Abstract
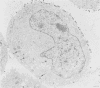
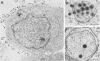
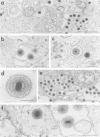
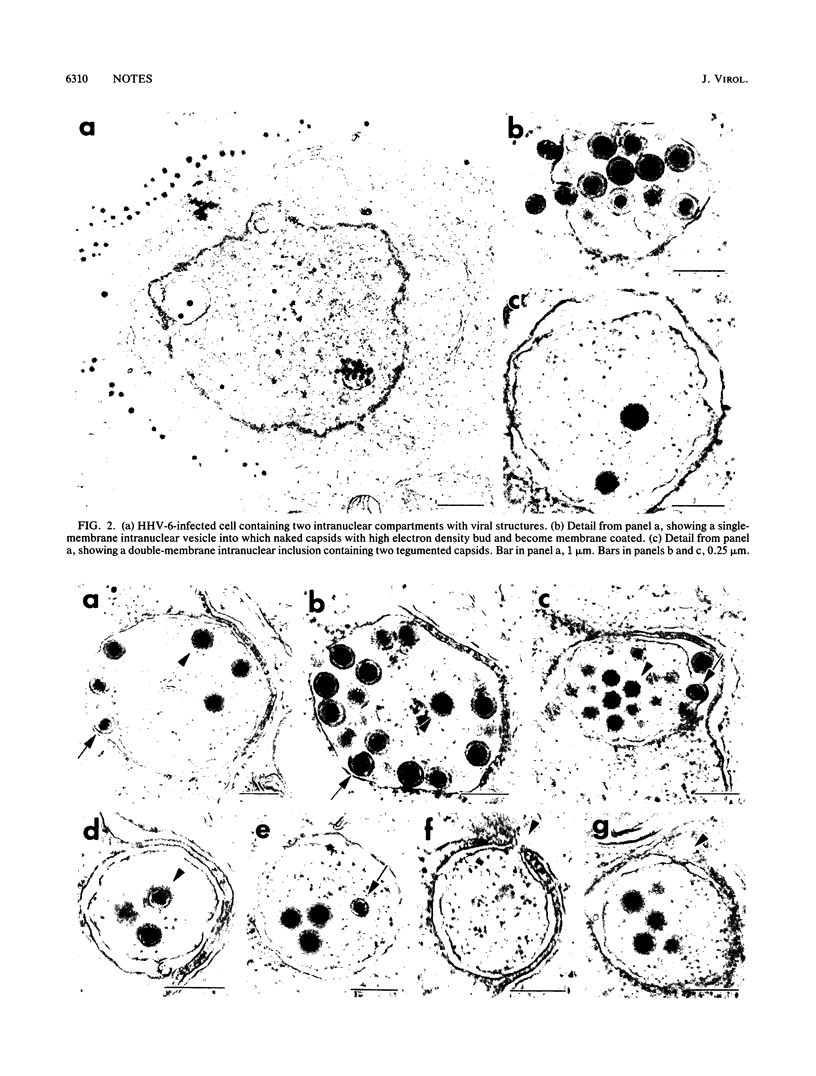
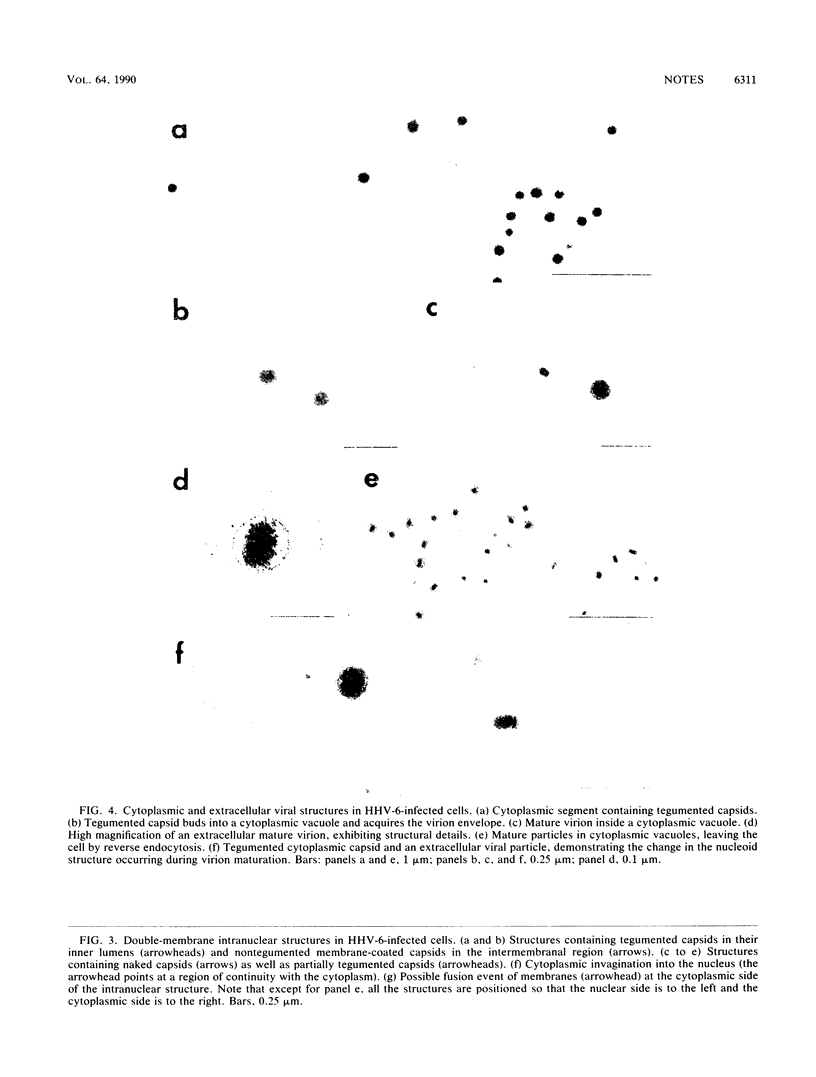
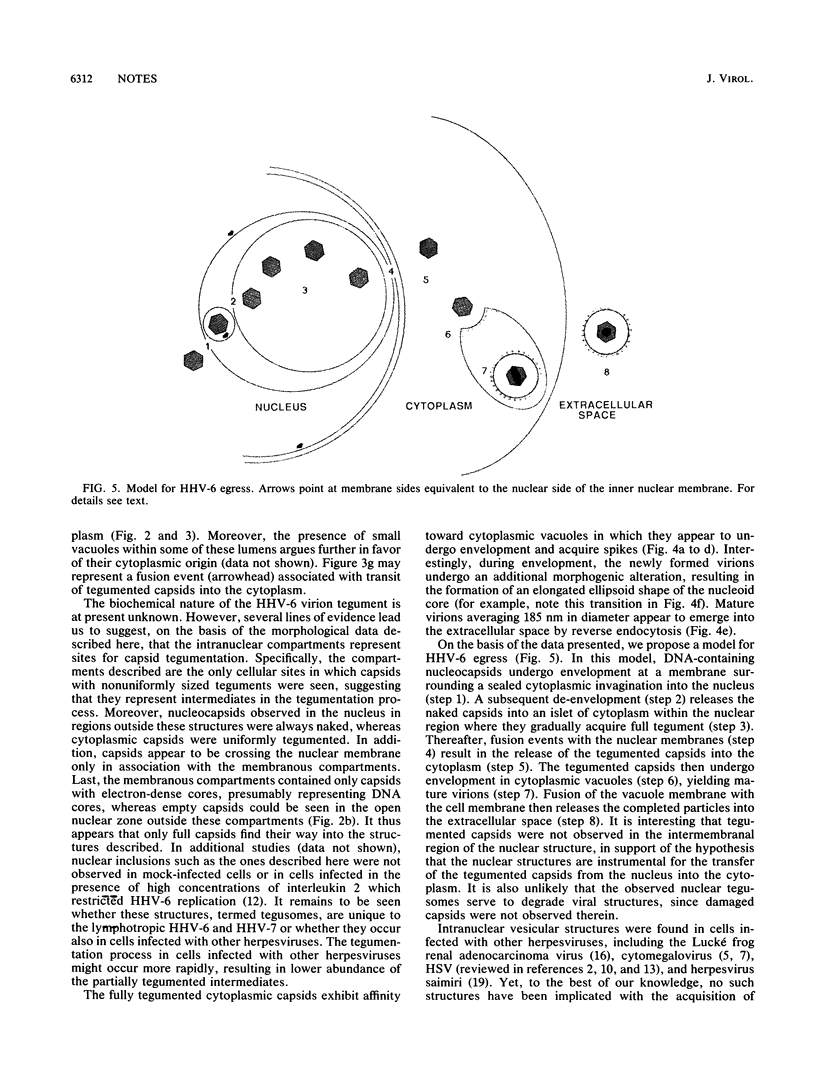
The virion of human herpesvirus 6 (HHV-6) contains a very distinct tegument layer, occupying the space between the nucleocapsid and the virion envelope. Ultrastructural analyses of thymocytes infected with HHV-6 revealed the presence of intranuclear spherical compartments, approximately 1.5 microns in diameter, in which tegumentation seems to take place. These compartments, termed tegusomes, were bounded by two membranes and contained ribosomes, consistent with their derivation by cytoplasmic invagination into the nucleus. Capsids located within the nucleus outside the tegusomes were all naked, while those located in the cytoplasm were uniformly tegumented. In contrast, capsids present inside the tegusomes contains teguments of variable thicknesses. In addition, nucleocapsids were documented in the process of budding into the tegusomes. We thus suggest that the tegusomes represent a cellular site in which HHV-6 virions acquire their tegument.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld P., Kramarsky B., Salahuddin S. Z., Gallo R. C. Ultrastructural characterization of a new human B lymphotropic DNA virus (human herpesvirus 6) isolated from patients with lymphoproliferative disease. J Natl Cancer Inst. 1987 Nov;79(5):933–941. [PubMed] [Google Scholar]
- Di Luca D., Katsafanas G., Schirmer E. C., Balachandran N., Frenkel N. The replication of viral and cellular DNA in human herpesvirus 6-infected cells. Virology. 1990 Mar;175(1):199–210. doi: 10.1016/0042-6822(90)90200-b. [DOI] [PubMed] [Google Scholar]
- Downing R. G., Sewankambo N., Serwadda D., Honess R., Crawford D., Jarrett R., Griffin B. E. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987 Aug 15;2(8555):390–390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Wyatt L. S., Katsafanas G., Roffman E., Danovich R. M., June C. H. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanich R. E., Craighead J. E. Human cytomegalovirus infection of cultured fibroblasts. II. Viral replicative sequence of a wild and an adapted strain. Lab Invest. 1972 Sep;27(3):273–282. [PubMed] [Google Scholar]
- Lopez C., Pellett P., Stewart J., Goldsmith C., Sanderlin K., Black J., Warfield D., Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988 Jun;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F. Molecular and functional significance of cellular modifications induced by herpes simplex virus infection. Electron Microsc Rev. 1988;1(2):279–339. doi: 10.1016/0892-0354(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Biniaminov M., Rosenthal E., Sharon N., Ramot B. Interaction of peanut agglutinin with normal human lymphocytes and with leukemic cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):447–451. doi: 10.1073/pnas.76.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman E., Frenkel N. Interleukin-2 inhibits the replication of human herpesvirus-6 in mature thymocytes. Virology. 1990 Apr;175(2):591–594. doi: 10.1016/0042-6822(90)90447-y. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973 Oct;12(4):919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole C. W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969 Jul;4(1):75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S., Yoshikawa T., Asano Y., Yazaki T., Hirata S. Human herpesvirus-6 infection (exanthem subitum) without rash. Pediatrics. 1989 Jun;83(6):1003–1006. [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Higashi K., Kondo T., Takahashi H., Takahashi M., Yamanishi K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989 Jul;63(7):3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tralka T. S., Costa J., Rabson A. Electron microscopic study of Herpesvirus saimiri. Virology. 1977 Jul 1;80(1):158–165. doi: 10.1016/0042-6822(77)90388-9. [DOI] [PubMed] [Google Scholar]
- Vlazny D. A., Kwong A., Frenkel N. Site-specific cleavage/packaging of herpes simplex virus DNA and the selective maturation of nucleocapsids containing full-length viral DNA. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1423–1427. doi: 10.1073/pnas.79.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Uno F., Bai Z. L., Yamada M., Nii S., Sata T., Kurata T., Yamanishi K., Takahashi M. Electron microscopic study of a herpes-type virus isolated from an infant with exanthem subitum. Microbiol Immunol. 1989;33(2):147–154. doi: 10.1111/j.1348-0421.1989.tb01508.x. [DOI] [PubMed] [Google Scholar]