyama M. Replication of the resident Marek's Disease virus genome in synchronized nonproducer MKT-1 cells. J Virol. 1980 Feb;33(2):912–914. doi: 10.1128/jvi.33.2.912-914.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. F., Kieff E. D., Bachenheimer S. L., Roizman B., Spear P. G., Burmester B. R., Nazerian K. Size and composition of Marek's disease virus deoxyribonucleic acid. J Virol. 1971 Mar;7(3):289–294. doi: 10.1128/jvi.7.3.289-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki Y., Bos T. J., Davis C., Starbuck M., Vogt P. K. Avian sarcoma virus 17 carries the jun oncogene. Proc Natl Acad Sci U S A. 1987 May;84(9):2848–2852. doi: 10.1073/pnas.84.9.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maotani K., Kanamori A., Ikuta K., Ueda S., Kato S., Hirai K. Amplification of a tandem direct repeat within inverted repeats of Marek's disease virus DNA during serial in vitro passage. J Virol. 1986 May;58(2):657–660. doi: 10.1128/jvi.58.2.657-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maray T., Malkinson M., Becker Y. RNA transcripts of Marek's disease virus (MDV) serotype-1 in infected and transformed cells. Virus Genes. 1988 Oct;2(1):49–68. doi: 10.1007/BF00569736. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat K. A., Buckmaster A., Ross L. J. Partial transcription map of Marek's disease herpesvirus in lytically infected cells and lymphoblastoid cell lines. Int J Cancer. 1989 Jul 15;44(1):101–109. doi: 10.1002/ijc.2910440119. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Smith M., Nonoyama M. Transcription of the Marek's disease virus genome in virus-induced tumors. J Virol. 1979 Apr;30(1):84–89. doi: 10.1128/jvi.30.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Tanaka A., Nonoyama M. Transcription of the Marek's disease virus genome in a nonproductive chicken lymphoblastoid cell line. Virology. 1979 Feb;93(1):127–133. doi: 10.1016/0042-6822(79)90281-2. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Silver S., Nonoyama M. Biochemical evidence of the nonintegrated status of Marek's disease virus DNA in virus-transformed lymphoblastoid cells of chicken. Virology. 1978 Jul 1;88(1):19–24. doi: 10.1016/0042-6822(78)90105-8. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]