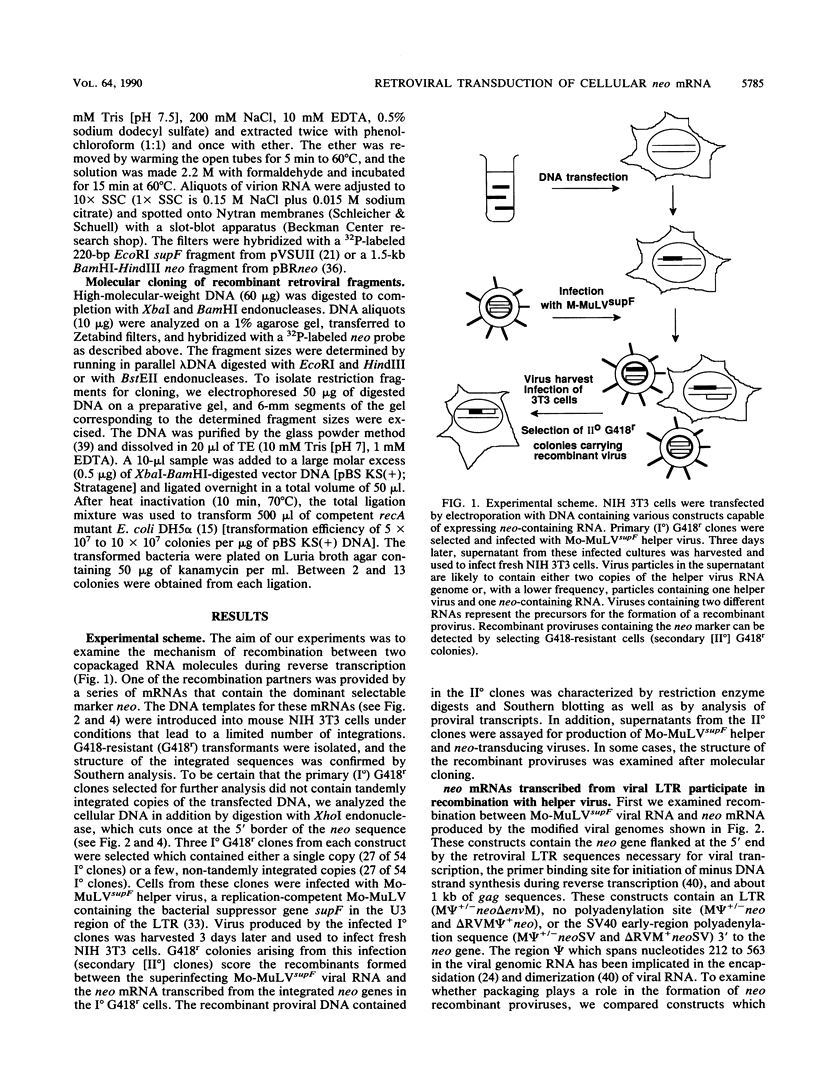
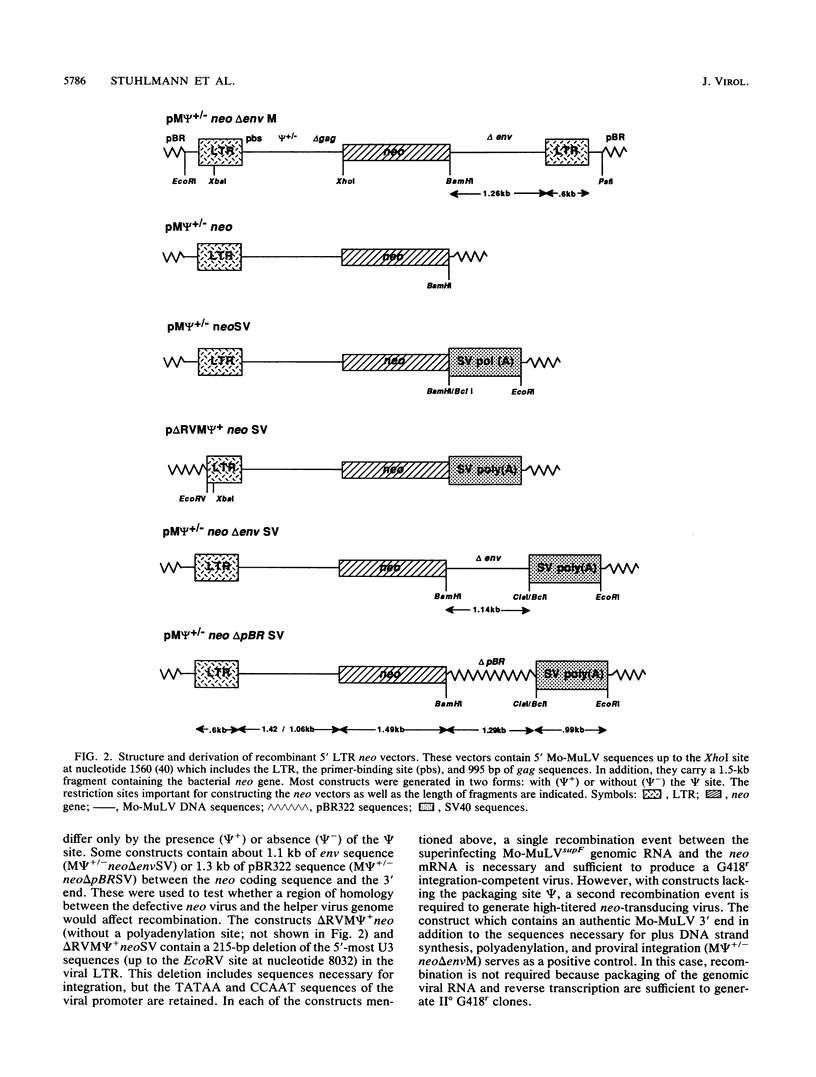
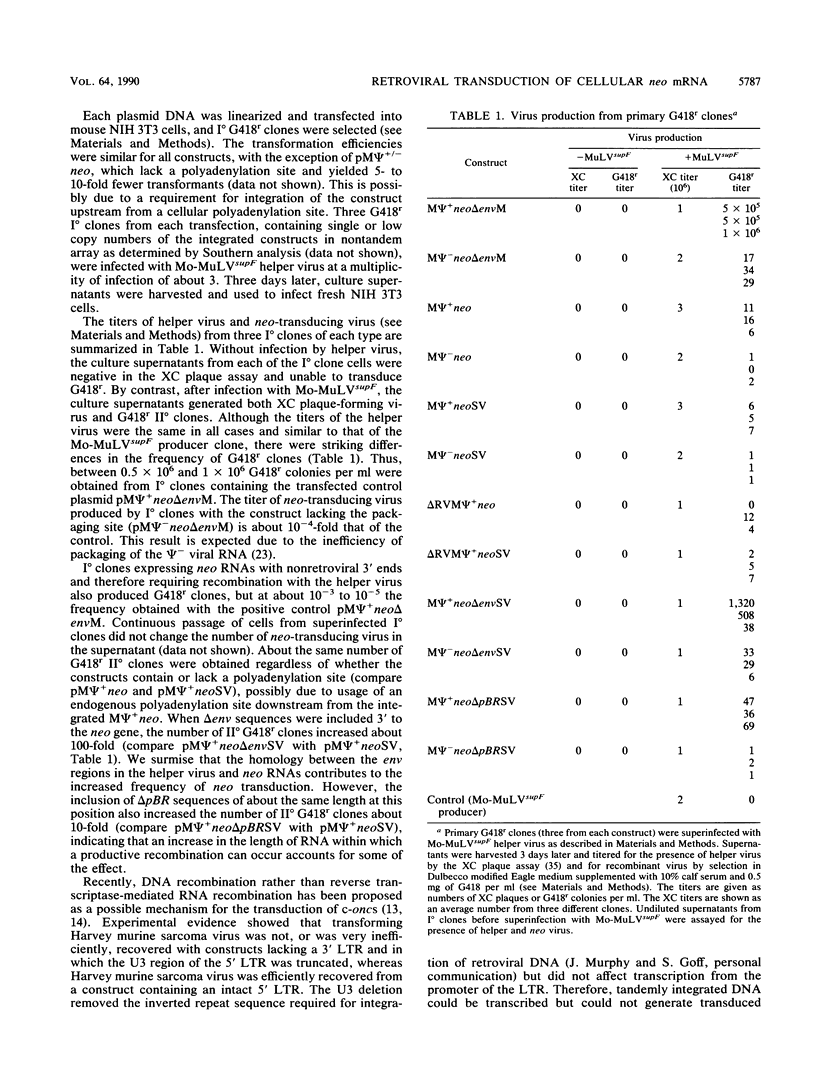
Abstract
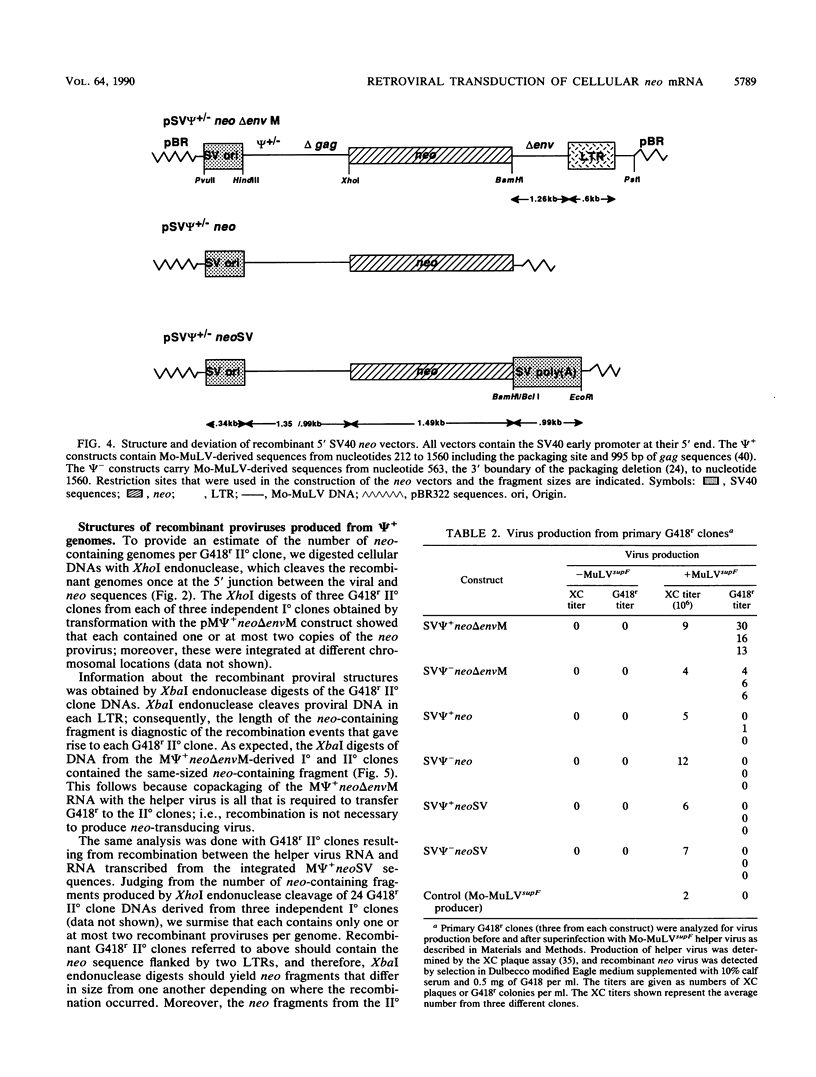
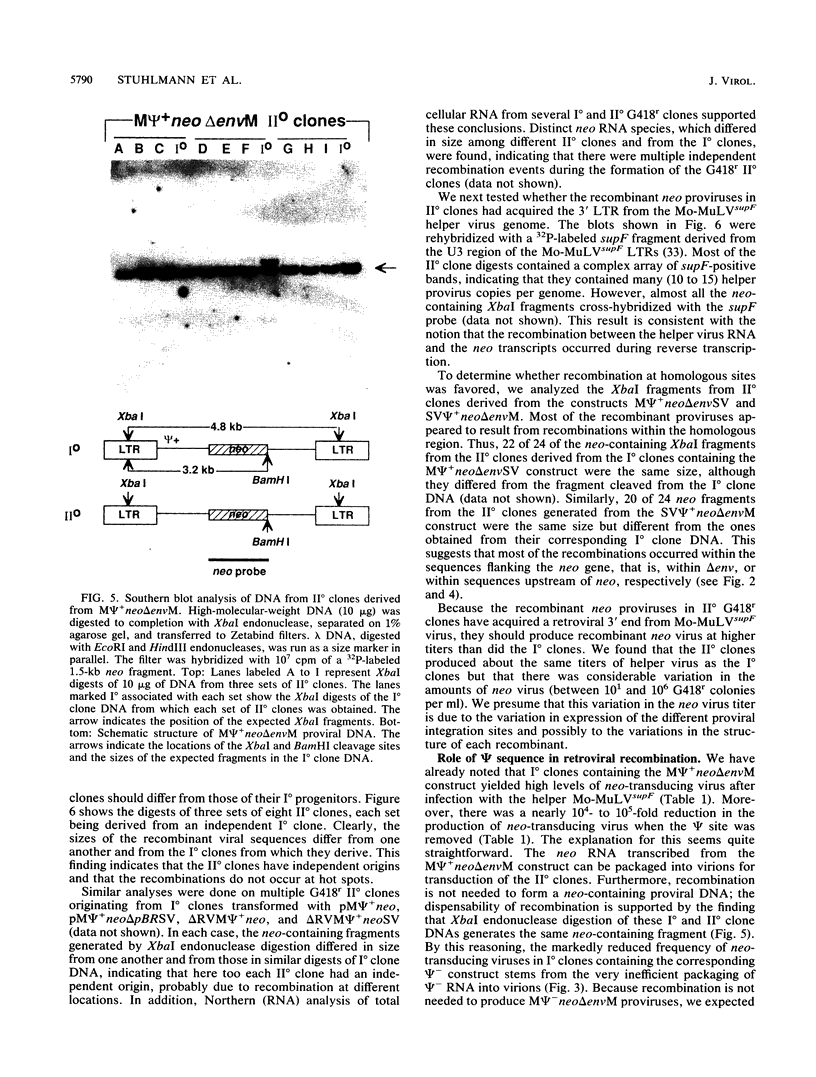
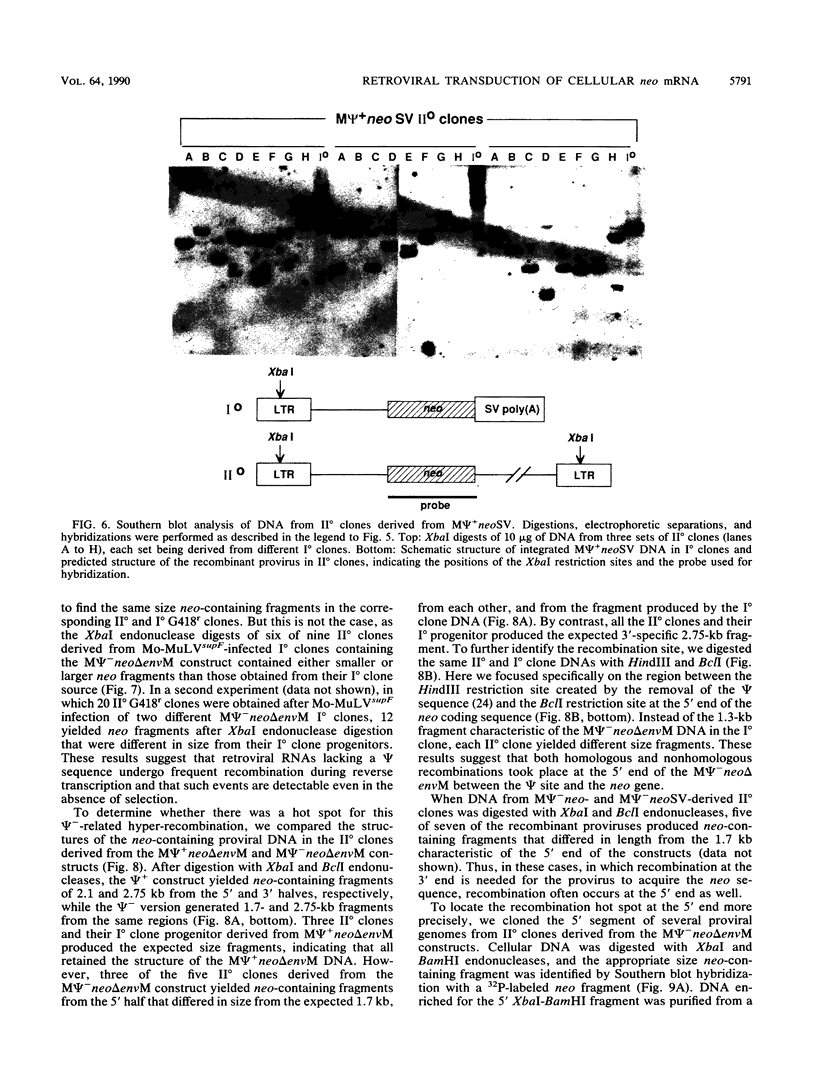
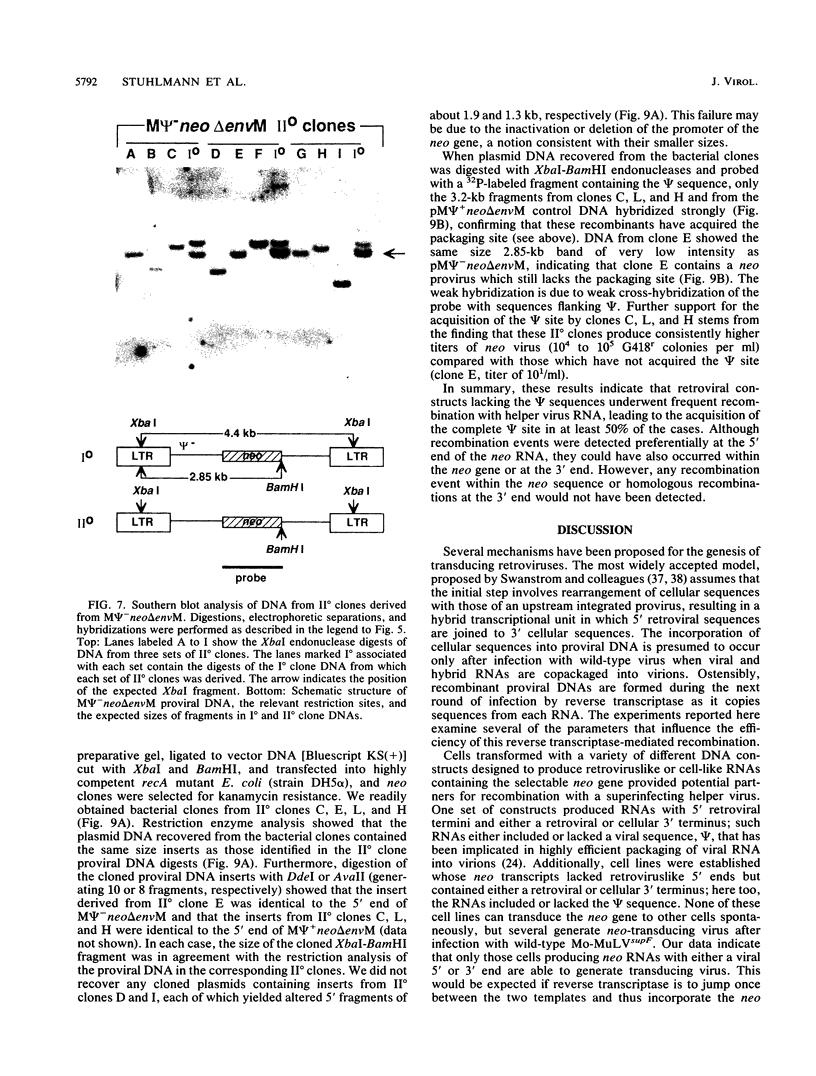
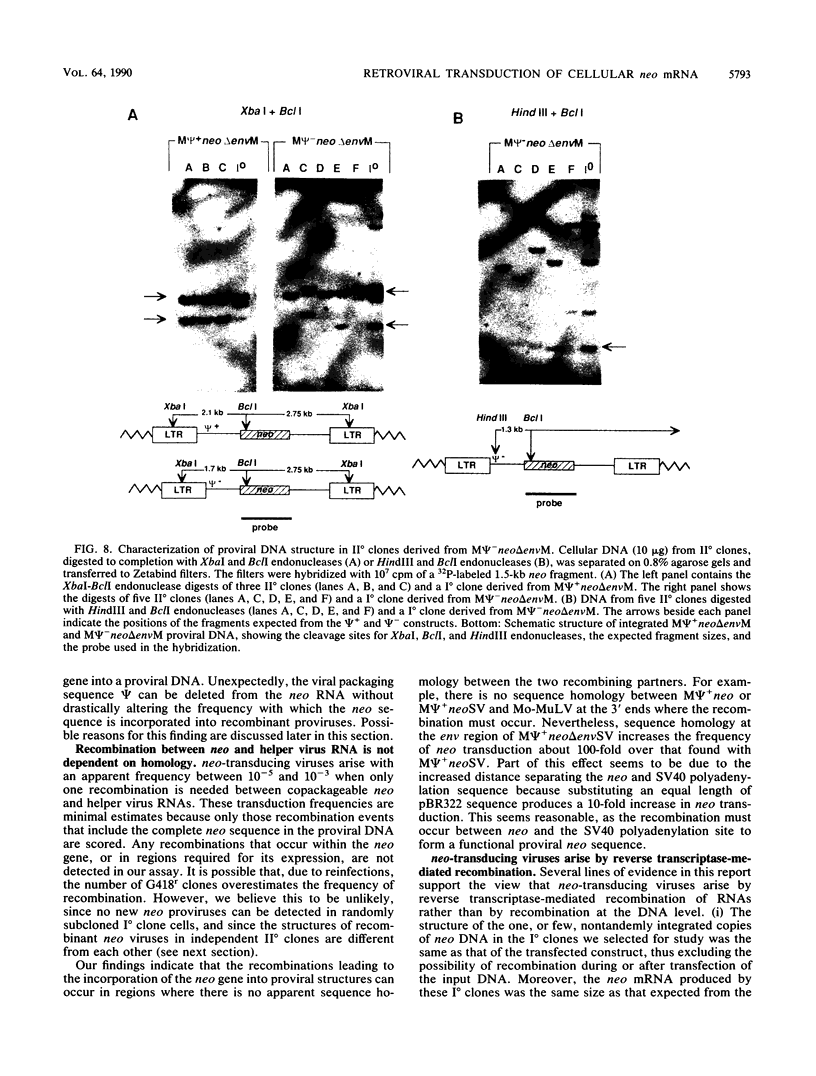
Transduction of cellular oncogenes by retroviruses is thought to be a multistep process, involving transcriptional activation of a cellular gene by upstream proviral integration and joining of cellular DNA to retroviral transcriptional signals, followed by copackaging and recombination with a helper virus genome during reverse transcription. To examine the molecular mechanism of the reverse transcriptase-mediated recombination, we introduced into mouse fibroblast cells a variety of constructs in which the neo selectable marker was joined to flanking retroviruslike or cell-like sequences. After superinfection and copackaging with a replication-competent Mo-MuLVsupF virus, the formation of recombinant neo transducing viruses was assessed in a second round of virus infection by the ability to confer G418 resistance to infected cells. Our results showed that recombinant neo proviruses were generated from neo RNA containing either a 5' or 3' retroviral end, implying that one recombination event with helper virus RNA was sufficient to incorporate the neo gene into proviral DNA. Recombination occurred with an apparent frequency of 10(-4) to 10(-5) per replication cycle in the absence of homology between the two recombining partners. This frequency, however, increased at least 100-fold if homology was provided at the site of recombination. Our results support the hypothesis that neo-transducing viruses arise via reverse transcriptase-mediated recombination of RNA rather than by recombination proceeding through DNA intermediates. Unexpectedly, removal of the retroviral packaging site psi reduced the number of neo recombinants only slightly. Our data indicated that although RNAs lacking the psi site are poorly packaged into virions, those RNAs that are included in the virions undergo frequent recombination, even if there is no selection for recombination. Many of the neo recombinants formed with the psi- constructs had undergone additional recombinations and often incorporated the psi site from the helper RNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Chien Y. H., Chattopadhyay S., Vogt P. K., Gardner M. B., Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978 Mar;25(3):888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F., Hoggan M. D., Willey R. L., Strebel K., Martin M. A., Repaske R. Genetic recombination of human immunodeficiency virus. J Virol. 1989 Mar;63(3):1455–1459. doi: 10.1128/jvi.63.3.1455-1459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fulton R., Forrest D., McFarlane R., Onions D., Neil J. C. Retroviral transduction of T-cell antigen receptor beta-chain and myc genes. Nature. 1987 Mar 12;326(6109):190–194. doi: 10.1038/326190a0. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Evidence that retroviral transduction is mediated by DNA not by RNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3604–3608. doi: 10.1073/pnas.87.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral transduction of oncogenic sequences involves viral DNA instead of RNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3733–3737. doi: 10.1073/pnas.85.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S. A., Coffin J. M. Efficient packaging of readthrough RNA in ALV: implications for oncogene transduction. Science. 1987 May 15;236(4803):845–848. doi: 10.1126/science.3033828. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hammond C., Bishop J. M. Nucleotide sequence and topography of chicken c-fps. Genesis of a retroviral oncogene encoding a tyrosine-specific protein kinase. J Mol Biol. 1985 Jan 20;181(2):175–186. doi: 10.1016/0022-2836(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hay N., Bishop J. M. The role of RNA molecules in transduction of the proto-oncogene c-fps. Cell. 1986 Mar 28;44(6):935–940. doi: 10.1016/0092-8674(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Boone L. R., Skalka A. M. Retroviral DNA H structures: displacement-assimilation model of recombination. Cell. 1982 Aug;30(1):53–62. doi: 10.1016/0092-8674(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Linial M. Creation of a processed pseudogene by retroviral infection. Cell. 1987 Apr 10;49(1):93–102. doi: 10.1016/0092-8674(87)90759-8. [DOI] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Baltimore D. Varying the position of a retrovirus packaging sequence results in the encapsidation of both unspliced and spliced RNAs. J Virol. 1985 May;54(2):401–407. doi: 10.1128/jvi.54.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miles B. D., Robinson H. L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985 May;54(2):295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. E., Goff S. P. Construction and analysis of deletion mutations in the U5 region of Moloney murine leukemia virus: effects on RNA packaging and reverse transcription. J Virol. 1989 Jan;63(1):319–327. doi: 10.1128/jvi.63.1.319-327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti K. G., Bondurant M., Tereba A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J Virol. 1981 Jan;37(1):411–419. doi: 10.1128/jvi.37.1.411-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988 Aug 26;241(4869):1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Crittenden L., Kung H. J. Mechanism of c-erbB transduction: newly released transducing viruses retain poly(A) tracts of erbB transcripts and encode C-terminally intact erbB proteins. J Virol. 1988 Jul;62(7):2437–2443. doi: 10.1128/jvi.62.7.2437-2443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W., Weiher H., Jaenisch R. Replication-competent Moloney murine leukemia virus carrying a bacterial suppressor tRNA gene: selective cloning of proviral and flanking host sequences. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1141–1145. doi: 10.1073/pnas.82.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson D., Dugan D., Reddy E. P. Aberrant splicing events that are induced by proviral integration: implications for myb oncogene activation. Proc Natl Acad Sci U S A. 1987 May;84(10):3171–3175. doi: 10.1073/pnas.84.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Beamand J. A. Genetic recombination in Rous sarcoma virus: the genesis of recombinants and lack of evidence for linkage between pol, env and src genes in three factor crosses. J Gen Virol. 1979 May;43(2):349–364. doi: 10.1099/0022-1317-43-2-349. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Bell J. G., Beamand J. A. Genetic recombination among temperature-sensitive mutnats of Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):897–905. doi: 10.1101/sqb.1974.039.01.104. [DOI] [PubMed] [Google Scholar]