Abstract
Infection of human erythroleukemic K562 cells by encephalomyocarditis virus readily resulted in establishment of persistently infected cultures. In contrast to the usual typical lytic infection by encephalomyocarditis virus, in which trypan blue staining of cells reaches close to 100% by about 15 h postinfection, K562 cell cultures required 3 to 4 days postinfection to reach a maximum of about 80 to 90% cell staining. The proportion of K562 cells taking up stain gradually decreased to about 10% of those present by about 13 days postinfection; during this time, virus yield per day measured by either plaque or hemagglutination titration fell about 10-fold. The decrease in percent staining was followed by waves of increased staining accompanied by increased virus production. Virus-producing cultures were maintained for over 3 months. Evolution of both virus and cells accompanied establishment of persistence in that plaque size changed from about 7 mm in diameter for the original virus to less than 1.5 mm by day 20 postinfection and most of the cells cloned from persistently infected cultures were resistant to superinfection with the original virus. Resistance was due, at least in part, to reduced virus attachment in that binding of 3H-labeled virus to cloned resistant cells was about 2% of that to uninfected cells.
Full text
PDF
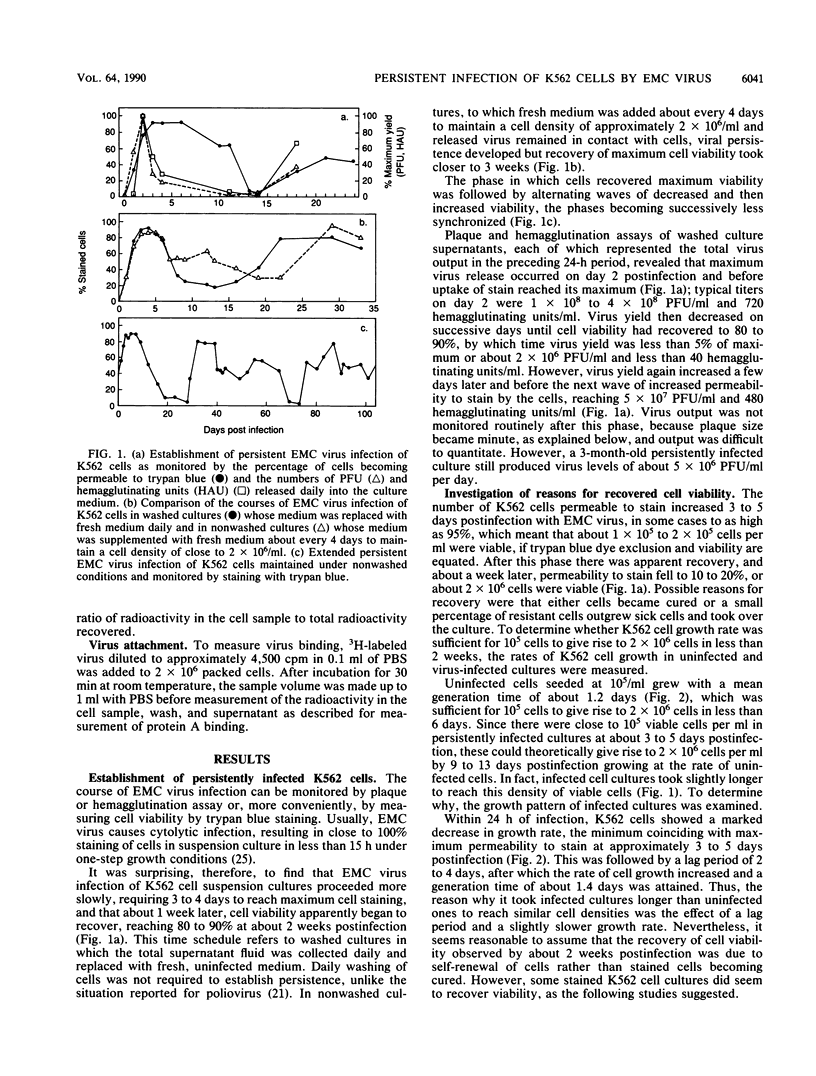
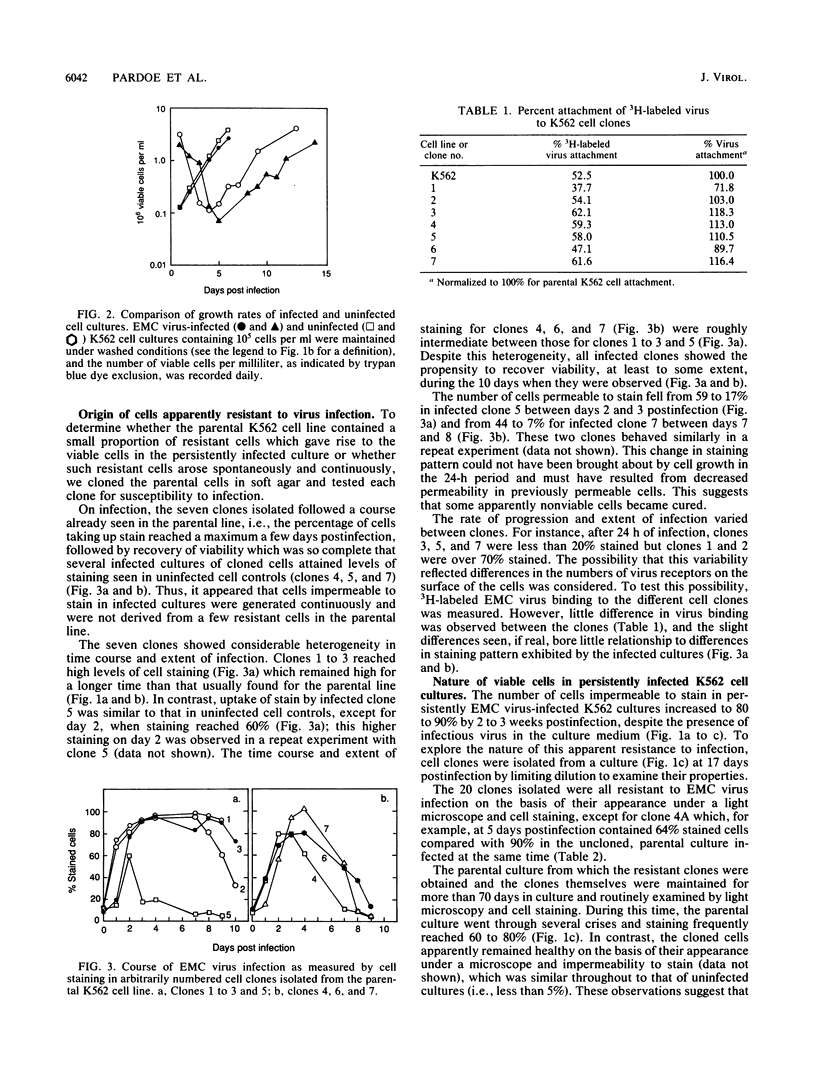


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., KURTZ H. Observations concerning a persisting infection of HeLa cells with poliomyelitis virus. J Exp Med. 1955 Nov 1;102(5):555–565. doi: 10.1084/jem.102.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Allaway G. P., Burness A. T. Site of attachment of encephalomyocarditis virus on human erythrocytes. J Virol. 1986 Sep;59(3):768–770. doi: 10.1128/jvi.59.3.768-770.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness A. T., Fox S. M., Pardoe I. U. The polypeptide composition of the encephalomyocarditis virus particle. J Gen Virol. 1974 Jun;23(3):225–236. doi: 10.1099/0022-1317-23-3-225. [DOI] [PubMed] [Google Scholar]
- Cao Y., Schnurr D. P. Persistent infection of YAC-1 cells by coxsackievirus B3. J Gen Virol. 1988 Jan;69(Pt 1):59–65. doi: 10.1099/0022-1317-69-1-59. [DOI] [PubMed] [Google Scholar]
- Cash E., Chamorro M., Brahic M. Theiler's virus RNA and protein synthesis in the central nervous system of demyelinating mice. Virology. 1985 Jul 15;144(1):290–294. doi: 10.1016/0042-6822(85)90327-7. [DOI] [PubMed] [Google Scholar]
- Cernescu C. Persistent virus infections in the human myeloid K-562 cell line: genetic implications and practical consequences. Virologie. 1982 Oct-Dec;33(4):257–265. [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINTER Z., PHILIPSON L., WESSLEN T. Persistent foot-and-mouth disease infections of cells in tissue culture. Virology. 1959 Aug;8:542–544. doi: 10.1016/0042-6822(59)90060-1. [DOI] [PubMed] [Google Scholar]
- Fujii N., Oguma K., Kimura K., Yamashita T., Ishida S., Fujinaga K., Yashiki T. Oligo-2',5'-adenylate synthetase activity in K562 cell lines persistently infected with measles or mumps virus. J Gen Virol. 1988 Aug;69(Pt 8):2085–2091. doi: 10.1099/0022-1317-69-8-2085. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Andersson L. C. Expression of the major red cell sialoglycoprotein, glycophorin A, in the human leukemic cell line K562. J Biol Chem. 1979 Aug 10;254(15):7442–7448. [PubMed] [Google Scholar]
- Gebauer F., de la Torre J. C., Gomes I., Mateu M. G., Barahona H., Tiraboschi B., Bergmann I., de Mello P. A., Domingo E. Rapid selection of genetic and antigenic variants of foot-and-mouth disease virus during persistence in cattle. J Virol. 1988 Jun;62(6):2041–2049. doi: 10.1128/jvi.62.6.2041-2049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. P., Righthand V. F. Persistence of echovirus 6 in cloned human cells. J Virol. 1985 Apr;54(1):219–223. doi: 10.1128/jvi.54.1.219-223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Levy A., Racaniello V. R. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J Virol. 1989 Jan;63(1):43–51. doi: 10.1128/jvi.63.1.43-51.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Maverakis N. H., Schmidt N. J., Riggs J. L., Lennette E. H. Carrier cultures of human fetal diploid cells infected with coxsackievirus type B2. Arch Gesamte Virusforsch. 1973;43(4):289–296. doi: 10.1007/BF01556144. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Viral persistence. Cell. 1989 Feb 24;56(4):517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- Roesing T. G., Landau B. J., Crowell R. L. Limited persistence of viral antigen in coxsackievirus B3 induced heart disease in mice. Proc Soc Exp Biol Med. 1979 Mar;160(3):382–386. doi: 10.3181/00379727-160-40455. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Green J., Ehrenfeld E. Characterization of a cell culture persistently infected with the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1982 Sep;43(3):1118–1122. doi: 10.1128/jvi.43.3.1118-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhon E. J., Wilson A. K., Jubelt B. Characterization of genetic changes occurring in attenuated poliovirus 2 during persistent infection in mouse central nervous systems. J Virol. 1984 Apr;50(1):137–144. doi: 10.1128/jvi.50.1.137-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERS F. K., HUPPERT J., HOSKINS J. M. Replication of an animal virus. Symp Soc Exp Biol. 1958;12:123–137. [PubMed] [Google Scholar]
- Taber R., Alexander V., Wald N., Jr The selection of virus-resistant Chinese hamster ovary cells. Cell. 1976 Aug;8(4):529–533. doi: 10.1016/0092-8674(76)90221-x. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Dávila M., Sobrino F., Ortín J., Domingo E. Establishment of cell lines persistently infected with foot-and-mouth disease virus. Virology. 1985 Aug;145(1):24–35. doi: 10.1016/0042-6822(85)90198-9. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Díez J., Domingo E. Extensive cell heterogeneity during persistent infection with foot-and-mouth disease virus. J Virol. 1989 Jan;63(1):59–63. doi: 10.1128/jvi.63.1.59-63.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


