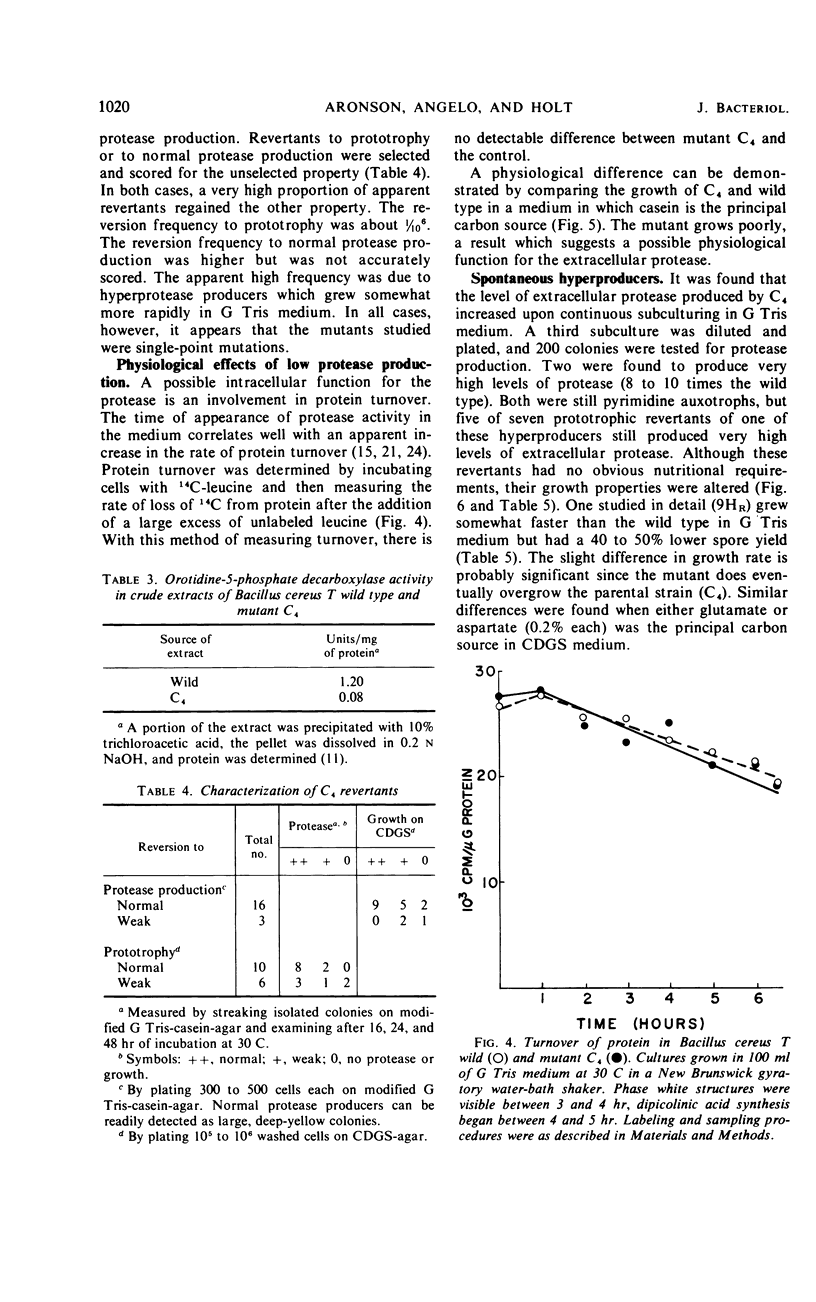
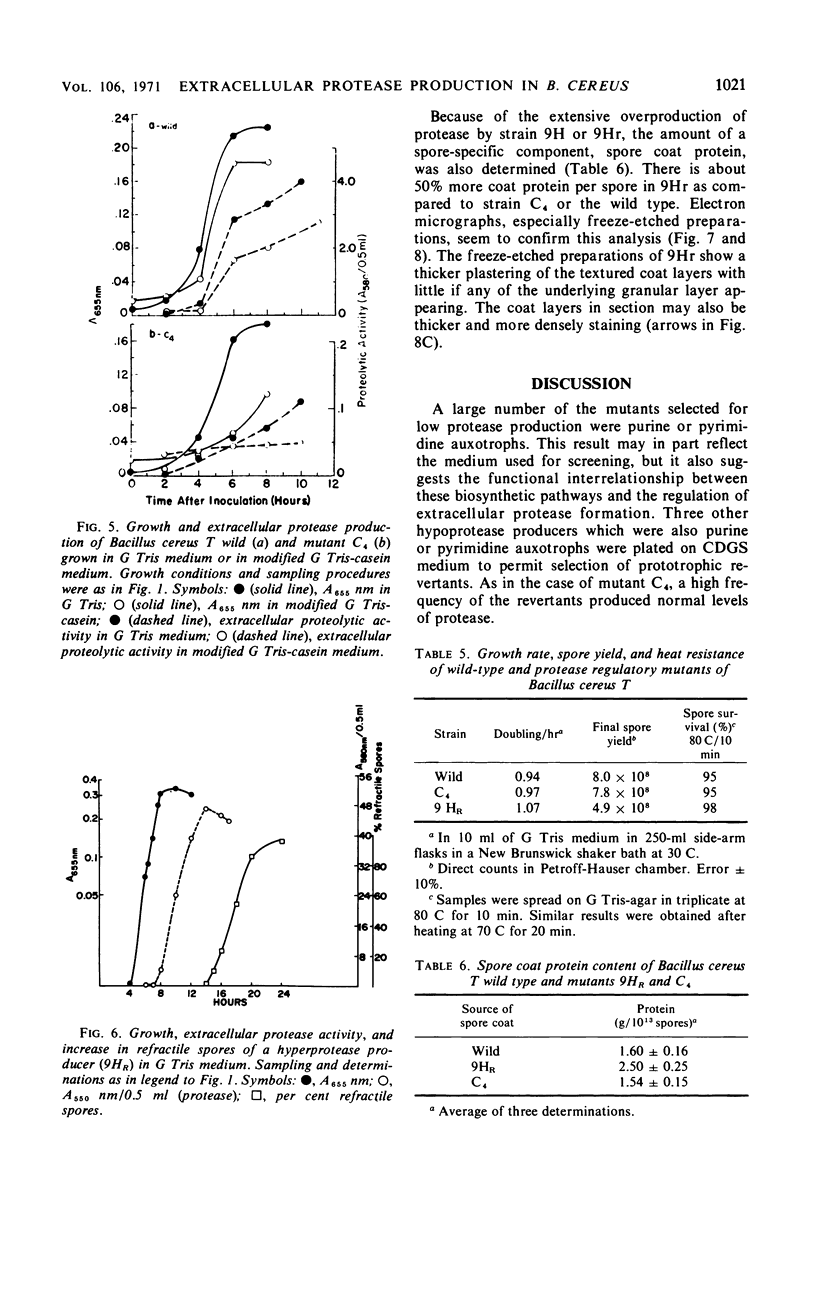
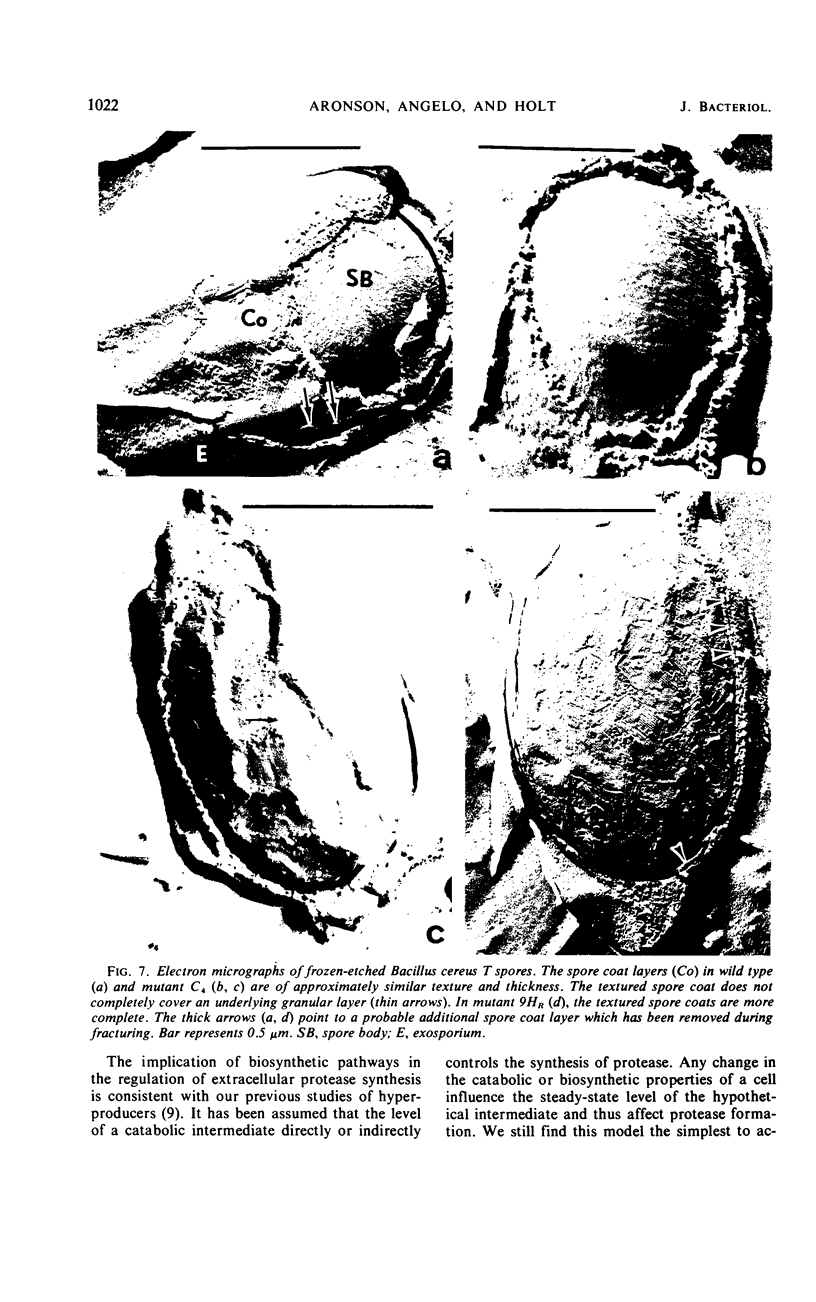
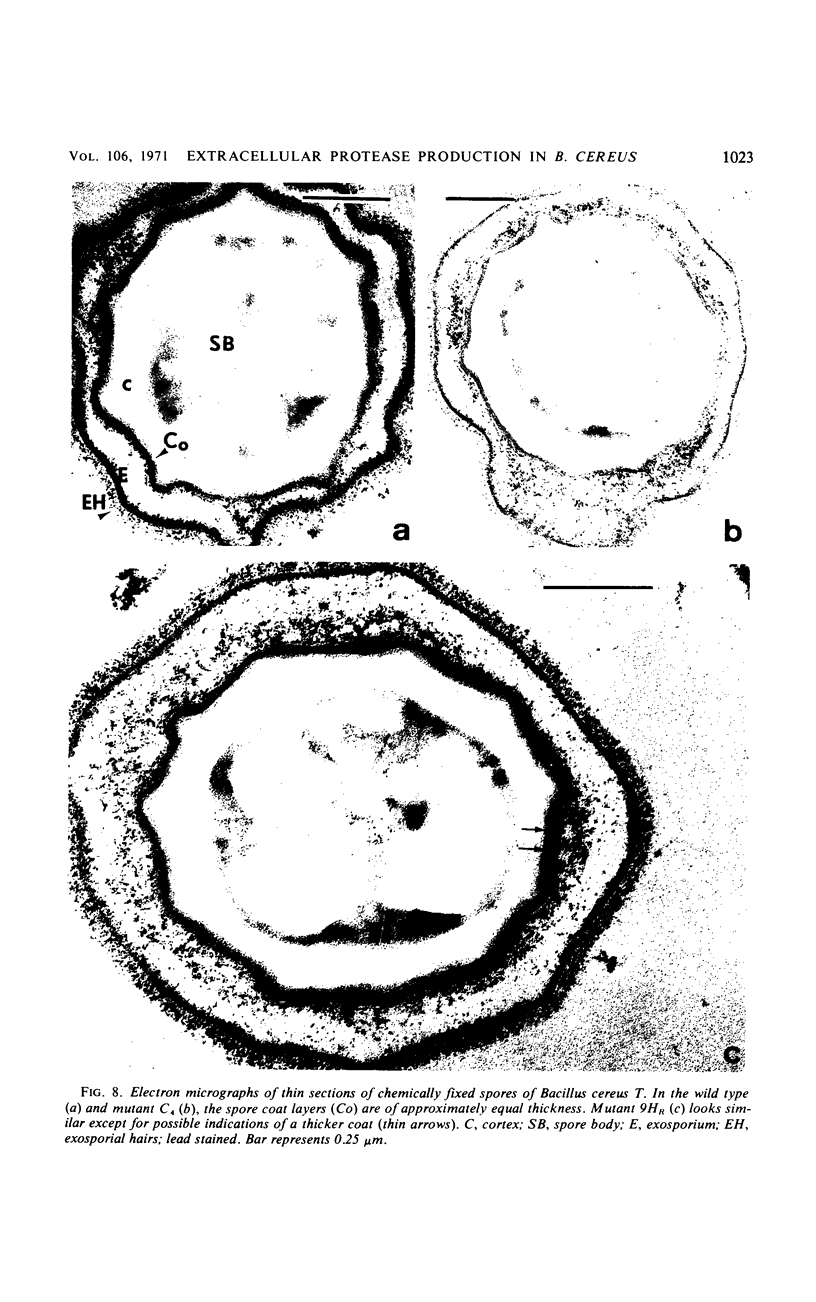
Abstract
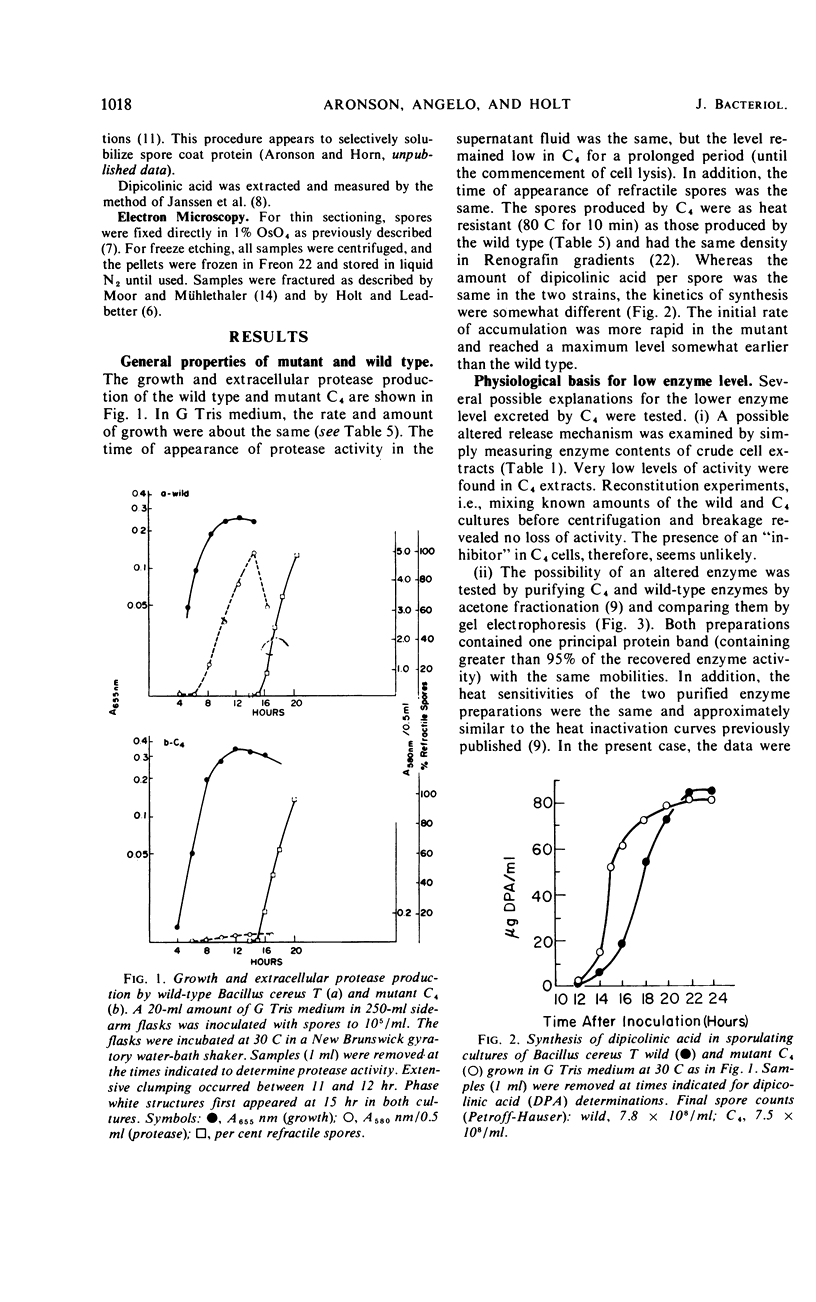
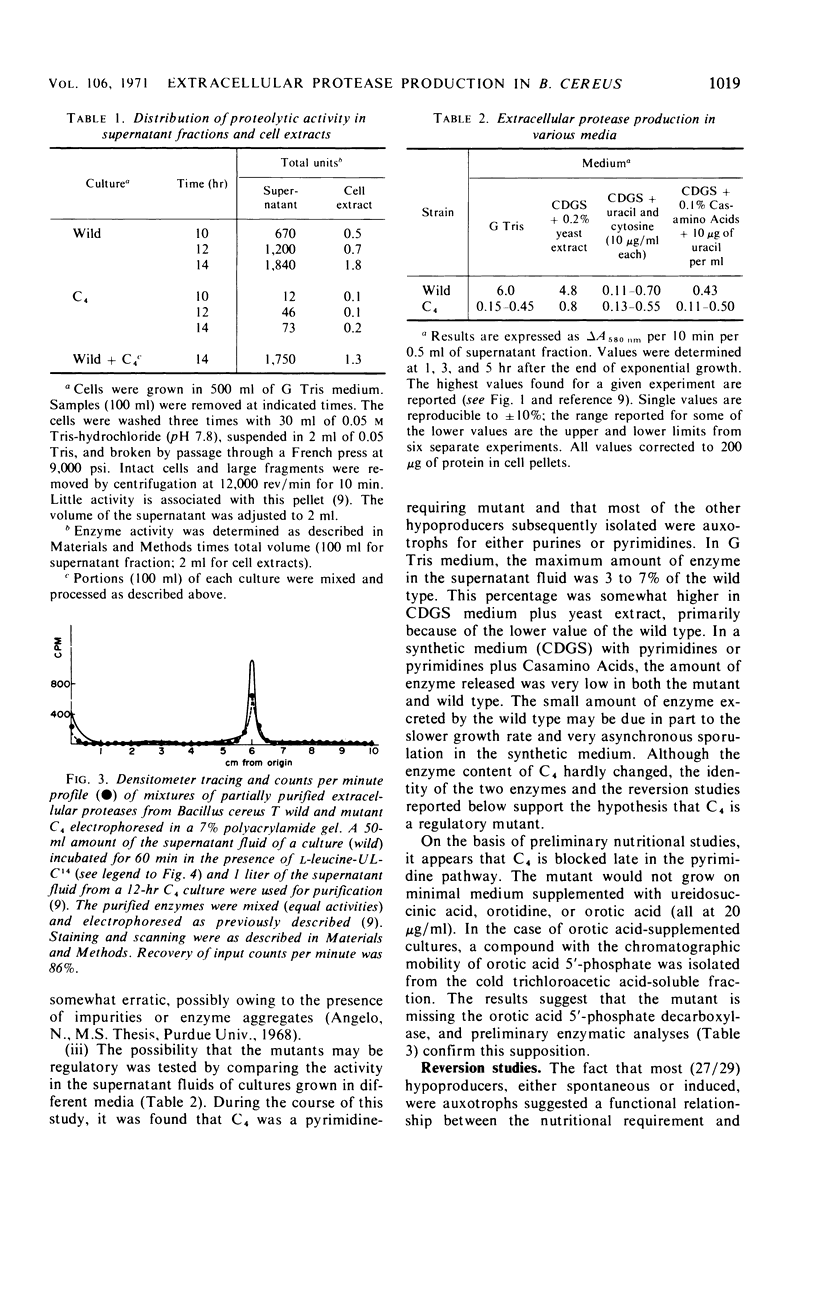
Twenty-nine mutants of Bacillus cereus T were selected on casein agar for their inability to produce large amounts of extracellular protease. They all formed spores, and 27 were also auxotrophs for purines or pyrimidines. Upon reversion to prototrophy, a large fraction regained the capacity to produce protease. Conversely, reversion to normal protease production resulted in loss of the purine or pyrimidine requirement in a large fraction of the revertants. One spontaneous low-protease-producing pyrimidine auxotroph studied in detail grew as well as the wild type and produced spores which were identical to those produced by the wild type on the basis of heat resistance, dipicolinic acid content, density, and appearance in the electron microscope. The rate of protein turnover in the mutant was the same as the wild type. The mutant did grow poorly, however, when casein was the principal carbon source. A mutant excreting 5 to 10 times as much protease as the wild type was isolated as a secondary mutation from the hypoproducer discussed above. Loss of the pyrimidine requirement in this case did not alter the regulation of protease production. Although the secondary mutant grew somewhat faster in most media than the wild type, the final cell yield was lower. The spores of this mutant appeared to have excess coat on the basis of both electron microscopic and chemical studies. There appear to be closely related but distinct catabolic controls for both extracellular protease and spore formation. These controls can be dissociated as for the hypoproducers but can also appear integrated as for the hyperprotease producer.
Full text
PDF



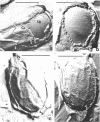
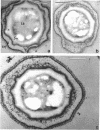
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Chaloupka J. Dual control of megateriopeptidase synthesis. Ann Inst Pasteur (Paris) 1969 Nov;117(5):631–636. [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Regulation of the formation of protease in Bacillus megaterium. I. The influence of amino acids on the enzyme formation. Folia Microbiol (Praha) 1966;11(2):82–88. doi: 10.1007/BF02878835. [DOI] [PubMed] [Google Scholar]
- Din F. U., Chaloupka J. Amino acid stimulation of proteinase synthesis in a sporogenous Bacillus megaterium KM. Biochem Biophys Res Commun. 1969 Oct 8;37(2):233–238. doi: 10.1016/0006-291x(69)90724-4. [DOI] [PubMed] [Google Scholar]
- GOLLAKOTA K. G., HALVORSON H. O. Biochemical changes occurring during sporulation of Bacillus cereus. Inhibition of sporulation by alpha-picolinic acid. J Bacteriol. 1960 Jan;79:1–8. doi: 10.1128/jb.79.1.1-8.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Fine structure of Sporocytophaga myxococcoides. Arch Mikrobiol. 1967 Jun 21;57(3):199–213. doi: 10.1007/BF00405947. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Trüper H. G., Takács B. J. Fine structure of Ectothiorhodospira mobilis strain 8113 thylakoids: chemical fixation and freeze-etching studies. Arch Mikrobiol. 1968;62(2):111–128. doi: 10.1007/BF00410398. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A., SIMMS E. S. Enzymatic synthesis of pyrimidine nucleotides; orotidine-5'-phosphate and uridine-5'-phosphate. J Biol Chem. 1955 Jul;215(1):403–451. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levisohn S., Aronson A. I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967 Mar;93(3):1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONRO R. E. Protein turnover and the formation of protein inclusions during sporulation of Bacillus thuringiensis. Biochem J. 1961 Nov;81:225–232. doi: 10.1042/bj0810225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J., Aubert J. P. Etude de la mégatériopeptidase, protéase exocellulaire de Bacillus megaterium. 3. Biosynthèse et rôle physiologique. Ann Inst Pasteur (Paris) 1969 Oct;117(4):461–473. [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKERSON W. J., NOVAL J. J., ROBISON R. S. KERATINASE. I. PROPERTIES OF THE ENZYME CONJUGATED ELABORATED BY STREPTOMYCES FRADIAE. Biochim Biophys Acta. 1963 Sep 3;77:73–86. doi: 10.1016/0006-3002(63)90470-0. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]
- Tamir H., Gilvarg C. Density gradient centrifugation for the separation of sporulating forms of bacteria. J Biol Chem. 1966 Mar 10;241(5):1085–1090. [PubMed] [Google Scholar]
- URBA R. C. Protein breakdown in Bacillus cereus. Biochem J. 1959 Mar;71(3):513–518. doi: 10.1042/bj0710513. [DOI] [PMC free article] [PubMed] [Google Scholar]