Abstract
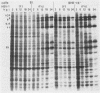
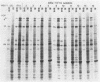
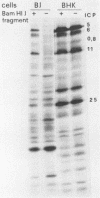
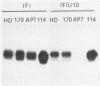
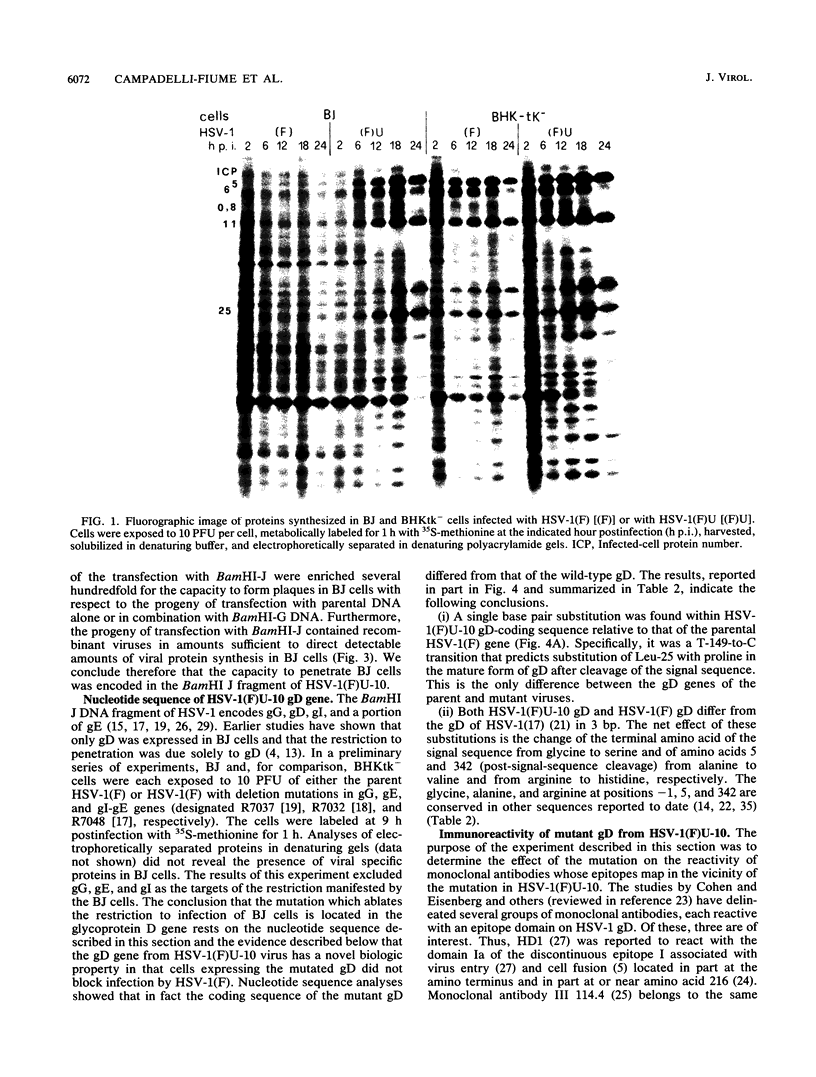
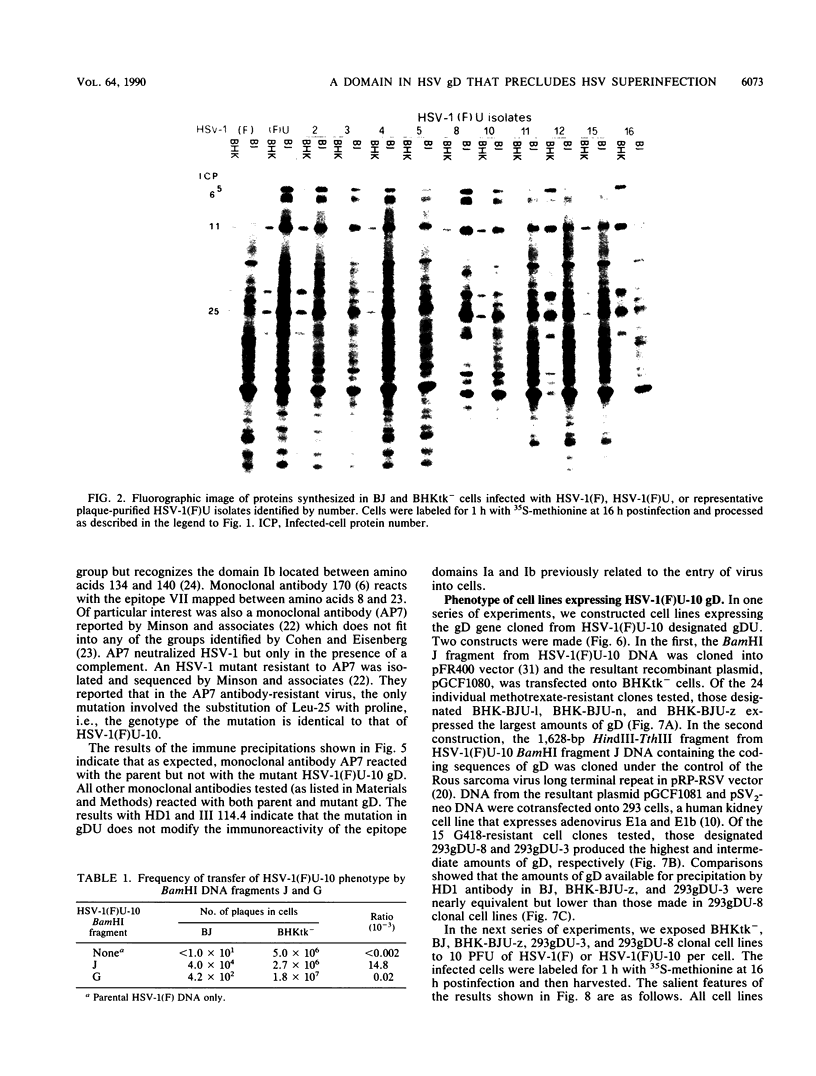
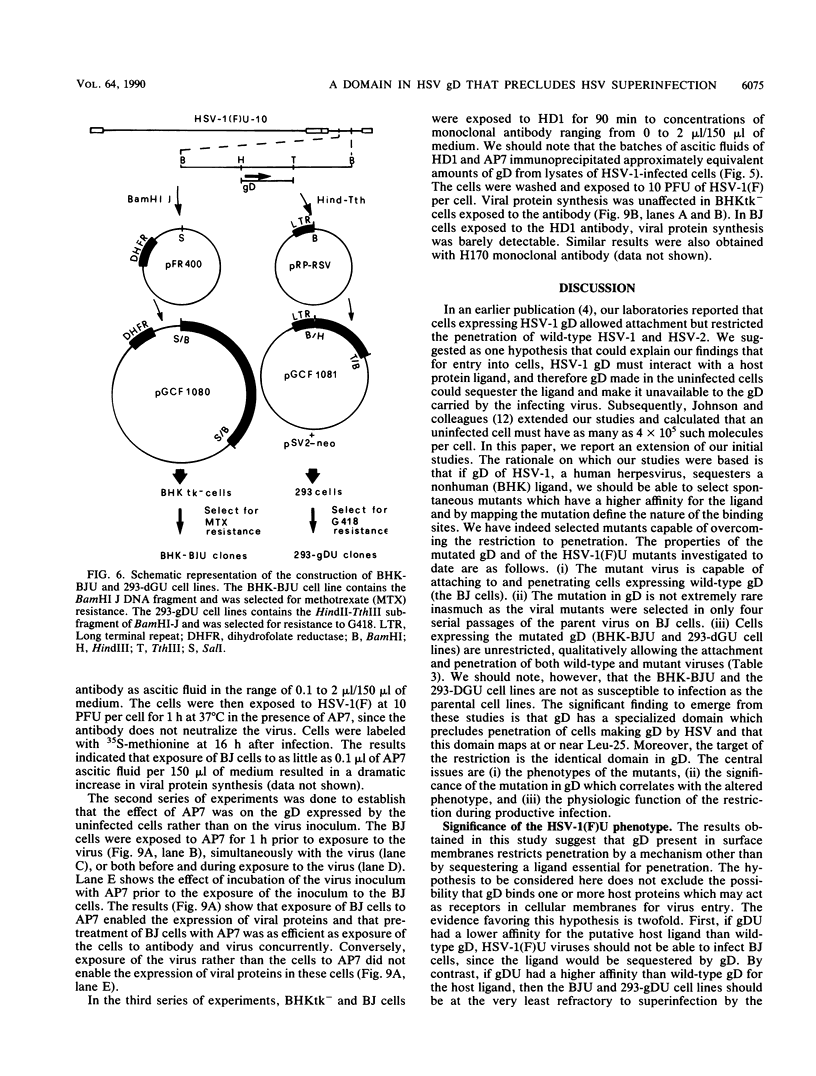
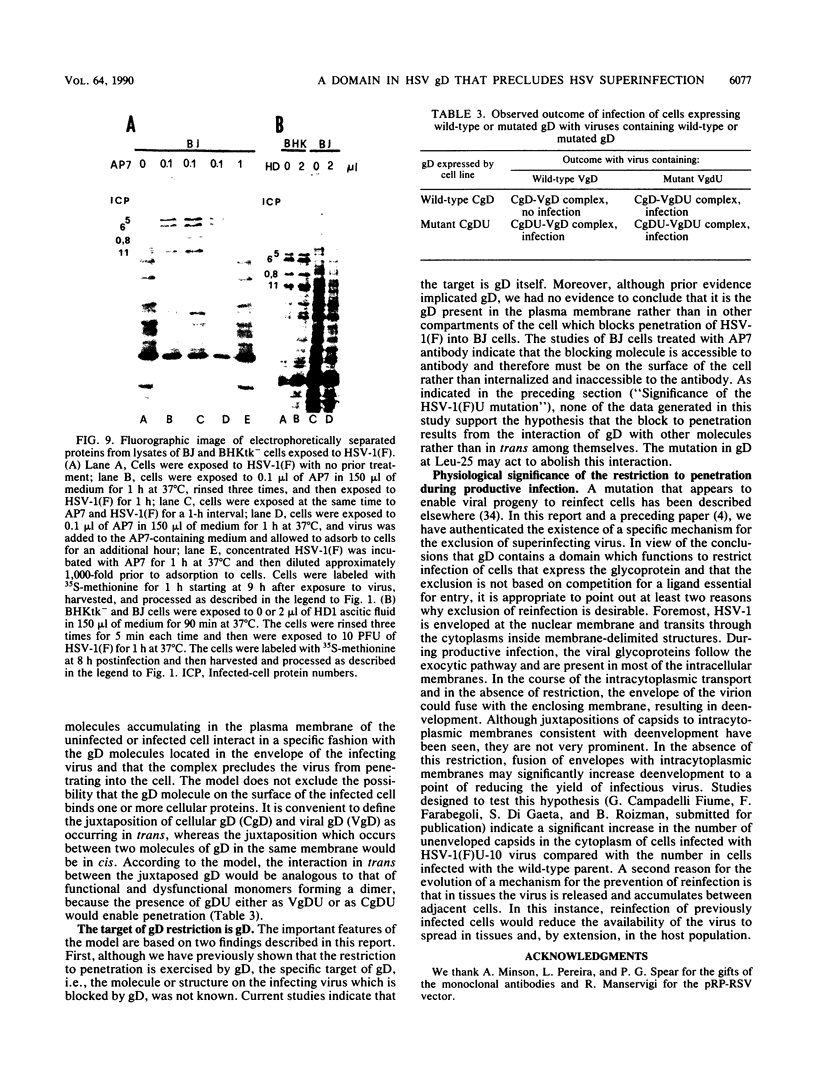
Earlier studies have shown that herpes simplex viruses adsorb to but do not penetrate permissive baby hamster kidney clonal cell lines designated the BJ series and constitutively expressing the herpes simplex virus 1 (HSV-1) glycoprotein D (gD). To investigate the mechanism of the restriction, the following steps were done. First, wild-type HSV-1 strain F [HSV-1(F)] virus was passaged blindly serially on clonal line BJ-1 and mutant viruses [HSV-1(F)U] capable of penetration were selected. The DNA fragment capable of transferring the capacity to infect BJ cells by marker transfer contains the gD gene. The mutant gD, designated gDU, differed from wild-type gD only in the substitution of Leu-25 by proline. gDU reacted with monoclonal antibodies which neutralize virus and whose epitopes encompass known functional domains involved in virus entry into cells. It did not react with the monoclonal antibody AP7 previously shown to react with an epitope which includes Leu-25. Second, cell lines expressing gDU constitutively were constructed and cloned. Unlike the clonal cell lines constitutively expressing gD (e.g., the BJ cell line), those expressing gDU were infectable by both HSV-1(F) and HSV-1(F)U. Lastly, exposure of BJ cells to monoclonal antibody AP7 rendered the cells capable of being infected with HSV-1(F). The results indicate that (i) gD expresses a specific function, determined by sequences at or around Leu-25, which blocks entry of virus into cells synthesizing gD, (ii) the gD which blocks penetration by superinfecting virus is located in the plasma membrane, (iii) the target of the restriction to penetration is the identical domain of the gD molecule contained in the envelope of the superinfecting virus, and (iv) the molecular basis of the restriction does not involve competition for a host protein involved in entry, as was previously thought.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Arsenakis M., Campadelli-Fiume G., Roizman B. Regulation of glycoprotein D synthesis: does alpha 4, the major regulatory protein of herpes simplex virus 1, regulate late genes both positively and negatively? J Virol. 1988 Jan;62(1):148–158. doi: 10.1128/jvi.62.1.148-158.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988 Jan;62(1):159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Avitabile E., Fini S., Stirpe D., Arsenakis M., Roizman B. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology. 1988 Oct;166(2):598–602. doi: 10.1016/0042-6822(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Stirpe D., Boscaro A., Avitabile E., Foá-Tomasi L., Barker D., Roizman B. Glycoprotein C-dependent attachment of herpes simplex virus to susceptible cells leading to productive infection. Virology. 1990 Sep;178(1):213–222. doi: 10.1016/0042-6822(90)90396-9. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Burke R. L., Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990 Jun;64(6):2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Spear P. G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989 Feb;63(2):819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Dowbenko D. J. DNA sequence analysis of the type-common glycoprotein-D genes of herpes simplex virus types 1 and 2. DNA. 1984;3(1):23–29. doi: 10.1089/dna.1.1984.3.23. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987 May 1;236(4801):573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Grossi M. P., Gualandri R., Balboni P. G., Marchini A., Rotola A., Rimessi P., Di Luca D., Cassai E., Barbanti-Brodano G. Protection from herpes simplex virus type 1 lethal and latent infections by secreted recombinant glycoprotein B constitutively expressed in human cells with a BK virus episomal vector. J Virol. 1990 Jan;64(1):431–436. doi: 10.1128/jvi.64.1.431-436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Muggeridge M. I., Wu T. T., Johnson D. C., Glorioso J. C., Eisenberg R. J., Cohen G. H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990 Feb;174(2):375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognon M., Furlong D., Conley A. J., Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral fraction which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981 Dec;40(3):870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]