Abstract
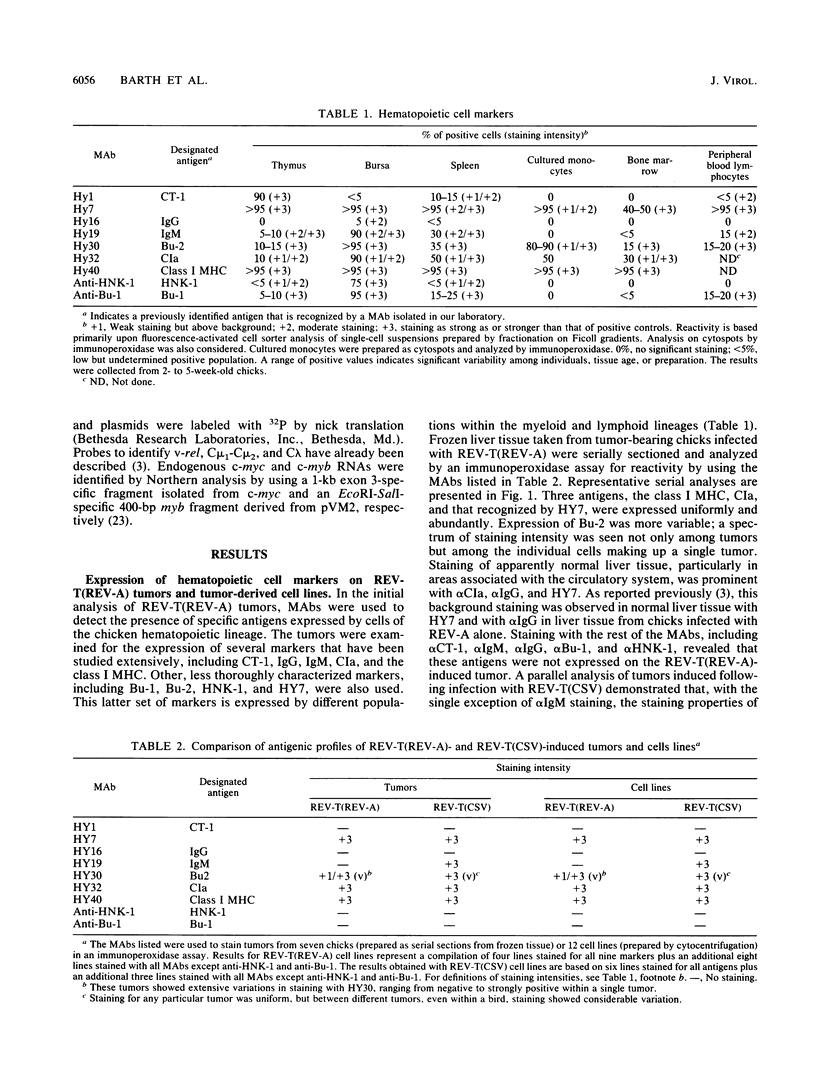
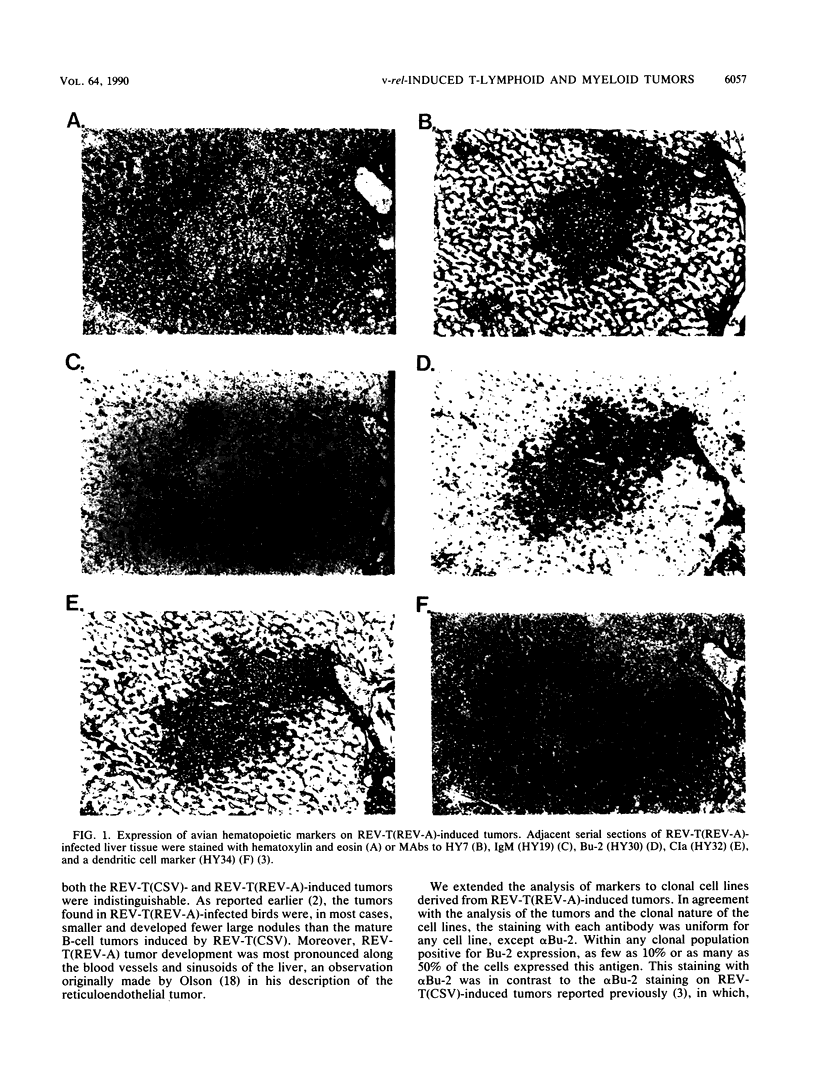
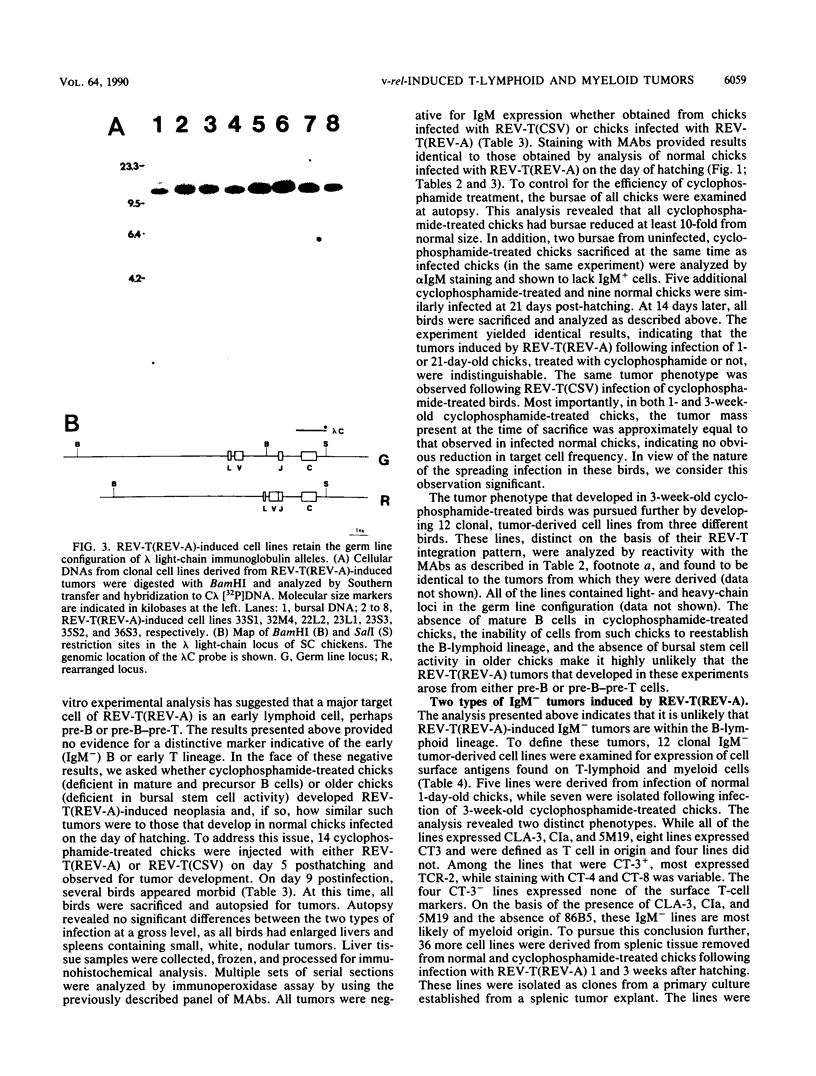
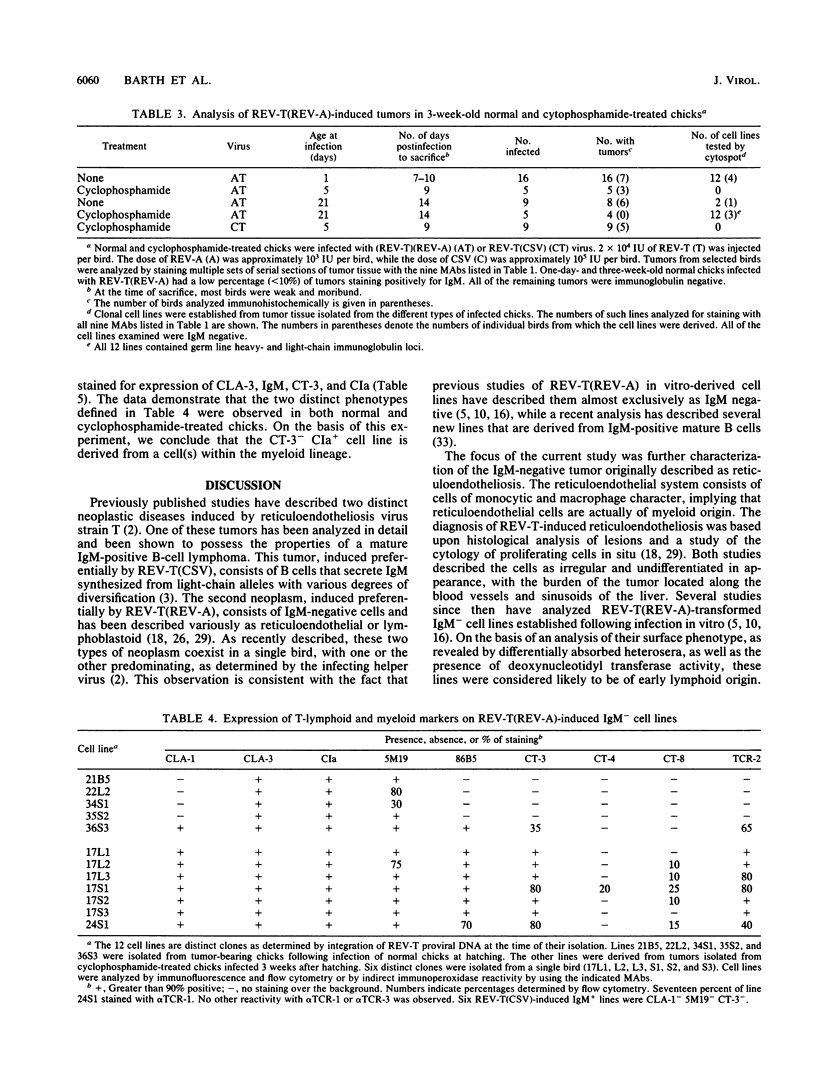
Infection of 1-day-old chicks with reticuloendotheliosis virus strain T induces a neoplastic disease that kills the chicks 7 to 14 days postinfection. In association with reticuloendotheliosis-associated virus (REV-A), reticuloendotheliosis virus T (REV-T) induces tumors that are predominantly immunoglobulin M (IgM) negative. We examined a variety of REV-T(REV-A)-induced tumors and tumor-derived cell lines and concluded that the principal IgM-negative tumors that develop in REV-T(REV-A)-infected chicks are neither pre-B or pre-B-pre-T but rather mature T lymphoid and myeloid. Without exception, the immunoglobulin heavy- and light-chain loci were in germ line configuration. Furthermore, the cell lines expressed neither sterile transcripts of the heavy- or light-chain immunoglobulin genes nor elevated levels of c-myb, two characteristics associated with murine pre-B lymphomas. Cell lines were also examined by using monoclonal antibodies for expression of a variety of cell surface markers expressed on B lymphocytes and/or T lymphocytes and/or myeloid cells. These reagents defined two types of IgM-negative tumor cell lines, one CIa+ CT-3+ (T lymphoid) and the other CIa+ CT-3-. By using the same approaches, tumor development was examined following REV-T(REV-A) infection at 1 and 3 weeks post-hatching of cyclophosphamide-treated chicks shown to be devoid of B-lymphoid cells. Again, the tumors that developed were either CIa+ CT-3+ (T lymphoid) or CIa+ CT-3-. Furthermore, the frequency and rate with which IgM-negative tumors developed in cyclophosphamide-treated chicks were not different from those observed in normal chicks. In 3-week-old cyclophosphamide-treated chicks, the presence of CIa+ CT-3- tumors bearing hematopoietic lineage markers, such as CLA-3 and 5M19, are most likely to have been derived from cells within the myeloid lineage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba T. W., Humphries E. H. Avian leukosis virus infection: analysis of viremia and DNA integration in susceptible and resistant chicken lines. J Virol. 1984 Jul;51(1):123–130. doi: 10.1128/jvi.51.1.123-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C. F., Humphries E. H. A nonimmunosuppressive helper virus allows high efficiency induction of B cell lymphomas by reticuloendotheliosis virus strain T. J Exp Med. 1988 Jan 1;167(1):89–108. doi: 10.1084/jem.167.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender T. P., Kuehl W. M. Differential expression of the c-myb proto-oncogene marks the pre-B cell/B cell junction in murine B lymphoid tumors. J Immunol. 1987 Dec 1;139(11):3822–3827. [PubMed] [Google Scholar]
- Beug H., Müller H., Grieser S., Doederlein G., Graf T. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981 Dec;115(2):295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Chan M. M., Chen C. L., Ager L. L., Cooper M. D. Identification of the avian homologues of mammalian CD4 and CD8 antigens. J Immunol. 1988 Apr 1;140(7):2133–2138. [PubMed] [Google Scholar]
- Chen C. L., Ager L. L., Gartland G. L., Cooper M. D. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986 Jul 1;164(1):375–380. doi: 10.1084/jem.164.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Cihak J., Lösch U., Cooper M. D. Differential expression of two T cell receptors, TcR1 and TcR2, on chicken lymphocytes. Eur J Immunol. 1988 Apr;18(4):539–543. doi: 10.1002/eji.1830180408. [DOI] [PubMed] [Google Scholar]
- Chen L., Lim M. Y., Bose H., Jr, Bishop J. M. Rearrangements of chicken immunoglobulin genes in lymphoid cells transformed by the avian retroviral oncogene v-rel. Proc Natl Acad Sci U S A. 1988 Jan;85(2):549–553. doi: 10.1073/pnas.85.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Huffnagle G. B., Ratcliffe M. J., Humphries E. H. Bu-2, a novel avian cell surface antigen on B cells and a population of non-lymphoid cells, is expressed homogeneously in germinal centers. Hybridoma. 1989 Dec;8(6):589–604. doi: 10.1089/hyb.1989.8.589. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Allen R., Glover C. Clonal analysis of the integration and expression of endogenous avian retroviral DNA acquired by exogenous viral infection. J Virol. 1981 Aug;39(2):584–596. doi: 10.1128/jvi.39.2.584-596.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L. H., Rufner R., Sevoian M. Isolation and development of a reticuloendotheliosis virus-transformed lymphoblastoid cell line from chicken spleen cells. Infect Immun. 1979 Aug;25(2):694–701. doi: 10.1128/iai.25.2.694-701.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. B., McClure J., Rup B., Niesel D. W., Garry R. F., Hoelzer J. D., Nazerian K., Bose H. R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981 Aug;25(2):421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Mussman H. C., Twiehaus M. J. Pathogenesis of reticuloendothelial virus disease in chicks--an acute runting syndrome. Avian Dis. 1971 Jul-Sep;15(3):483–502. [PubMed] [Google Scholar]
- Olson L. D. Histopathologic and hematologic changes in moribund stages of chicks infected with T-virus. Am J Vet Res. 1967 Sep;28(126):1501–1507. [PubMed] [Google Scholar]
- Olson W. C., Ewert D. L. Markers of B lymphocyte differentiation in the chicken. Hybridoma. 1990 Aug;9(4):331–350. doi: 10.1089/hyb.1990.9.331. [DOI] [PubMed] [Google Scholar]
- Peault B., Chen C. L., Cooper M. D., Barbu M., Lipinski M., Le Douarin N. M. Phylogenetically conserved antigen on nerve cells and lymphocytes resembles myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1987 Feb;84(3):814–818. doi: 10.1073/pnas.84.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Pizer E., Humphries E. H. RAV-1 insertional mutagenesis: disruption of the c-myb locus and development of avian B-cell lymphomas. J Virol. 1989 Apr;63(4):1630–1640. doi: 10.1128/jvi.63.4.1630-1640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rup B. J., Spence J. L., Hoelzer J. D., Lewis R. B., Carpenter C. R., Rubin A. S., Bose H. R., Jr Immunosuppression induced by avian reticuloendotheliosis virus: mechanism of induction of the suppressor cell. J Immunol. 1979 Sep;123(3):1362–1370. [PubMed] [Google Scholar]
- Scofield V. L., Bose H. R., Jr Depression of mitogen response in spleen cells from reticuloendotheliosis virus-infected chickens and their suppressive effect on normal lymphocyte response. J Immunol. 1978 Apr;120(4):1321–1325. [PubMed] [Google Scholar]
- Sowder J. T., Chen C. L., Ager L. L., Chan M. M., Cooper M. D. A large subpopulation of avian T cells express a homologue of the mammalian T gamma/delta receptor. J Exp Med. 1988 Feb 1;167(2):315–322. doi: 10.1084/jem.167.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Zeigel R. F., Twiehaus M. J. Biological studies with RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quail. J Natl Cancer Inst. 1966 Dec;37(6):731–743. [PubMed] [Google Scholar]
- Veromaa T., Vainio O., Eerola E., Toivanen P. Monoclonal antibodies against chicken Bu-1a and Bu-1b alloantigens. Hybridoma. 1988 Feb;7(1):41–48. doi: 10.1089/hyb.1988.7.41. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Lee L. F., Bacon L. D., Smith E. J. Depression of vaccinal immunity to Marek's disease by infection with reticuloendotheliosis virus. Infect Immun. 1979 Oct;26(1):90–98. doi: 10.1128/iai.26.1.90-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Lai M. M. Avian reticuloendotheliosis virus contains a new class of oncogene of turkey origin. Virology. 1981 May;111(1):289–293. doi: 10.1016/0042-6822(81)90674-7. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Bargmann W., Bose H. R., Jr Rearrangement and diversification of immunoglobulin light-chain genes in lymphoid cells transformed by reticuloendotheliosis virus. Mol Cell Biol. 1989 Nov;9(11):4970–4976. doi: 10.1128/mcb.9.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]






