Abstract
High-efficiency entry of the enteropathogenic bacterium Yersinia pseudotuberculosis into nonphagocytic cells is mediated by the bacterial outer membrane protein invasin. Invasin-mediated uptake requires high affinity binding of invasin to multiple β1 chain integrin receptors on the host eukaryotic cell. Previous studies using inhibitors have indicated that high-efficiency uptake requires tyrosine kinase activity. In this paper we demonstrate a requirement for focal adhesion kinase (FAK) for invasin-mediated uptake. Overexpression of a dominant interfering form of FAK reduced the amount of bacterial entry. Specifically, the autophosphorylation site of FAK, which is a reported site of c-Src kinase binding, is required for bacterial internalization, as overexpression of a derivative lacking the autophosphorylation site had a dominant interfering effect as well. Cultured cells expressing interfering variants of Src kinase also showed reduced bacterial uptake, demonstrating the involvement of a Src-family kinase in invasin-promoted uptake.
Entry into host cells is a common property of a number of bacterial pathogens (1, 2). Investigation of cellular penetration provides a powerful model system to analyze unique characteristics of microbial pathogens and the host cell response to bacterial interaction (3, 4). Efficient entry into cultured cells by the enteropathogenic bacterium Yersina pseudotuberculosis is mediated by the protein invasin (2). This 108-kDa outer membrane protein binds at least five members of the β1 chain integrin family cell adhesion molecules on the mammalian cell surface (5). The specificity of invasin for integrin receptors appears to be responsible for the cell tropism of the organism after oral ingestion by the host. Shortly after introduction into the small intestine, bacteria encoding invasin are found exclusively in M cells located within the epithelium overlying intestinal Peyer’s patches (6). These are the only cells that present β1 integrins on the luminal side of the intestine (6, 7).
Escherichia coli strains encoding the Y. pseudotuberculosis invasin protein are internalized efficiently by a wide variety of cultured cells (5). Previous studies have indicated that invasin-mediated uptake requires endocytic factors and is modulated by interaction of the integrin β1 chain with the cytoskeleton (8). Entry in this fashion is sensitive to inhibitors of tyrosine kinases (9) as well as to the action of a bacterially produced tyrosine phosphatase, YopH (10, 11). This phosphatase has been shown to localize to focal adhesions and dephosphorylate proteins localized at this site, such as focal adhesion kinase (FAK) and p130CAS (10, 11). There also appears to be some increase in phosphorylation of selected mammalian proteins in response to invasin-mediated adhesion (11).
FAK is a 125-kDa protein that contains an amino-terminal autophosphorylation site (Y397), a central kinase domain, and a carboxyl-terminal region required for its localization to focal adhesions (FAT site) (12). The amino terminus is believed to interact directly with the cytoplasmic domain of the β1 chain (13), and in a FAT site-dependent fashion has been shown to be recruited to sites of integrin adhesion to substrates (13, 14). Integrin ligation leads to an overall increase in cellular tyrosine phosphorylation levels concomitant with activation of FAK (15, 16). Although the role of this protein in regulating adhesion events is not completely understood, cell lines harboring homozygous insertion mutations in the gene encoding FAK have slower migration kinetics (17), and such lesions result in embryonic lethality in mice. Expression of dominant interfering variants of FAK reduces cellular migration rates as well as depresses the rate of cell spreading on matrix (18). FAK appears to be an important factor in sending downstream phosphorylation signals that are initiated by cell adhesion, with resulting phosphorylation of mitogen-activated protein kinases and other proteins involved in growth regulation (19).
In this paper, we investigate the role of FAK in invasin-mediated uptake. The results indicate that efficient uptake via the invasin pathway requires FAK, as derivatives of this protein that are defective for interaction with a src-kinase family member interfere with the uptake process.
MATERIALS AND METHODS
Cell Culture.
Chicken embryo fibroblast (CEF) cells were a kind gift of J. Coffin (Tufts University School of Medicine). Culture medium for CEF cells consisted of modified Richter’s MEM, 10% (vol/vol) fetal bovine serum, (HyClone), 4 μg/ml of insulin, 100 units/ml of penicillin, and 100 μg/ml of streptomycin. Transfection was performed by using Lipofectamine (GIBCO) following the manufacturer’s instructions. For replicating retroviral RCAS DNA (20), cells were cultured for 7–9 days after transfection to allow for uniform infection of monolayers. For coexpression of two transfected genes, methods followed those described for RCAS vectors (20). All RCAS derivatives were kindly provided by J. T. Parsons (Univ. of Virginia Health Science Center). To allow cotransfection of two derivatives on RCAS vectors, CEF cells were transfected with either RCAS DNA viral subgroup A encoding FRNK, or RCAS DNA subgroup B encoding FAK. The transfected cells were cultured for 5–7 days, mixed with each other, and grown for an additional 7–10 days with routine passaging to allow coinfection of cells in monolayer. This strategy has been estimated to result in approximately 86% of the monolayer being infected by the appropriate RCAS vector (20). For FAK/Src mixing experiments, CEF cells were transfected with RCAS DNA subgroup B encoding FAK derivatives or with RCAS DNA encoding Src derivatives viral subgroup A, and coinfection proceeded as above. Based on the previously observed efficiency of single vector infections, approximately 75% of the cells should harbor both subgroups of virus (20).
Internalization Assay.
After transfection and culture of CEF cells to allow uniform infection of monolayers with RCAS derivatives, cells were seeded in triplicate in 24-well dishes at a density of 1.2 × 105 cells/well for vector and FAK-transfected cells or 1.7 × 105 cells/well for FRNK-transfected cells. After 1 day of additional culture, the monolayers were washed three times in PBS and incubated with 0.5 ml of DMEM, 10% fetal bovine serum. The cells then were challenged at a multiplicity of infection (MOI) of three bacteria/cultured cell with E. coli strain MC4100(pRI203), expressing invasin (21). Bacterial internalization was determined after 1-hr incubation at 37°C by gentamicin protection as described (21). An uptake index was determined for each transfectant as the number of bacteria internalized (determined by gentamicin protection) per cultured cell. The total number of cultured cells was determined by crystal violet staining of parallel samples as described (22), and uptake indices were expressed as a percentage uptake relative to vector control.
Analysis of the Amount of Protein Expression by CEF Transfectants.
Transfected cells were seeded in a 60-cm dish simultaneously with cells seeded for the uptake assay so as to be confluent after 24 hr. Cells were rinsed twice with cold PBS, lysed with 0.5 ml of modified RIPA buffer (50 mM Tris, pH 8.0/150 mM NaCl/1 mM EDTA/1% Nonidet P-40/0.1% SDS/0.5% deoxycholic acid/20 μM leupeptin/1 μM pepstatin/10 μg/ml of chymostatin/200 μM 4-(2-aminoethyl)benzenesulfonyl fluoride/10 μg/ml of E64) for 10 min on ice and scraped with a cell scraper into a microfuge tube. The lysates then were cleared at 14,000 rpm in a microfuge for 10 min, and supernatants were transferred to fresh tubes. The protein concentration was determined by BCA reagent (Pierce), and equal amounts of protein were loaded on 10% SDS/PAGE. After transfer to Immobilon (Millipore), the filter replicas were probed with rabbit anti-FAK serum C-20 (1/2,000 dilution, Santa Cruz Biotechnology). Filters were used to analyze cells that were cotransfected with FAK, and Src were stripped by incubation for 30 min at 50°C with shaking in 100 mM 2-mercaptoethanol, 62.5 mM Tris⋅HCL, pH 6.7, 2% SDS after probing with anti-FAK. The membranes were washed with PBS-0.1% Tween, blocked overnight in PBS-5% nonfat dry milk at 4°C, and reprobed with mouse mAb anti-v-Src (Ab-1, 1/1,000 dilution, Oncogene Science). All blots were visualized by enhanced chemiluminesence (ECL, Amersham).
Cell Binding Assay.
Transfected CEF cells were cultured for 7–9 days and seeded on coverslips in 24-well dishes at a density of 2 × 104 cells/well. The next day, cells were washed twice with PBS, challenged with MC4100(pRI203inv+) at a MOI of ≈10 in DMEM-10% fetal bovine serum, and incubated at 37°C for 1 hr. The MOI used was slightly higher than that in the uptake assay, as lower multiplicities did not yield sufficient bacterial binding to obtain meaningful histograms. The coverslips then were processed for bacterial binding assay using Giemsa staining (22). The number of bound bacteria per cell was determined by light microscopy.
Bead Uptake Assay.
The FAK knockout mouse cell line (DU3, −/−) (17) was transfected on coverslips at a density of 2 × 104 with pMA10, a pcDNA3.1 Hygro (Invitrogen) derivative that expresses FAK. To construct this plasmid, the FAK ORF from chicken was PCR-amplified from the RCAS (subgroup A) FAK plasmid, using primers 5′-GCGGGATCCGCACCATGGCAGCAGCTTACCTTG-3′ and 5′-CGCTCTAGATTAGTGGGGCCTGGACTG-3′. The PCR product was purified, digested with BamHI and XbaI, and ligated into the identically digested pcDNA3.1 Hygro vector backbone to obtain pMA10. The transiently transfected cells then were challenged with beads to determine the efficiency of invasin-promoted uptake. This assay was used because the Lipofectamine transfection resulted in considerable autofluorescent debris that interfered with the ability to accurately detect fluorescence of immunoprobed bacteria. Latex beads have a uniform shape and can be easily distinguished from autofluorescent debris. For preparation of beads, 2 × 108 latex beads (1 μm, Polysciences) were coated for 1 hr at 37°C with 0.5 μg maltose binding protein-Invasin497 (23) in 400 μl of coupling buffer (0.2 M NaHCO3, pH 8.6/0.5 M NaCl) and washed twice with PBS containing 1 mg/ml of BSA. Cells were challenged with invasin-coated beads 16 hr posttransfection at a MOI of ≈100 beads/cell, allowing 30-min binding at room temperature followed by three washes with PBS. Prewarmed media then was added to the cells, and the adhesion mix was shifted to 37°C for 1 hr. The coverslips then were washed and processed for immunofluorescence.
The coverslips were blocked with PBS + 4% goat serum for 1 hr at room temperature, washed with PBS, and probed with a 1:1,000 diluted rabbit anti-MBP (New England Biolabs) for 1 hr, followed by washing and probing with anti-rabbit IgG- tetramethylrhodamine B isothiocyanate (TRITC) (1/500 dilution, Boehringer Mannheim) for 1 hr at room temperature. Cells were permeablized with methanol (−20°C) for 10 sec and washed and blocked with PBS + 4% goat serum for 1 hr at room temperature. The coverslips then were probed again with anti-MBP as well as with mouse anti-FAK mAb (1/100 dilution, clone 77, Transduction Laboratories, Lexington, KY) for 1 hr at room temperature, washed, and probed with goat anti-rabbit IgG- fluorescein isothiocyanate (FITC) and goat anti-mouse IgG-cascade blue (1/500, Molecular Probes). Internalization was determined for FAK and mock-transfected cells. Percent uptake is defined as the number of bound beads that resisted staining with TRITC (inside) versus the number of FITC-stained (total bound). SDs were calculated for triplicate coverslips.
RESULTS
Dominant Interfering FAK Inhibits Invasin-Mediated Uptake.
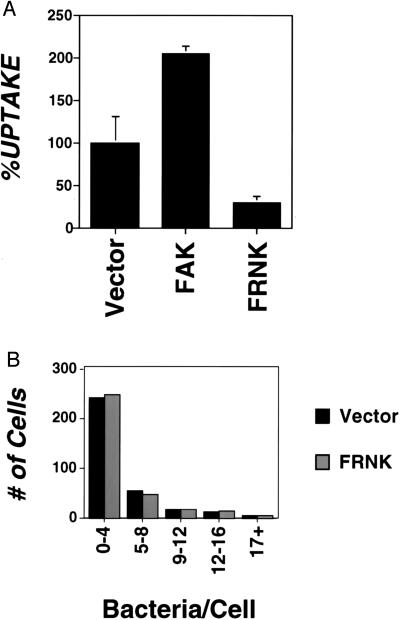
To study the role of FAK in invasin-mediated uptake, internalization of invasin-encoding E. coli was examined in CEF cells overexpressing either wild-type FAK or a derivative of FAK (FRNK) containing a carboxyl-terminal region shown to interfere with the function of resident FAK (Materials and Methods). Uptake of bacteria was strikingly dependent on the concentration of FAK in these cells (Fig. 1A). Overexpression of FRNK was shown to inhibit uptake, with the internalization efficiency approximately 30% that of vector-transfected cells (Fig. 1A, compare Vector to FRNK). Cells overexpressing wild-type FAK consistently showed increased bacterial internalization (Fig. 1A, compare FAK to Vector). The amount of stimulation varied among different transfection pools, but could approach 200% of vector control in a typical experiment (Fig. 1A). Comparison of vector vs. overproduced wild-type FAK from four different experiments performed in triplicate indicated that there was a significant difference in the percent uptake of vector = 99.9% ± 23.5 vs. FAK = 155% ± 42.2 (P < 0.001). Western blot analysis with an antibody that recognizes both FAK and FRNK confirmed overexpression of FAK constructs relative to FAK levels in vector-transfected cells (see below, Fig. 3B). The observed inhibition of bacterial uptake by FRNK was not caused by decreased bacterial binding by FRNK-transfected cells (Fig. 1B). The total number of bacteria bound to both vector and FRNK-transfected cells was identical, and histograms of the distribution of bacteria binding individual cells were almost superimposible after allowing binding for 1 hr at a MOI = 10 (vector = 1.75 ± 0.10 vs. FRNK = 1.99 ± 0.38 bacteria bound/cell).
Figure 1.
Carboxyl-terminal fragment of FAK (FRNK) inhibits invasin-mediated uptake. (A) The amount of bacterial internalization was determined for CEF cells that were transfected with either RCAS vector alone or RCAS overexpressing FAK or FRNK (Materials and Methods). %UPTAKE refers to the amount of internalization observed relative to that of the vector-transfected control. A representative experiment is shown with error bars indicating the SE for samples prepared in triplicate. For vector-transfected control, 1.3 ± 0.3% of the initial bacterial inoculum was internalized during the course of the experiment. (B) FRNK overexpression does not inhibit invasin-mediated binding to CEFs. The amount of bacterial binding was determined (Materials and Methods) for vector-transfected cells (filled bars) and FRNK overexpressing CEF cells (empty bars) of Giemsa-stained preparations.
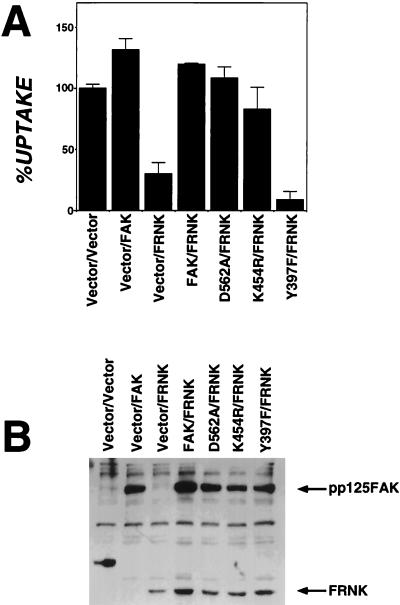
Figure 3.
Tyr-397 substitution of FAK is unable to overcome FRNK inhibition of invasin-mediated uptake. (A) Bacterial internalization was determined for vector-transfected cells or cells overexpressing a mixture of FAK derivatives (Materials and Methods). FAK: wild-type FAK transfectants; FRNK: transfectants expressing FRNK interfering fragment; D562A or K464R: FAK derivatives lacking kinase activity; Y397F: FAK substitution lacking autophosphorylation site. %UPTAKE refers to the amount of internalization observed relative to that of the vector-transfected control. (B) Cell lysates of vector-transfected cells or cells overexpressing FAK derivatives were examined by immunoblotting with anti-FAK serum. Samples were identical to those in A.
FAK Is Required for High-Efficiency Uptake of Invasin-Coated Beads.
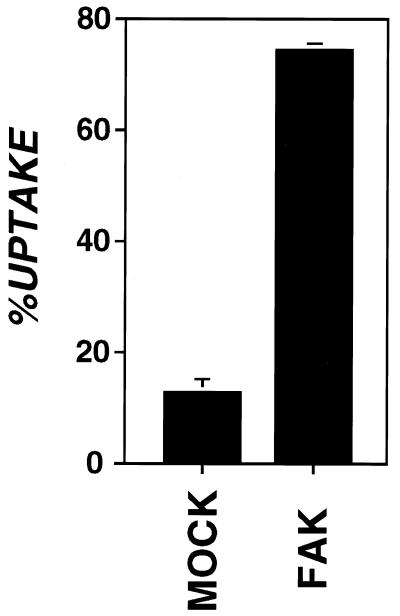
The requirement for FAK in invasin-mediated uptake was further demonstrated by analyzing uptake in cells unable to express FAK because of the presence of a homozygous insertion mutation. The FAK knockout cell line (DU3, −/−) was tested for invasin-mediated uptake in a bead challenge assay (Fig. 2) (P. Dersch and R.R.I., unpublished work). DU3 was transfected with either vector alone (Mock) or vector expressing FAK (FAK+) and challenged with invasin-coated beads (Materials and Methods). In this assay, FAK mouse knockout cells re-expressing FAK were able to internalize roughly six times the number of bound beads as vector-transfected cells (vector = 12.7% ± 2.3, FAK = 74.1% ± 1.2). FAK expression had no affect on binding of beads to cells (vector = 1.05 ± 0.17, FAK+ =1.03 ± 0.04 average beads/cell) and did not substantially increase internalization of nonspecifically bound (MBP-coated) beads (vector = 16.4% ± 5.2, FAK = 20.4% ± 1.6). In fact, for the FAK knockout cell line, the internalization efficiency of nonspecifically bound (MBP-coated) beads was similar to that of invasin-coated beads.
Figure 2.
FAK is required for high-efficiency uptake of invasin-coated beads. Internalization of invasin-coated beads by FAK knockout mouse cells (DU3, −/−) transfected with vector (Mock) or expressing FAK (FAK+) was determined by using immunoflourescence protection assay (Materials and Methods). %UPTAKE refers to percentage of beads bound to cells that were internalized. Error bars represent SE calculated for triplicate coverslips.
Tyr-397 Substitution of FAK Is Unable to Overcome FRNK Inhibition of Invasin-Mediated Uptake.
The interference of uptake by FRNK could be overcome by coordinately overexpressing a wild-type copy of FAK. In a double transfection experiment, wild-type FAK and FRNK overexpressed in the same cell restored uptake to levels greater than vector-transfected controls (Fig. 3A, FAK/FRNK vs. Vector/FRNK). The catalytic activity of FAK was not required to restore uptake to high levels in FRNK-expressing cells (Fig. 3A). Overexpression of FAK mutants that lack FAK kinase activity (D562A and K464R) rescued uptake in cells overexpressing FRNK (Fig. 3A, D562A/FRNK and K464R/FRNK). Coexpression of one particular FAK derivative with FRNK, however, indicated that the autophosphorylation site (Y397 of FAK) (24) is required for uptake. Overexpression of the derivative FAK Y397F having a substitution mutation in this site could not restore uptake in FRNK-transfected cells (Fig. 3A, Y397F/FRNK). In fact, cells overexpressing FAK Y397F with FRNK resulted in the most severe defect in bacterial uptake observed in these transfection experiments (Fig. 3A, Y397F/FRNK vs. Vector/FRNK).
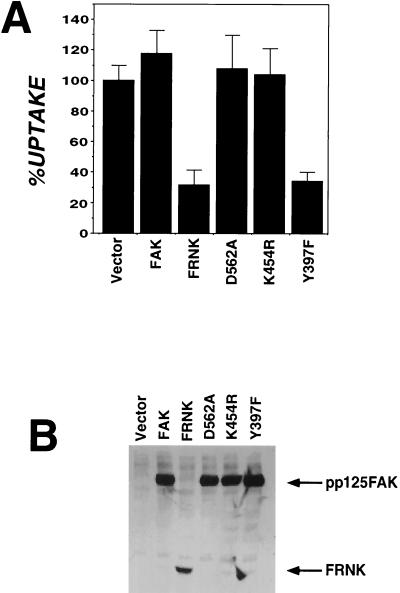
Overexpression of FAK Y397F Inhibits Invasin-Mediated Uptake.
Consistent with the strong phenotype associated with coexpression of the FAK Y397F and FRNK constructs is the fact that when overexpressed by itself, the FAK Y397F construct showed a dominant interfering affect on uptake. The level of interference for this autophosphorylation mutant was similar to that observed with FRNK overexpression (Fig. 4A, FRNK, Y397F). As expected from their ability to rescue cells from FRNK interference, overexpression of kinase-defective forms of FAK did not inhibit invasin-mediated uptake (Fig. 4A, D562A and K464R), consistent with the previously demonstrated lack of inhibition of these variants on cell migration (25) (see Discussion). Western blot analysis confirmed that the levels of FAK expression were similar for all samples and the effects observed were not caused by overexpression of Y397F relative to the kinase-defective forms (Fig. 4B).
Figure 4.
Overexpression of Y397F has dominant interfering effect on uptake. (A) Bacterial internalization was determined for CEF cells transfected with vector (RCAS), FAK, FRNK, kinase-defective FAK (D562A and K454R), or FAK lacking the autophosphorylation site (Y397F) (Materials and Methods). A representative experiment is shown with error bars indicating the SE for samples prepared in triplicate. %UPTAKE refers to the amount of internalization observed relative to that of the vector-transfected control. (B) Cell lysates from vector transfected, FAK, FRNK, kinase-defective FAK (D562A and K464R), and FAK lacking the autophosphorylation site (Y397F) were examined by immunoblotting with anti-FAK antibody.
c-Src Kinase Is Required for Invasin-Mediated Uptake.
FAK is known to strongly associate with c-Src kinase upon integrin engagement (26). As a result of integrin engagement, a binding site for the Src SH2-domain is created when FAK becomes tyrosine-phosphorylated at Y397. This association has been shown to result in increased Src kinase activity as well as the observed phosphorylation of Src substrates (27). As expression of the Y397F derivative interfered with uptake, a role for c-Src kinase in this process was suspected.
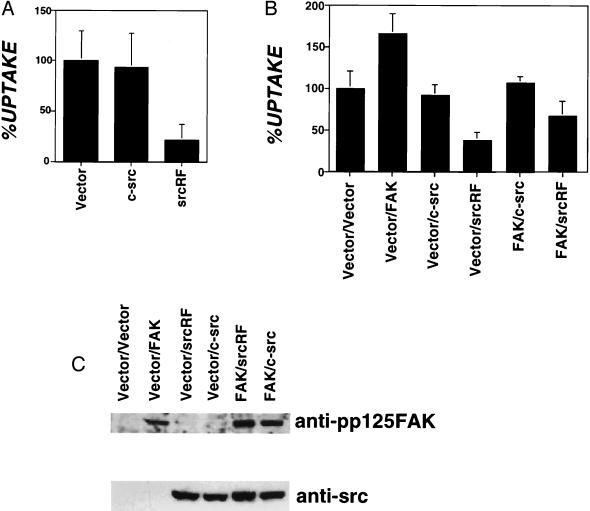
Bacterial internalization was determined for CEF cells overexpressing Src isoforms (Fig. 5A). Overexpression of an interfering form of Src that lacks kinase activity (SrcRF) lowered invasin-mediated uptake to levels seen with the interfering form of FAK (30% compared with vector transfected, Fig. 5A, srcRF). Although cells overexpressing full-length c-Src kinase consistently showed a moderate reduction in levels of uptake (80–90%) relative to vector-transfected cells (Fig. 5A, c-src), this effect did not seem statistically significant (P = 0.06). Bacterial binding assays performed with CEF cells transfected with either vector alone or cells overexpressing SrcRF indicated that the decreased bacterial uptake observed for SrcRF overexpressing cells was not caused by a decrease in bacterial binding (vector = 1.23 ± 0.53 vs. SrcRF = 1.21 ± 0.55 bacteria bound/cell).
Figure 5.
c-Src kinase is required for invasin-mediated uptake. (A) Kinase defective Src interferes with bacterial internalization. The amount of bacterial internalization was determined for CEF cells transfected with RCAS derivatives including vector alone (vector) or overexpressing c-Src kinase or kinase-defective Src (srcRF). %UPTAKE refers to the amount of internalization observed relative to that of the vector-transfected control. A representative experiment is shown with error bars indicating the SE for samples prepared in triplicate. (B) Src coexpression blocks FAK stimulation of uptake. Bacterial internalization was determined in CEF cells for vector-transfected cells or cells overexpressing mixtures of FAK or c-src derivatives. %UPTAKE refers to the amount of internalization observed relative to that of the vector-transfected control. Results are the mean of four independent experiments with error bars representing the SE for 13 samples. (C) Cell lysates of RCAS vector transfected and cells overexpressing FAK, c-Src, kinase-defective Src (srcRF), or cooverexpressing FAK and c-Src (FAK/c-Src), FAK and kinase-defective Src (FAK/srcRF) were immunoblotted with anti-FAK and anti-Src antibodies.
The above data suggest a model for invasin-mediated uptake in which FAK recruits a Src-family kinase to bind and phosphorylate substrate. To further examine the interaction of FAK and Src during uptake we examined the uptake characteristics of CEF cells coexpressing FAK and Src derivatives. Specifically, cells coexpressing wild-type FAK and either c-Src or SrcRF were assayed for the ability to internalize bacteria. Overexpression of SrcRF abolished the observed FAK stimulation of invasin-mediated uptake. Entry into the FAK/SrcRF coexpressing cells was at levels similar to those observed for cells that express SrcRF alone (Fig. 5B). Furthermore, coexpression of c-Src in the same cells as overexpressed FAK resulted in uptake levels similar to those observed for c-Src alone (Fig. 5B). Expression of FAK was not affected by coexpression of Src isoforms, and vice versa, as determined by immunoblot analysis (Fig. 5C). These data are consistent with a model of FAK/Src interaction in which a Src family member acts downstream of FAK to promote uptake.
DISCUSSION
Invasin-mediated bacterial internalization has been shown to require tight binding to β1 integrins as well as poorly defined subsequent integrin signaling events (2). In this paper we have presented evidence indicating that FAK and c-Src kinase are key players in the signaling process needed for high-efficiency invasin-mediated uptake.
Inhibition of either FAK or c-Src kinase via overexpression of dominant interfering isoforms inhibited bacterial internalization without effecting bacterial binding to eukaryotic cells, indicating a defect in either initiation or completion of the phagocytic cup. Furthermore, overexpression of FAK stimulated bacterial uptake, presumably enhancing the rate of delivery of a critical component to the nascent phagosome. This finding indicates a direct role for FAK in modulating the internalization efficiency of integrin-mediated uptake. Src kinase appears to be required for uptake, although the participation of other Src family kinases is equally likely (26).
The ability of the kinase-inactive variants to reverse the inhibition of uptake by FRNK, and the observed lack of interference by the kinase-inactive forms, is presumably the result of their retained ability to become tyrosine-phosphorylated (20). Lack of interference by such variants has been noted previously, as they fail to inhibit both cell spreading and cell migration (20, 25). In contrast, transfectants expressing the Tyr-397 autophosphorylation mutant show an overall decrease in FAK phosphorylation as well as that of the cytoskeletal protein paxillin (20), consistent with the uptake defect observed with this variant. The Tyr-397 variant had not been previously demonstrated to have a dominant interfering phenotype on either cell spreading or cell migration, although inhibition of integrin-mediated ERK2 stimulation by overexpression of a Tyr-397 mutant has been reported (28).
The exact nature of FAK and Src action on invasin-promoted uptake remains to be determined. Cells overexpressing FAK may have an increased disposition for bacterial uptake, perhaps stimulating phosphorylation of several key proteins, or increasing the mobility and availability of the integrin receptor in the membranes. This model, in which a large number of cytoskeletal factors throughout the cell would be activated, proposes that FAK enhances the phagocytic capability of the cell. An alternate mechanism, in which FAK acts directly in the uptake process, can be envisioned as well. In this scenario, FAK is recruited to the site of, or is activated by, invasin binding to the integrin receptor. This event then would lead to a cascade of signals at the site of the phagocytic cup, resulting in uptake. Although an increase in FAK phosphorylation in response to bacterial binding can be observed, it has been difficult to demonstrate that the direct result of this signal leads to internalization of bacteria (10, 11) (P. Dersch, M.A.A., and R.R.I., unpublished work).
The binding of FAK to c-Src in response to integrin engagement has been shown to result in the Src-dependent phosphorylation of the cytoskeletal components paxillin and p130CAS (27, 29). Concordantly, paxillin phosphorylation is reduced in cells expressing interfering variants of FAK and Src (18, 20). Therefore, the sites of action of FAK and a Src kinase family member in invasin-mediated uptake potentially occur upstream of the cytoskeletal rearrangements necessary for entry. Engagement of β1 integrins by invasin may lead to the recruitment and activation of pp125FAK and src-family kinases, followed by phosphorylation of signaling intermediates that are associated with actin rearrangements such as paxillin and p130CAS. In support of this model, phosphorylation of paxillin has been observed upon cellular challenge with bacteria expressing invasin (30), an event considered to be downstream of FAK action (27, 29).
The results in this paper provide identification of kinase molecules required for invasin-mediated uptake. Our data are consistent with previous reports implicating participation of FAK in uptake, which were based on the correlation of reduced bacterial uptake after dephosphorylation of several host cell proteins by YopH (10, 11). The major target of dephosphorylation in YopH-treated cells appears to be a signaling intermediate, p130CAS, whose phosphorylation and participation in the process of cellular migration depends on the actions of FAK and c-Src (31). Presumably, p130CAS lies downstream in a signal cascade that leads to invasin-promoted uptake. Further work should determine the specific role these proteins play in modulating invasin-promoted uptake.
Acknowledgments
We thank A. Richardson and J.T. Parsons for the kind gift of RCAS vectors, D. Ilic for the generous gift of the DU3 FAK knockout cell line, D. Schlaepfer for helpful discussion, and J. Solomon for critical reading of the manuscript. This work was supported by Grant AI23538 from the National Institute of Allergy and Infectious Diseases (to R.R.I.). M.A.A. was supported by Training Grant 5T32 A107422–5 from the National Institute of Allergy and Infectious Diseases, and R.R.I. is an Investigator of Howard Hughes Medical Institute.
ABBREVIATIONS
- FAK
focal adhesion kinase
- CEF
chicken embryo fibroblast
- MBP
maltose binding protein
- MOI
multiplicity of infection
References
- 1.Falkow S, Isberg R R, Portnoy D A. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 2.Isberg R R, Tran Van Nhieu G. Trends Microbiol. 1994;2:10–14. doi: 10.1016/0966-842x(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen L M, Hobbie S, Galan J E. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 4.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 5.Isberg R R, Leong J M. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 6.Marra A, Isberg R R. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark M A, Hirst B H, Jepson M A. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran Van Nhieu G, Krukonis E S, Reszka A A, Horwitz A F, Isberg R R. J Biol Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- 9.Rosenshine I, Duronio V, Finlay B B. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black D S, Bliska J B. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson C, Carballeira N, Wolf-Watz H, Fallman M. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrand J D, Schaller M D, Parsons J T. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaller M D, Otey C A, Hildebrand J D, Parsons J T. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto S, Akiyama S K, Yamada K M. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 15.Guan J L, Shalloway D. Nature (London) 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 16.Howe A, Aplin A E, Alahari S K, Juliano R L. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 17.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Nature (London) 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 18.Richardson A, Parsons T. Nature (London) 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 19.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 20.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong J M, Morrissey P E, Marra A, Isberg R R. EMBO J. 1995;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Merriam J J, Mueller J P, Isberg R R. Infect Immun. 1996;64:2483–2489. doi: 10.1128/iai.64.7.2483-2489.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong J M, Fournier R S, Isberg R R. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaller M D, Parsons J T. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 25.Cary L A, Chang J F, Guan J L. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 26.Cobb B S, Schaller M D, Leu T H, Parsons J T. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlaepfer D D, Broome M A, Hunter T. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlaepfer D D, Hunter T. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 29.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson K, Carballeira N, Magnusson K E, Persson C, Stendahl O, Wolf-Watz H, Fallman M. Mol Microbiol. 1996;20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 31.Cary L A, Han D C, Polte T R, Hanks S K, Guan J L. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]