Abstract
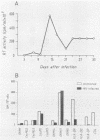
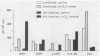
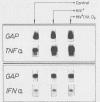
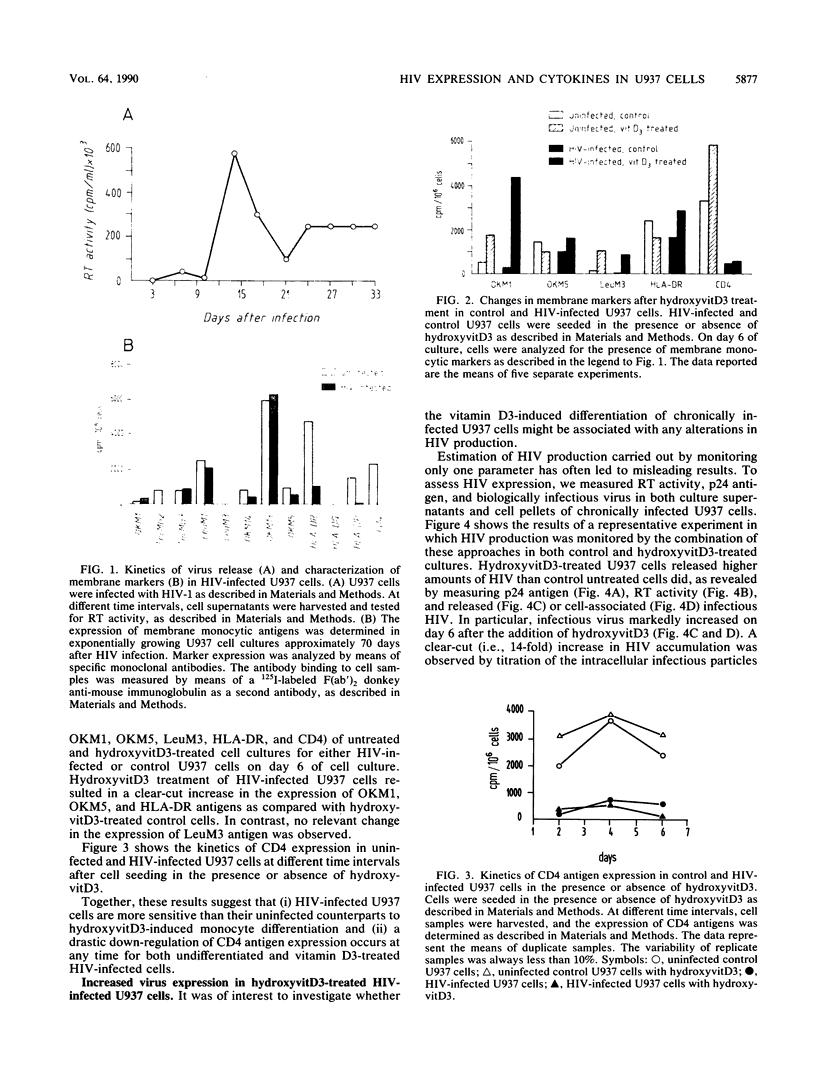
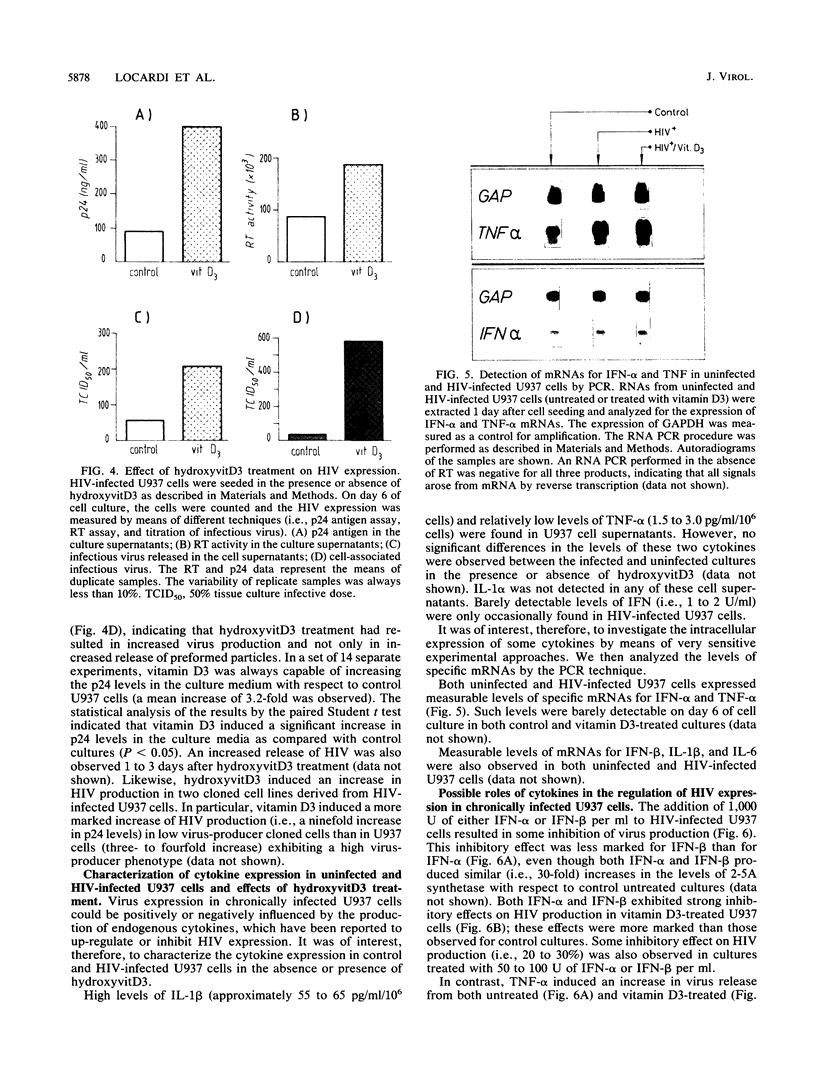
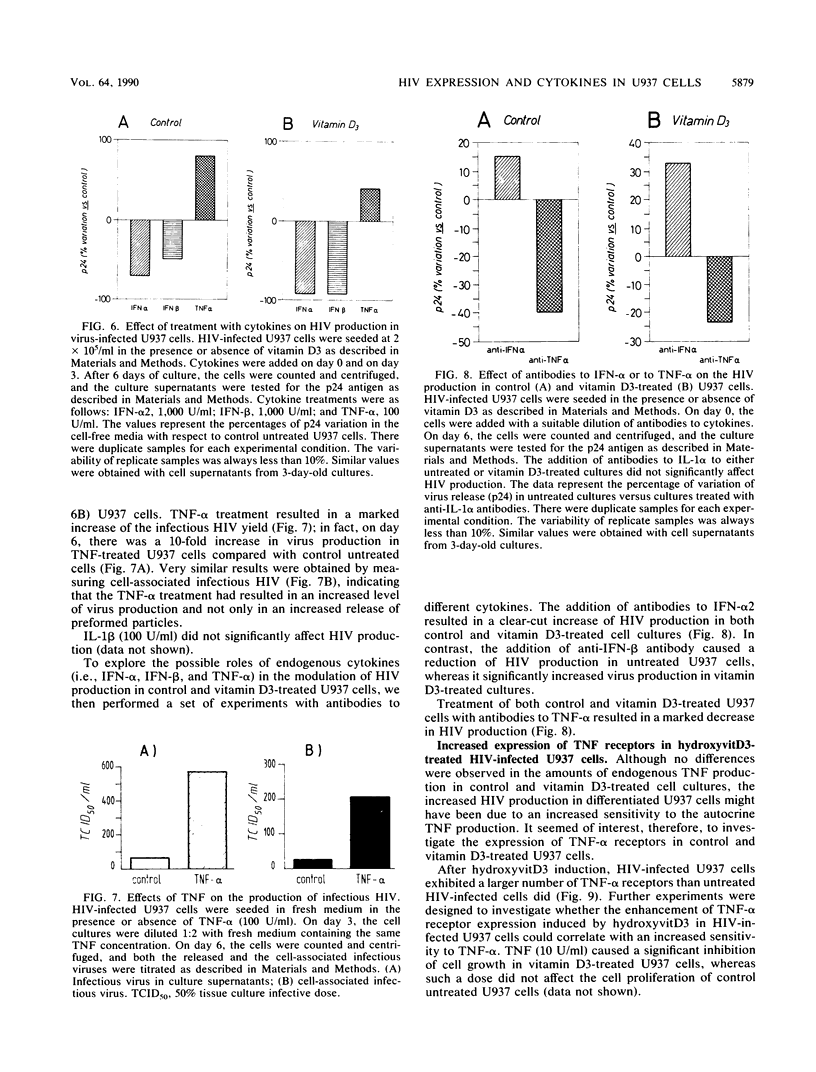
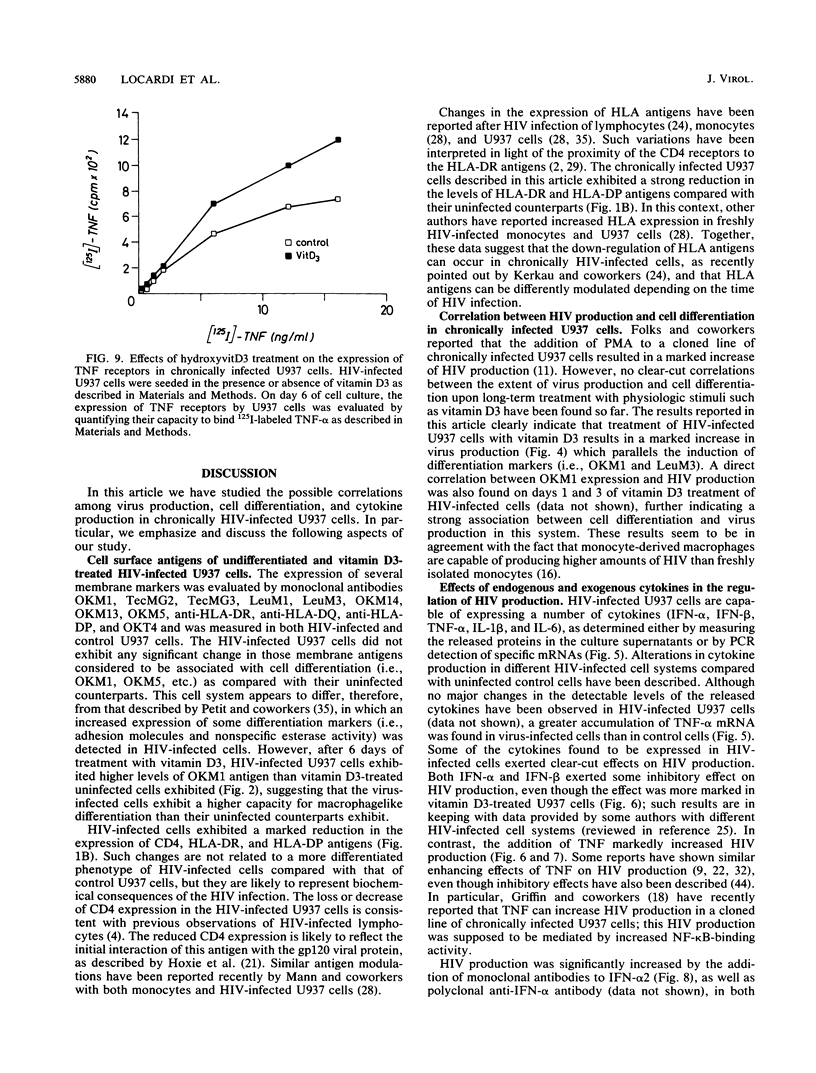
We have investigated the roles of cytokines in the modulation of human immunodeficiency virus (HIV) production in chronically infected U937 cells upon in vitro differentiation by hydroxyvitamin D3. HIV-infected U937 cells exhibited markedly lower levels of CD4 and HLA-DR antigens than uninfected cells did. Vitamin D3 induced a time-dependent macrophagelike differentiation, as determined by monitoring the expression of some surface antigens by means of the monoclonal antibodies OKM1, OKM5, OKM13, OKM14, OKT4, anti-HLA-DR, TecMG2, TecMG3, LeuM3, LeuM1, anti-HLA-DP, and anti-HLA-DQ. Treatment with hydroxyvitamin D3 resulted in a marked increase in HIV production compared with control cultures. Interleukin 1 beta (IL-1 beta) and tumor necrosis factor alpha (TNF-alpha) were detected in the culture media, whereas interferon (IFN) was not generally found. Using the polymerase chain reaction technique, we found HIV-infected U937 cells to express detectable levels of mRNAs for alpha interferon (IFN-alpha), IFN-beta, TNF-alpha, and IL-1 beta. The addition of TNF resulted in a marked increase of HIV production, whereas IL-1 beta was ineffective. In contrast, both IFN-alpha and IFN-beta exerted some inhibitory effect on HIV production, which was more marked in vitamin D3-treated cultures than in untreated cultures. HIV production was significantly increased by antibodies to IFN-alpha in both untreated and vitamin D3-treated cultures. Anti-IFN-beta antibody increased HIV production only in vitamin D3-treated cells. In contrast, anti-TNF-alpha antibodies markedly decreased HIV production in both control and differentiating U937 cells. Vitamin D3 treatment resulted in a higher expression of TNF receptors in differentiating cells than in control HIV-infected cells. These data demonstrate a strong correlation between HIV production and macrophagelike differentiation in chronically infected U937 cells and suggest that endogenous IFN and TNF exert opposite effects in the regulation of virus production in both undifferentiated and vitamin D3-treated cell cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chayt K. J., Harper M. E., Marselle L. M., Lewin E. B., Rose R. M., Oleske J. M., Epstein L. G., Wong-Staal F., Gallo R. C. Detection of HTLV-III RNA in lungs of patients with AIDS and pulmonary involvement. JAMA. 1986 Nov 7;256(17):2356–2359. [PubMed] [Google Scholar]
- Clayton L. K., Sieh M., Pious D. A., Reinherz E. L. Identification of human CD4 residues affecting class II MHC versus HIV-1 gp120 binding. Nature. 1989 Jun 15;339(6225):548–551. doi: 10.1038/339548a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Antonelli G., Capobianchi M. R., De Marco F. Replication of human immunodeficiency virus: yield of infectious virus under single growth cycle conditions. Arch Virol. 1988;103(1-2):127–131. doi: 10.1007/BF01319814. [DOI] [PubMed] [Google Scholar]
- Dodd R. C., Cohen M. S., Newman S. L., Gray T. K. Vitamin D metabolites change the phenotype of monoblastic U937 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7538–7541. doi: 10.1073/pnas.80.24.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Ferrantini M., Belardelli F., Locardi C. Role of endogenous alpha/beta interferon in the selection of virus nonproducer Friend leukemia cells after serial intraperitoneal passages in syngeneic mice. J Virol. 1988 Feb;62(2):600–605. doi: 10.1128/jvi.62.2.600-605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Schnittman S., Orenstein J., Poli G., Fauci A. S. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988 Feb 15;140(4):1117–1122. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Betts R. F., Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986 Nov 7;256(17):2365–2371. [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Ito M., Baba M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., De Clercq E. Tumor necrosis factor enhances replication of human immunodeficiency virus (HIV) in vitro. Biochem Biophys Res Commun. 1989 Jan 16;158(1):307–312. doi: 10.1016/s0006-291x(89)80213-x. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Mestan J., Mittnacht S., Dieffenbach C. W. Beta interferon subtype 1 induction by tumor necrosis factor. Mol Cell Biol. 1989 Jul;9(7):3037–3042. doi: 10.1128/mcb.9.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkau T., Schmitt-Landgraf R., Schimpl A., Wecker E. Downregulation of HLA class I antigens in HIV-1-infected cells. AIDS Res Hum Retroviruses. 1989 Dec;5(6):613–620. doi: 10.1089/aid.1989.5.613. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Macé K., Duc Dodon M., Gazzolo L. Restriction of HIV-1 replication in promonocytic cells: a role for IFN-alpha. Virology. 1989 Feb;168(2):399–405. doi: 10.1016/0042-6822(89)90282-1. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Gartner S., LeSane F., Blattner W. A., Popovic M. Cell surface antigens and function of monocytes and a monocyte-like cell line before and after infection with HIV. Clin Immunol Immunopathol. 1990 Feb;54(2):174–183. doi: 10.1016/0090-1229(90)90079-6. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Read-Connole E., Arthur L. O., Robey W. G., Wernet P., Schneider E. M., Blattner W. A., Popovic M. HLA-DR is involved in the HIV-1 binding site on cells expressing MHC class II antigens. J Immunol. 1988 Aug 15;141(4):1131–1136. [PubMed] [Google Scholar]
- Michaelis B., Levy J. A. HIV replication can be blocked by recombinant human interferon beta. AIDS. 1989 Jan;3(1):27–31. [PubMed] [Google Scholar]
- Michaelis B., Levy J. A. HIV replication can be blocked by recombinant human interferon beta. AIDS. 1989 Jan;3(1):27–31. [PubMed] [Google Scholar]
- Moscicki R. A., Amento E. P., Krane S. M., Kurnick J. T., Colvin R. B. Modulation of surface antigens of a human monocyte cell line, U937, during incubation with T lymphocyte-conditioned medium: detection of T4 antigen and its presence on normal blood monocytes. J Immunol. 1983 Aug;131(2):743–748. [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Galindo J., Richman D. D. Human immunodeficiency virus infection of monoblastoid cells: cellular differentiation determines the pattern of virus replication. J Virol. 1988 Oct;62(10):3558–3564. doi: 10.1128/jvi.62.10.3558-3564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Petit A. J., Terpstra F. G., Miedema F. Human immunodeficiency virus infection down-regulates HLA class II expression and induces differentiation in promonocytic U937 cells. J Clin Invest. 1987 Jun;79(6):1883–1889. doi: 10.1172/JCI113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Orenstein J. M., Kinter A., Folks T. M., Fauci A. S. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989 May 5;244(4904):575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Induction of expression of HIV in latently or chronically infected cells. AIDS Res Hum Retroviruses. 1989 Feb;5(1):1–4. doi: 10.1089/aid.1989.5.1. [DOI] [PubMed] [Google Scholar]
- Roy S., Wainberg M. A. Role of the mononuclear phagocyte system in the development of acquired immunodeficiency syndrome (AIDS). J Leukoc Biol. 1988 Jan;43(1):91–97. doi: 10.1002/jlb.43.1.91. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Stanton G. J., Langford M. P., Dianzani F. Virus yield-reduction assay for interferon by titration of Sindbis virus hemagglutinin. Methods Enzymol. 1981;78(Pt A):351–357. doi: 10.1016/0076-6879(81)78141-2. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Testa U., Ferbus D., Gabbianelli M., Pascucci B., Boccoli G., Louache F., Thang M. N. Effect of endogenous and exogenous interferons on the differentiation of human monocyte cell line U937. Cancer Res. 1988 Jan 1;48(1):82–88. [PubMed] [Google Scholar]
- Wong G. H., Krowka J. F., Stites D. P., Goeddel D. V. In vitro anti-human immunodeficiency virus activities of tumor necrosis factor-alpha and interferon-gamma. J Immunol. 1988 Jan 1;140(1):120–124. [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- Yarden A., Shure-Gottlieb H., Chebath J., Revel M., Kimchi A. Autogenous production of interferon-beta switches on HLA genes during differentiation of histiocytic lymphoma U937 cells. EMBO J. 1984 May;3(5):969–973. doi: 10.1002/j.1460-2075.1984.tb01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]