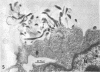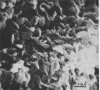Abstract
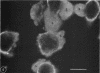
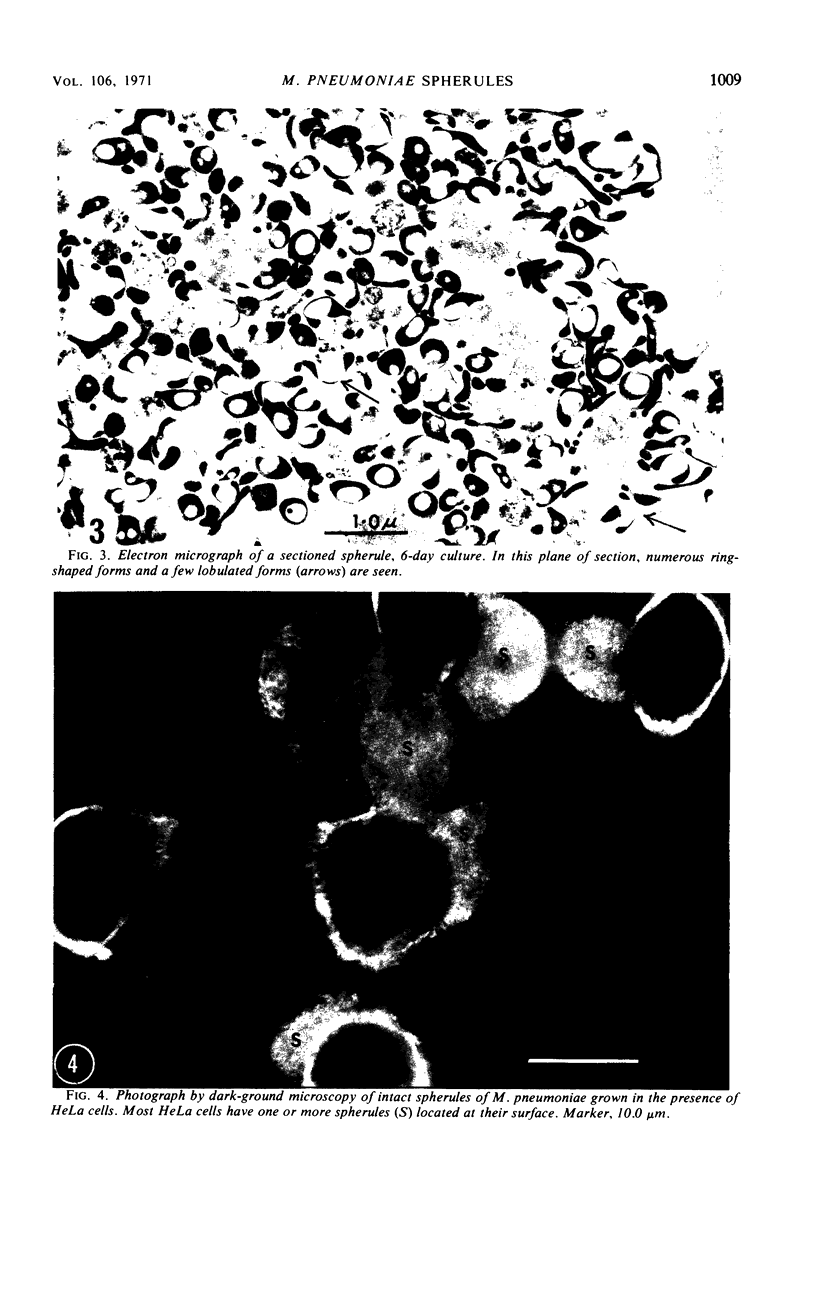
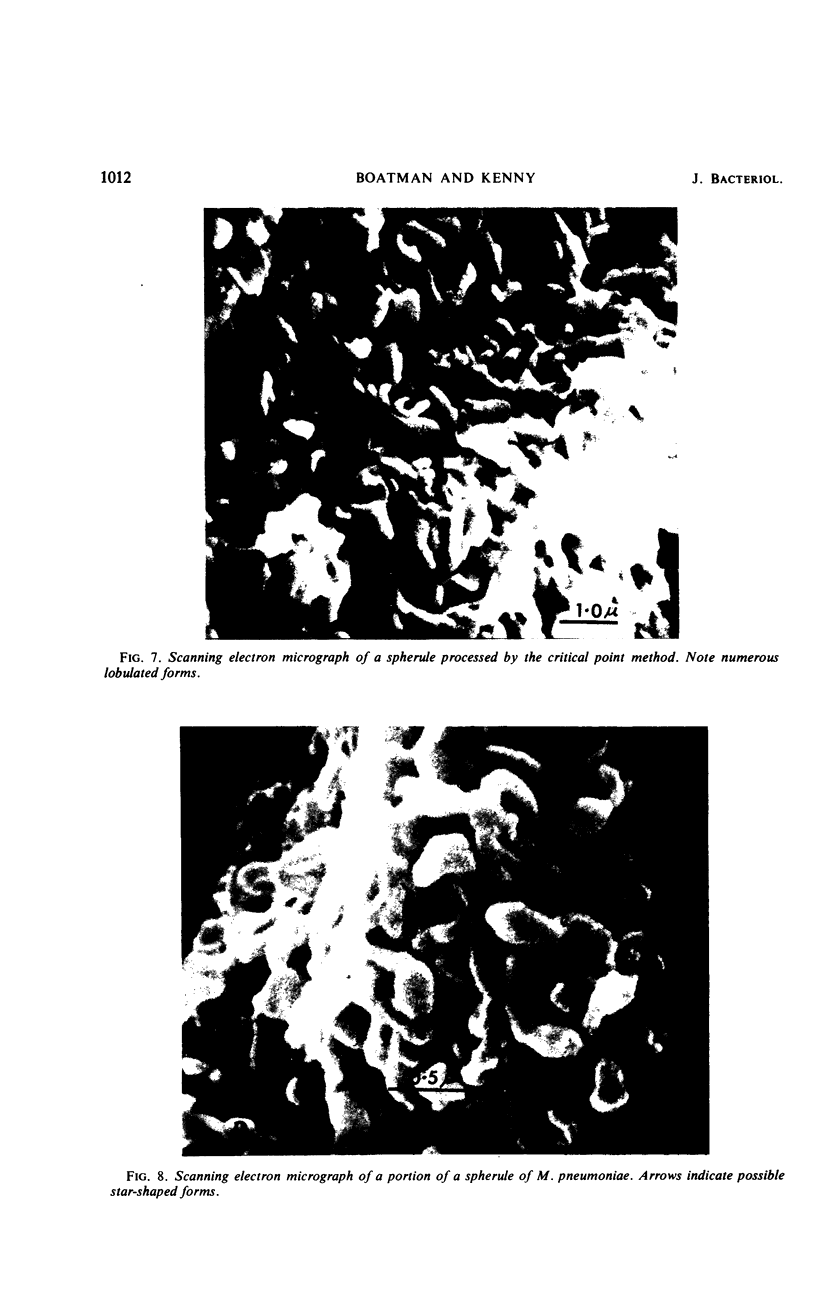
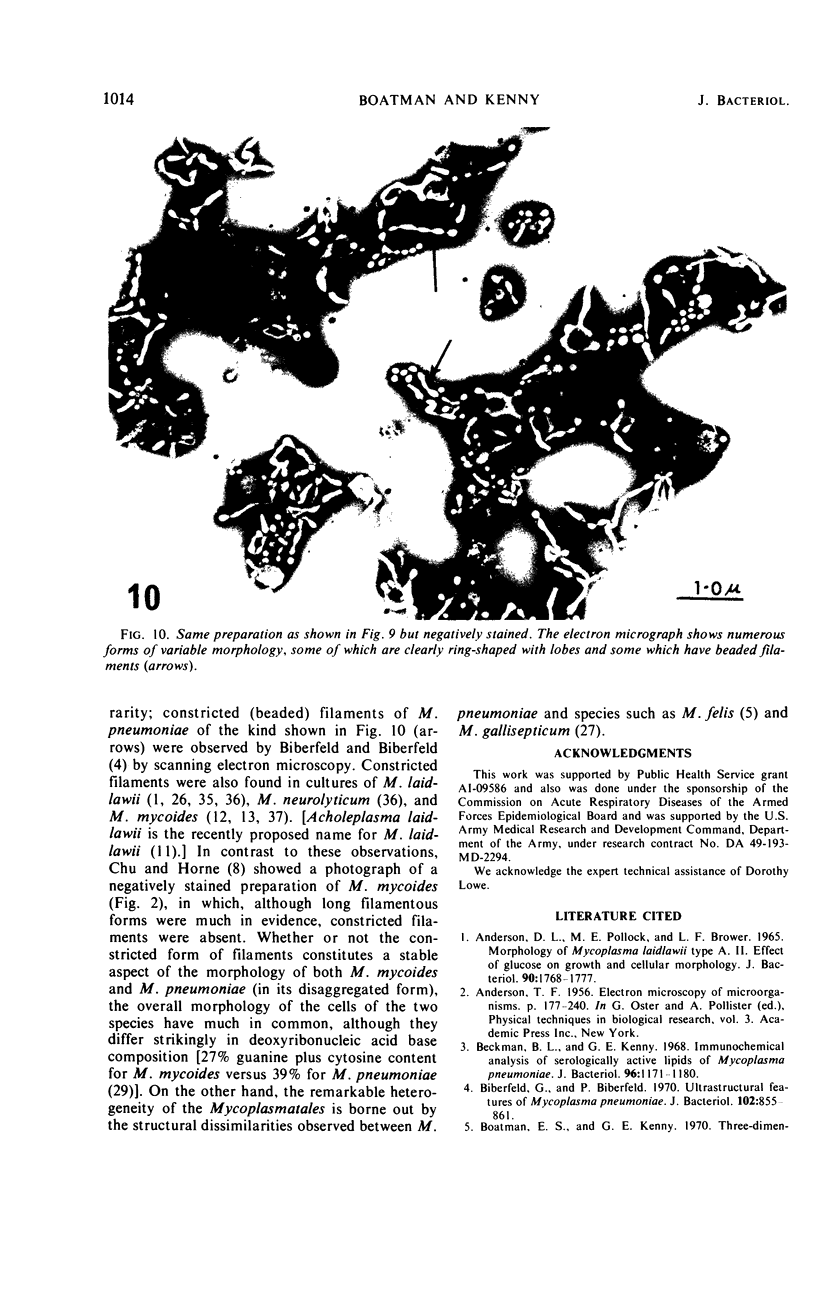
During growth in fluid medium, most strains of Mycoplasma pneumoniae produce free-floating granules which become larger with time. We have called these granules “spherules.” This study describes the morphological and ultrastructural features of M. pneumoniae strain AP-164 spherules, both free and in association with HeLa cells in cell culture. In thin section, spherules were composed of lobulated cells, connected together by membranes, and ring-shaped cells. The two-dimensional morphology observed varied according to the plane of section and to the age of the culture. In HeLa cell cultures, mycoplasmata were found attached to plasma membranes of cells; in sections, individual mycoplasmata were often aligned in radial apposition to the membranes. Mycoplasmata were not found intracellularly. The three-dimensional morphology of spherules was examined by the critical point method and by scanning electron microscopy. Both methods revealed lobulated forms, ring-shaped forms, and star-shaped forms. Treatment of the spherules with crude porcine pancreatic lipase effectively released large numbers of free organisms. Phosphotungstic acid preparations of these uncentrifuged forms revealed a morphology in agreement with the other methods used. Lobulated ring forms with “beaded” filaments were prominent. In respect to morphology, M. pneumoniae under our conditions resembles that of the type species M. mycoides.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Pollock M. E., Brower L. F. Morphology of Mycoplasma laidlawii type A. II. Effect of glucose on growth and cellular morphology. J Bacteriol. 1965 Dec;90(6):1768–1777. doi: 10.1128/jb.90.6.1768-1777.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman B. L., Kenny G. E. Immunochemical analysis of serologically active lipids of Mycoplasma pneumoniae. J Bacteriol. 1968 Oct;96(4):1171–1180. doi: 10.1128/jb.96.4.1171-1180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman E. S., Kenny G. E. Three-dimensional morphology, ultrastructure, and replication of Mycoplasma felis. J Bacteriol. 1970 Jan;101(1):262–267. doi: 10.1128/jb.101.1.262-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt W. Growth morphology of Mycoplasma pneumoniae strain FH on glass surface. Proc Soc Exp Biol Med. 1968 Jun;128(2):338–340. doi: 10.3181/00379727-128-33009. [DOI] [PubMed] [Google Scholar]
- Bredt W. Motility and multiplication of Mycoplasma pneumoniae. A phase contrast study. Pathol Microbiol (Basel) 1968;32(6):321–326. doi: 10.1159/000162074. [DOI] [PubMed] [Google Scholar]
- CLYDE W. A., Jr Demonstration of Eaton's agent in tissue culture. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:715–718. doi: 10.3181/00379727-107-26732. [DOI] [PubMed] [Google Scholar]
- Chu H. P., Horne R. W. Electron microscopy of Mycoplasma gallisepticum and Mycoplasma mycoides using the negative staining technique and their comparison with myxovirus. Ann N Y Acad Sci. 1967 Jul 28;143(1):190–203. doi: 10.1111/j.1749-6632.1967.tb27658.x. [DOI] [PubMed] [Google Scholar]
- Clyde W. A., Jr, Kim K. S. Biophysical characterization of human mycoplasma species. Ann N Y Acad Sci. 1967 Jul 28;143(1):425–435. doi: 10.1111/j.1749-6632.1967.tb27687.x. [DOI] [PubMed] [Google Scholar]
- ENNY G. E., GRAYSTON J. T. EATON PLEUROPNEUMONIA-LIKE ORGANISM (MYCOPLASMA PNEUMONIAE) COMPLEMENT-FIXING ANTIGEN: EXTRACTION WITH ORGANIC SOLVENTS. J Immunol. 1965 Jul;95:19–25. [PubMed] [Google Scholar]
- FREUNDT E. A. Morphology and classification of the PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:312–325. doi: 10.1111/j.1749-6632.1960.tb42693.x. [DOI] [PubMed] [Google Scholar]
- Gois M., Cerny M., Veznikova D. Relationship between metabolism-inhibiting antibodies and colonial growth of Mycoplasma hyorhinis and other porcine mycoplasma in the presence of rabbit and pig sera. Res Vet Sci. 1970 Mar;11(2):161–167. [PubMed] [Google Scholar]
- HAYFLICK L., STINEBRING W. R. Intracellular growth of pleuropneumonialike organisms (PPLO) in tissue culture and in ovo. Ann N Y Acad Sci. 1960 Jan 15;79:433–449. doi: 10.1111/j.1749-6632.1960.tb42709.x. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Serological comparison of ten glycolytic Mycoplasma species. J Bacteriol. 1969 Jun;98(3):1044–1055. doi: 10.1128/jb.98.3.1044-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Clyde W. A., Jr, Denny F. W. Physical properties of human Mycoplasma species. J Bacteriol. 1966 Jul;92(1):214–219. doi: 10.1128/jb.92.1.214-219.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson D. L., MacLeod R. Mycoplasma pneumoniae and Mycoplasma salivarium: electron microscopy of colony growth in agar. J Bacteriol. 1970 Feb;101(2):609–617. doi: 10.1128/jb.101.2.609-617.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773–1783. [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Ultrastructure and life cycle of Mycoplasma gallisepticum A5969. Ann N Y Acad Sci. 1967 Jul 28;143(1):59–65. doi: 10.1111/j.1749-6632.1967.tb27644.x. [DOI] [PubMed] [Google Scholar]
- Maniloff J. Ultrastructure of Mycoplasma laidlawii during culture development. J Bacteriol. 1970 May;102(2):561–572. doi: 10.1128/jb.102.2.561-572.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Rogul M., Wittler R. G. Molecular genetic studies of relationships among mycoplasma, L-forms and bacteria. Ann N Y Acad Sci. 1967 Jul 28;143(1):21–30. doi: 10.1111/j.1749-6632.1967.tb27639.x. [DOI] [PubMed] [Google Scholar]
- Neimark H. Heterogeneity among the mycoplasma and relationships to bacteria. Ann N Y Acad Sci. 1967 Jul 28;143(1):31–37. doi: 10.1111/j.1749-6632.1967.tb27640.x. [DOI] [PubMed] [Google Scholar]
- Organick A. B., Siegesmund K. A., Lutsky I. I. Pneumonia due to mycoplasma in gnotobiotic mice. II. Localization of Mycoplasma pulmonis in the lungs of infected gnotobiotic mice by electron microscopy. J Bacteriol. 1966 Oct;92(4):1164–1176. doi: 10.1128/jb.92.4.1164-1176.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. E., KENNY G. E. Mammalian cell cultures contaminated with PPLO III. Elimination of PPLO with specific antiserum. Proc Soc Exp Biol Med. 1963 Jan;112:176–181. doi: 10.3181/00379727-112-27985. [DOI] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Effect of pH on the immunogenicity of Mycoplasma pneumoniae. J Bacteriol. 1969 Feb;97(2):612–619. doi: 10.1128/jb.97.2.612-619.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Cosenza B. J. Growth phases of Mycoplasma in liquid media observed with phase-contrast microscope. J Bacteriol. 1966 Feb;91(2):858–869. doi: 10.1128/jb.91.2.858-869.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Cosenza B. J., Tourtellotte M. E. Filamentous growth of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):66–72. doi: 10.1111/j.1749-6632.1967.tb27645.x. [DOI] [PubMed] [Google Scholar]
- Razin S. Mycoplasma taxonomy studiedy electrophoresis of cell proteins. J Bacteriol. 1968 Sep;96(3):687–694. doi: 10.1128/jb.96.3.687-694.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell A. W. The stability of Mycoplasma mycoides. J Gen Microbiol. 1965 Aug;40(2):227–234. doi: 10.1099/00221287-40-2-227. [DOI] [PubMed] [Google Scholar]
- Shedden W. I., Cole B. C. Rapid method for demonstrating intracellular pleuropneumonia-like organisms in a strain of hamster kidney cells (BHK 21 C13). Nature. 1966 May 21;210(5038):868–868. doi: 10.1038/210868a0. [DOI] [PubMed] [Google Scholar]
- Steinberg P., Horswood R. L., Chanock R. M. Temperature-sensitive mutants of Mycoplasma pneumoniae. I. In vitro biologic properties. J Infect Dis. 1969 Aug;120(2):217–224. doi: 10.1093/infdis/120.2.217. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. II. Monocytes and lymphocytes. J Exp Med. 1966 Sep 1;124(3):533–542. doi: 10.1084/jem.124.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]