Abstract
The finding that ADP-ribosylation factor (ARF) can activate phospholipase D has led to debate as to whether ARF recruits coat proteins through direct binding or indirectly by catalytically increasing phosphatidic acid production. Here we test critical aspects of these hypotheses. We find that Golgi membrane phosphatidic acid levels do not rise—in fact they decline—during cell-free budding reactions. We confirm that the level of membrane-bound ARF can be substantially reduced without compromising coat assembly [Ktistakis, N. T., Brown, H. A., Waters, M. G., Sternweis, P. C. & Roth, M. G. (1996) J. Cell Biol. 134, 295–306], but find that under all conditions, ARF is present on the Golgi membrane in molar excess over bound coatomer. These results do not support the possibility that the activation of coat assembly by ARF is purely catalytic, and they are consistent with ARF forming direct interactions with coatomer. We suggest that ARF, like many other G proteins, is a multifunctional protein with roles in trafficking and phospholipid signaling.
The ADP-ribosylation factor (ARF) family of small GTPases plays a key role in triggering the assembly of coated vesicles (1, 2). Once activated by a nucleotide exchange factor (3, 4), these proteins exchange GTP for GDP and translocate from the cytosol onto the membrane. The presence of ARF-GTP on the membrane leads to the subsequent assembly of the vesicle coat, and hydrolysis of bound GTP results in uncoating before fusion. Myristoylated ARF1 and the coat protein, coatomer, are the principal components of coat protomer I (COPI)-coated vesicles, that bud from Golgi membranes (5, 6). Indeed, ARF1 and the coatomer are the only cystosolic proteins required for the formation of functional COPI-coated vesicles (7, 8).
The above findings together with the facts that first, ARF binding precedes and is necessary for coatomer binding (9, 10), second, ARF binding leads to the binding of stoichiometric quantities of coatomer (10), and third, coatomer and ARF are clustered together in coated buds (5, 7), have led to the model that during coat assembly coatomer binds directly to ARF. The membrane-bound coatomers then self-assemble into coats, driving budding in the process (6, 11). More recent experiments demonstrating a direct interaction between ARF and the β-COP subunit of coatomer strongly support this mechanism for vesicle assembly (12). This model has become standard and is generally applicable as has been demonstrated for the assembly of AP1/clathrin-coated vesicles (13, 14) and COPII-coated vesicles (15).
ARF in its GTP-bound form is also an activator of the phospholipid hydrolytic enzyme phospholipase D (PLD), which cleaves phosphatidylcholine (PC) to release choline thereby producing phosphatidic acid (PA) (16, 17). Isoforms of PLD are localized to the Golgi apparatus (18), and PLD has been suggested to facilitate vesicle formation from the Golgi apparatus, the endoplasmic reticulum (ER), the trans-Golgi network, and endosomes (19–23).
Other data that on the face of it suggest that COPI-coated vesicles can bud without ARF, either when Golgi membranes are preincubated with ARF, which is then largely removed before adding coatomer in two-stage-budding reactions, or when Golgi membranes from PtK1 cells (which have very high basal PLD activity) are used, led to the proposal of an alternative model for coat assembly in which ARF has a purely catalytic role (19, 20). In this model, ARF activates PLD, which then hydrolyzes PC, leading to an increase in PA levels in the membrane. After that, according to this model, ARF is no longer necessary because increased PA levels are proposed to directly (or indirectly) result in coatomer binding without the participation of ARF.
A prediction of the standard model is that ARF levels on the membrane must be equal to or greater than coatomer levels on a molar basis under all conditions. An equally clear prediction of the PLD model is that the level of Golgi PA should increase in the presence of ARF-GTP and that sub-stoichiometric levels of ARF should be sufficient for coat assembly. To distinguish between these models, we have reexamined the stoichiometry of ARF and coatomer in the Golgi membranes during COPI-coat assembly by using an anti-ARF antibody while carefully establishing the lower detection limits with this antibody and have directly measured the level of PA in Golgi.
MATERIALS AND METHODS
ARF and Coatomer-Binding Assay.
Recombinant myristoylated ARF1 was prepared by coexpression in E. coli with N-myristoyl transferase (24) as described in Weiss et al. (25) and Helms et al. (26). Coatomer was purified as previously described in Waters et al. (27). Golgi membranes were isolated from rat liver as described in Malhotra et al. (28). The final binding reaction conditions were 25 mM Hepes (pH 7.2), 2.5 mM magnesium acetate, 15 mM potassium chloride, and 0.2 M sucrose, and the reaction volume was 50 μl for all reaction stages. For multistage reactions, the Golgi membranes were reisolated by centrifugation at 15,000 × g for 20 min. Coatomer (15 μg/ml), ARF (12 μg/ml), and GTPγS (50 μM) were added to each reaction stage as indicated in the figure legends. Unless otherwise indicated, the incubation time was 15 min and the reaction temperature was 37°C. After the final incubation, the reaction mixture was layered onto a 150-μl sucrose cushion (15% wt/wt sucrose/25 mM Hepes, pH 7.2/2.5 mM magnesium acetate) in 0.5-ml tubes and the membranes were pelleted by centrifugation at 15,000 × g for 30 min at 4°C in a refrigerated microfuge. The membrane pellets were resuspended in Laemmli sample buffer and analyzed by Western blotting.
Western Blotting.
Proteins were fractionated by using 12% SDS/PAGE and blotted onto nitrocellulose by using standard protocol for the Bio-Rad minigel and blotting apparatuses. After the transfer, the membranes were blocked and incubated with an anti β-COP mAb, M3A5 (29), and an anti-ARF antibody 2048 (10), affinity purified from rabbit sera. The signal was visualized by using horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) and ECL (Amersham). The signals were quantitated by using densitometry.
PA Levels in Golgi Fractions.
CHO cells were grown in suspension to a density of 3 × 105 cells/ml in 1 liter of media (MEMα plus 10% fetal calf serum). The cells were washed one time with low phosphate Krebs-Ringer’s solution and resuspended in 250 ml of low phosphate Krebs-Ringer’s solution containing 100 μM sodium phosphate and 2.5 mCi [32P]orthophosphate. The cells were incubated for 9 hr at 37°C. After the incubation, the cells were recovered and washed two times with PBS. Approximately 60% of the labeled orthophosphate was incorporated into cells. Golgi membranes were isolated from the 32P-labeled Chinese hamster ovary cells as described in Balch et al. (30). The 32P-labeled membranes were incubated by using the conditions described above for the binding assay. After the incubation, the phospholipids were extracted from the reaction by adding CaCl2 to 1.5 mM, 6 vol chloroform:methanol (1:2), 4 vol chloroform, and 4 vol 2.4 M HCl, with vortexing between each addition. After centrifugation for 5 min at 2,000 × g, the organic phase was removed and the aqueous phase was reextracted three times with 2 vol of chloroform. The organic phases were pooled and washed twice with 1 ml of methanol:1 M HCl (1:1). The samples were evaporated and resuspended in chloroform:methanol:H2O (20:9:1) and loaded onto silica HPTLC plates (Merck). The plates were developed by using chloroform:acetone:methanol:acetic acid:water (25:20:20:8:2) as solvent. PA was identified by comparing the migration of the labeled phospholipids to dioleoyl- and dipalmitoyl-PA standards (Avanti Polar Lipids). The amount of labeled PA was determined by using a PhosphoImager (Molecular Dynamics).
RESULTS
Membrane-Bound ARF Is Present in Amounts at Least Stoichiometric with Assembling Coatomer.
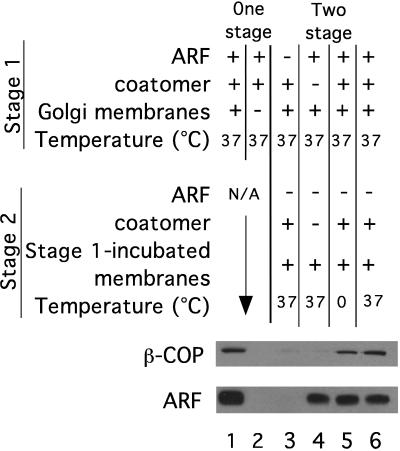
The assembly of coatomer on the Golgi membrane was measured by using a binding assay (9, 10) based on conditions that bud COPI-coated vesicles and reconstitute intra-Golgi transport (31). As expected, when Golgi membranes were incubated with purified myristoylated ARF and coatomer at 37°C in the presence of GTPγS, both ARF and coatomer bound to the membrane (Fig. 1, lane 1). Consistent with published data (19), once Golgi membranes have been incubated in the presence of ARF in a first stage incubation without added coatomer, nearly as much coatomer can be bound to the reisolated membranes in a second stage reaction that is devoid of additional ARF (Fig. 1, lane 5). Coatomer binding in stage 2 is dependent on the presence of ARF during stage 1 (lane 3). All of the bound coatomer detected in these blots is derived from the added coatomer and not from any coatomer endogenous to the membrane fraction because β-COP is not detected when exogenous coatomer is omitted from both stages (lane 4). Coatomer binding in stage 2 occurs almost as efficiently at 0°C as at 37°C (lanes 5 and 6, respectively) suggesting that once ARF is bound to the membrane, no additional enzymatic catalysis is necessary for coatomer binding.
Figure 1.
ARF and coatomer binding in one- and two-stage reactions. The amount of membrane-bound ARF and coatomer (β-COP) determined by Western blot analysis of binding reactions. Lanes 1 and 2 show one-stage reactions in which ARF and coatomer were incubated together. Lanes 3–6 show the results from two-stage reactions in which the membranes were first incubated with ARF but not coatomer, reisolated, and then incubated in a second stage with coatomer but not ARF. As controls, membranes (lane 2) or ARF (lane 3) were excluded from stage 1 or coatomer (lane 4) was excluded from both stages. All incubations were carried out at 37°C except lane 5, which was carried out at 0°C.
Coatomer binds comparably well in a one-stage incubation when ARF and coatomer are added simultaneously as in a two-stage reaction when coatomer is added subsequent to ARF (Fig. 1, lanes 1 vs. 6). The levels of bound ARF, on the other hand, are markedly lower after stage 2 than they were at the end of stage 1 (Fig. 1, lane 1 vs. lane 6); the extent of this reduction was variable among different experiments. The previous study (19) reported that ARF levels can even fall below detection limits in such a two-stage-budding reactions. From this, the authors concluded that ARF was absent under conditions in which coatomer could bind, after prior treatment of the membrane with ARF plus GTPγS, and therefore that the role of ARF is completed before coatomer binding. However, these authors did not determine the lower detection limit for the ARF antibodies to determine whether stoichiometric amounts of ARF (with respect to coatomer) could have been detected.
The interpretation of the Western blot analysis of ARF levels is in principle difficult because the molecular weight of ARF is 30 times less than that of coatomer so that very small mass quantities of ARF can still be potentially relevant on a molar basis. Therefore, we set out to quantitate more carefully the reduction in ARF levels in multistage reactions.
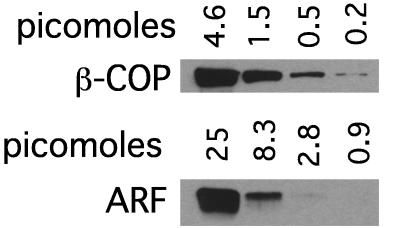
Differences in Western blot signals can be misleading because the steepness of antibody-binding curves can vary greatly among different antibodies, and signals on x-ray film can rapidly saturate in particular when using chemiluminescence detection systems. To assess these parameters for the antibodies directed against coatomer and ARF used in these studies, Western blot signals were compared after a 3-fold serial dilution of purified coatomer and ARF. Whereas the M3A5 mAb against the β-COP subunit of coatomer bound linearly over a broad concentration range >100-fold, the linear range for the polyclonal ARF antibody 2048 was over a much narrower 9-fold range (Fig. 2). The detection limit was also at an ≈10-fold lower concentration for the anti-β-COP antibody compared with the anti-ARF antibody.
Figure 2.
Signal linearity for Western blot analysis of ARF and coatomer. Western blot analysis of serial dilutions of coatomer (β-COP) and ARF. The amount of protein loaded in each lane is indicated.
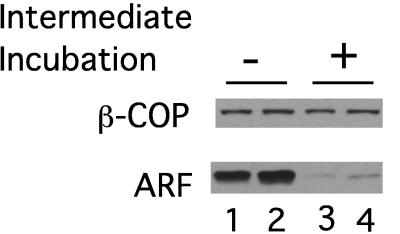
With an understanding of the limitations of these antibodies for quantitation, we proceeded to determine the amount of coatomer and ARF bound to the membrane after multistage-budding incubations (Fig. 3 and Table 1). To create more stringent conditions and to reduce variation in the extent of the reduction in bound ARF levels after the first stage incubation with ARF, an additional “intermediate” second stage incubation was carried out at 37°C in the absence of both ARF and coatomer to allow a period during which all readily dissociating ARF could do so (Fig. 3, lanes 3 and 4). After this “intermediate” incubation, the membranes were once again pelleted and incubated with coatomer in the absence of ARF in a second stage incubation as before.
Figure 3.
The reduction of bound ARF in multistage reactions. Western blot indicating the amount of ARF and coatomer bound to Golgi membranes as a result of multistage incubations. Lanes 1 and 2 show duplicate, independent reactions carried out in two stages exactly as described for Fig. 1. Lanes 3 and 4 show duplicate results obtained when an “intermediate” incubation (15 min at 37°C), to allow ARF to dissociate, is interposed between stages 1 and 2 in the absence of both ARF and coatomer.
Table 1.
ARF and coatomer stoichiometry in multistage budding reactions
| Intermediate incubation | − | + |
|---|---|---|
| Coatomer | 1.5 pmol | 1.5 pmol |
| ARF | 8 pmol | 3 pmol |
| Molar ratio ARF:coatomer | 5.3 | 2.0 |
Fig. 3 shows that coatomer bound to a similar extent in stage 2 whether or not the intermediate incubation was carried out (compare duplicate experiments in lanes 1 and 2 with duplicate experiments in lanes 3 and 4). However, there was a significant decrease in the Western blot signal for ARF as a result of the intermediate (second stage) incubation. Comparison with standard curves derived as in Fig. 2 shows that the amount of Golgi-bound ARF decreased by 2.6-fold. Importantly, the decreased ARF levels still corresponded to a 2-fold molar excess over the levels of bound coatomer (Table 1). Thus, although we can confirm the finding of Ktistakis et al. (19) that ARF can be reduced without compromising coatomer-dependent budding in a second stage, we find that ARF still is present on the membrane in amounts that are stoichiometrically similar to the number of assembling coatomer molecules.
ARF-GTP Does Not Increase in Situ Levels of Golgi PA.
Since coatomer binding is not temperature dependent once ARF has been bound to the membrane in a two-stage reaction, any enzymatic catalysis involved in vesicle assembly, e.g., ARF induced changes in the phospholipid composition due to the stimulation of PLD activity, is likely to have occurred during the first stage incubation with ARF and GTPγS. As a further test of the model that ARF-mediated increase in PA levels can account for coatomer binding to the Golgi membrane, we have examined the changes in PA levels on Golgi membranes during incubations with or without ARF and GTPγS.
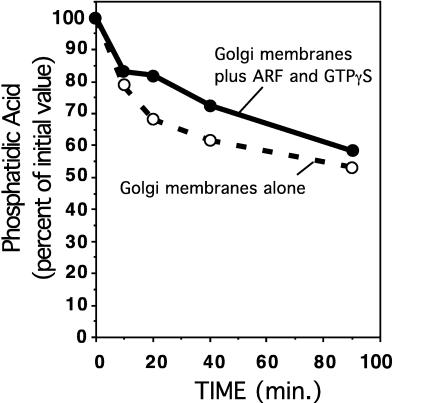
For these experiments, we isolated Golgi membranes from cells in which the phospholipids had been metabolically labeled with 32P-orthophosphate. These Golgi membranes were incubated at 37°C in buffer alone or in the presence of ARF and GTPγS as for the stage 1 reactions above. After this incubation, the phospholipids were extracted from the reaction and fractionated by thin layer chromatography. The labeled PA spot was identified by comparison with PA standards and quantitated by autoradiography with a PhosphoImager.
Fig. 4 shows the change in PA levels as a function of time. PA levels were found to be highest on the input membranes and decreased over time. In the presence of ARF and GTPγS, PA still decreased although at a slightly slower rate. This small effect could be due to the activation of PLD, which would generate labeled PA through the hydrolysis of the labeled PC in the membranes. Since the PA level was highest at the outset of the incubation, and since coatomer does not bind to these membranes unless they have been incubated with ARF, coatomer binding to Golgi membranes cannot be mediated solely through a general increase in PA levels on the membrane generated through the activation of PLD by ARF.
Figure 4.
PA levels in the Golgi membrane fractions. 32P-labeled Golgi membranes were incubated in the presence and absence of ARF and GTPγS as indicated. Shown is the amount of 32P-labeled PA present after incubations at the indicated times. PA levels are expressed relative to the 32P-PA present in the Golgi membranes before incubation.
DISCUSSION
In the standard model for coat assembly (11), upon activation and nucleotide exchange, myristoylated ARF-GTP binds to receptors on the membrane (26) and subsequently recruits coatomer through a direct-binding interaction. The coat then self-assembles, possibly using additional interactions with selected membrane proteins such as members of the p24 family of integral membrane proteins (32–36), to form a coated vesicle. In the more recently proposed PLD model (19), after activated ARF binds to the membrane, it does not bind to coatomer. Rather, it activates PLD leading to the hydrolysis of PC and the concomitant formation of PA (or possibly other derived lipids) on the membrane. The proposed resulting changes in the phospholipid bilayer composition would then allow direct coatomer binding to the bilayer, coat assembly, and vesicle budding.
If ARF-GTP recruits coatomer to the membrane via direct binding, and if GTP cannot be hydrolyzed (as is the case with GTPγS, which is used in place of GTP in our experiments), then ARF should always be present in equal or greater molar amounts than coatomer. If ARF acts catalytically to activate PLD, sub-stoichiometric levels of ARF would be sufficient if enough time were allowed for hydrolysis. Initial studies on the ratio of ARF to coatomer during vesicle-budding reactions demonstrated that ARF was present on the membrane in molar excess over coatomer (10).
However, more recent studies have reported that vesicles can be assembled with apparently substoichiometric or, even apparently, no ARF on the parental membrane. Ktistakis et al. (19) reported that ARF levels are reduced in two-stage binding reactions in which Golgi membranes are incubated sequentially with ARF and coatomer, and we have reproduced this result qualitatively here. Although we find that ARF levels are indeed reduced, they are still in a molar excess over coatomer. A similar reduction in ARF levels may have occurred under budding of COPI-coated vesicles as reported by Ktistakis et al. (19), but the results from this study are difficult to interpret because the detection limits of the ARF antibody used was not reported. Another example of apparently ARF-independent coat assembly was reported for AP-2-adaptor coats on endosomal membranes (22) and suffers the same reservation.
Ktistakis et al. (19) also reported that coated vesicles could be produced in vitro, without the addition of any exogenous ARF, from PtK1 cell Golgi membranes which reportedly have a high endogenous PLD activity. Isolated PtK1 cell Golgi membranes also retain high endogenous levels of bound ARF during incubations (19), which could easily explain the fact that exogenous ARF is not required for budding reactions without challenging the standard model. We suggest therefore that the apparent discrepancy between the substantial molar excess initially determined for ARF and more recent studies indicating ARF-independent coat assembly may in every case derive from the difficulties of measuring small quantities of ARF by Western blotting.
Our results also are consistent with previous studies finding significant ARF levels in COPI-coated vesicles isolated from two-stage-budding experiments when ARF and coatomer were added together in a first stage (8) and that ARF can be crosslinked to the β subunit of coatomer in a GTP-dependent manner (12). Furthermore, it has been found that ARF isoforms that are only poor activators of PLD are nonetheless able to trigger assembly of coatomer coats (37). Finally, the recent reconstitution of COPII-coated vesicle budding from liposomes, indicates that the ARF relative, Sar1p, is required even though PA is already present on chemically defined liposomes (38) and therefore must have a distinct role in coat binding and assembly. Along these same lines, both isoforms of PLD present in yeast are neither regulated by ARF nor required for cell growth (39–41).
Previously, it has been shown that ARF-GTP is present on the membrane in both a loose-bound and a tight-bound pool, as revealed by extracting ARF-bound membranes with liposomes (26). The ARF protein that remains bound to the membrane after multiple incubations likely represents this previously characterized tight-bound pool of ARF. This tight-bound ARF pool likely mediates coat assembly because coatomer binding is not reduced as a result of the loss of the dissociated pool (Fig. 3).
In light of our data, what role, if any, does the well-documented activation of PLD by ARF-GTP play in vesicle assembly? Although the global level of PA does not increase in an ARF-GTP-dependent fashion during budding reactions (Fig. 4), the possibility of large but localized changes in phospholipid composition in membranes in or surrounding budding sites is as hard to rule out as it would be to establish. There could in fact be a general role for negatively charged phospholipids in vesicle formation (19–23, 38), but our results indicate that it is unlikely that a role for ARF can be bypassed by changes in the phospholipid composition of the membrane.
The simplest remaining possibility is that PLD activation by ARF is a distinct use of ARF that is unrelated to vesicle assembly. Along these lines, activation of PLD by ARF has been implicated in several signaling cascades including signaling from the insulin receptor (42) and from G protein-coupled receptors (43, 44). ARF’s activation of PLD also can involve other signaling molecules such as rho and ralA (45, 46). Such signaling events have been shown to regulate cytoskeletal rearrangements (47). Thus, ARF in all likelihood plays at least two distinct roles in cells—one as a stoichiometric regulator of vesicle coat assembly and release and a second role mediating PLD-dependent cell-signaling cascades. ARF would be one of many examples of multifunctional G proteins that, depending on context or subcellular location, have one or another physiologically unrelated downstream effects.
Acknowledgments
This work was supported by a National Institutes of Health grant (to J.E.R.) and by Fellowships from the European Molecular Biology Organization (to G. Schiavo) and the Deutsche Forschungsgemeinschaft (G. Stenbeck).
ABBREVIATIONS
- ARF
ADP ribosylation factor
- COPI
coat protomer I
- PLD
phospholipase D
- PA
phosphatidic acid
- PC
phosphatidylcholine
References
- 1.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 2.Schekman R, Orci L. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 3.Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson C L, Chabre M. Nature (London) 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 4.Peyroche A, Paris S, Jackson C L. Nature (London) 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- 5.Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman J E, Wieland F T. Nature (London) 1991;349:215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- 6.Serafini T, Orci L, Amherdt M, Brunner M, Kahn R A, Rothman J E. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 7.Orci L, Palmer D J, Amherdt M, Rothman J E. Nature (London) 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- 8.Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman J E. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson J G, Cassel D, Kahn R A, Klausner R D. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer D J, Helms J B, Beckers C J, Orci L, Rothman J E. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- 11.Rothman J E. Nature (London) 1994;372:59–67. [Google Scholar]
- 12.Zhao L, Helms J B, Brügger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland F T. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamnes M A, Rothman J E. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 14.Traub L M, Ostrom J A, Kornfeld S J. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlowe C L, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M F, Ravazzola M, Amherdt M, Schekman R. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 16.Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft S, Thomas G M, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty N F, Truong O, Hsuan J J. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 18.Colley W C, Sung T C, Roll R, Jenco J, Hammond S M, Altshuller Y, Bar-Sagi D, Morris A J, Frohman M A. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 19.Ktistakis N T, Brown H A, Waters M G, Sternweis P C, Roth M G. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi K, Roth M G, Ktistakis N T. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y G, Siddhanta A, Austin C D, Hammond S M, Sung T C, Frohman M A, Morris A J, Shields D. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West M A, Bright N A, Robinson M S. J Cell Biol. 1997;138:1239–1254. doi: 10.1083/jcb.138.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddhanta A, Shields D. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- 24.Duronio R J, Jackson-Machelski E, Heuckeroth R O, Olins P O, Devine C S, Yonemoto W, Slice L W, Taylor S S, Gordon J I. Proc Natl Acad Sci USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss O, Holden J, Rulka C, Kahn R A. J Biol Chem. 1989;264:21066–21072. [PubMed] [Google Scholar]
- 26.Helms J B, Palmer D J, Rothman J E. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters M G, Beckers C J, Rothman J E. Methods Enzymol. 1992;219:331–337. doi: 10.1016/0076-6879(92)19033-3. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra V, Serafini T, Orci L, Shepherd J C, Rothman J E. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 29.Allan V J, Kreis T E. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balch W E, Dunphy W G, Braell W A, Rothman J E. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 31.Orci L, Glick B S, Rothman J E. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- 32.Stamnes M A, Craighead M W, Hoe M H, Lampen N, Geromanos S, Tempst P, Rothman J E. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schimmöller F, Singer K B, Schröder S, Krüger U, Barlowe C, Riezman H. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms J B, Wieland F T. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiedler K, Veit M, Stamnes M A, Rothman J E. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud J P, Thomas D Y, Bergeron J J, Nilsson T J. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang J O, Sung T C, Morris A J, Frohman M A, Kornfeld S J. J Biol Chem. 1997;272:33001–33008. doi: 10.1074/jbc.272.52.33001. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka K, Orci L, Amherdt M, Bednarek S Y, Hamamoto S, Schekman R, Yeung T. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 39.Waksman M, Tang X, Eli Y, Gerst J E, Liscovitch M. J Biol Chem. 1997;271:2361–2364. doi: 10.1074/jbc.271.5.2361. [DOI] [PubMed] [Google Scholar]
- 40.Mayr J A, Kohlwein S D, Paltauf F. FEBS Lett. 1996;393:236–240. doi: 10.1016/0014-5793(96)00893-9. [DOI] [PubMed] [Google Scholar]
- 41.Rudge S A, Cavenagh M M, Kamath R, Sciorra V A, Morris A J, Kahn R A, Engebrecht J. Mol Biol Cell. 1998;9:2025–2036. doi: 10.1091/mbc.9.8.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shome K, Vasudevan C, Romero G. Curr Biol. 1997;7:387–396. doi: 10.1016/s0960-9822(06)00186-2. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon S C. Nature (London) 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- 44.Fensome A, Whatmore J, Morgan C, Jones D, Cockcroft S J. J Biol Chem. 1998;273:13157–13164. doi: 10.1074/jbc.273.21.13157. [DOI] [PubMed] [Google Scholar]
- 45.Luo J Q, Liu X, Frankel P, Rotunda T, Ramos M, Flom J, Jiang H, Feig L A, Morris A J, Kahn R A, Foster D A. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bacon K B, Schall T J, Dairaghi D J. J Immunol. 1998;160:1894–1900. [PubMed] [Google Scholar]
- 47.Radhakrishna H, Klausner R D, Donaldson J G. J Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]