Abstract
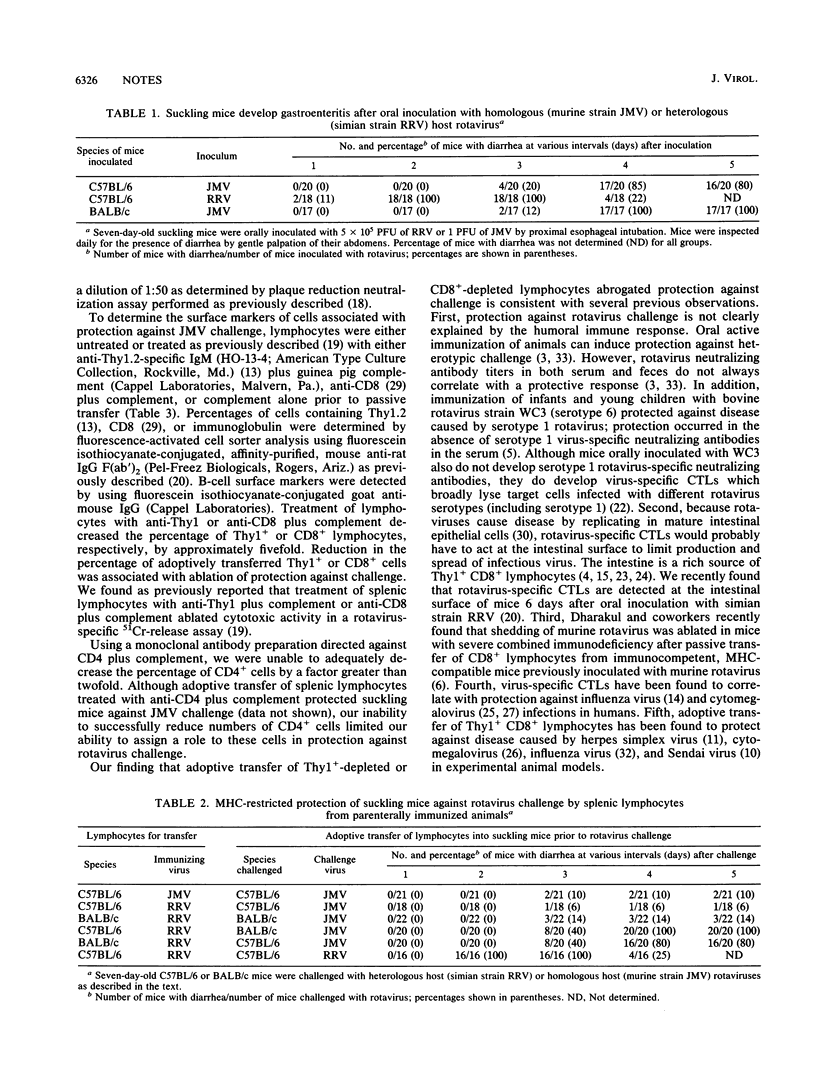
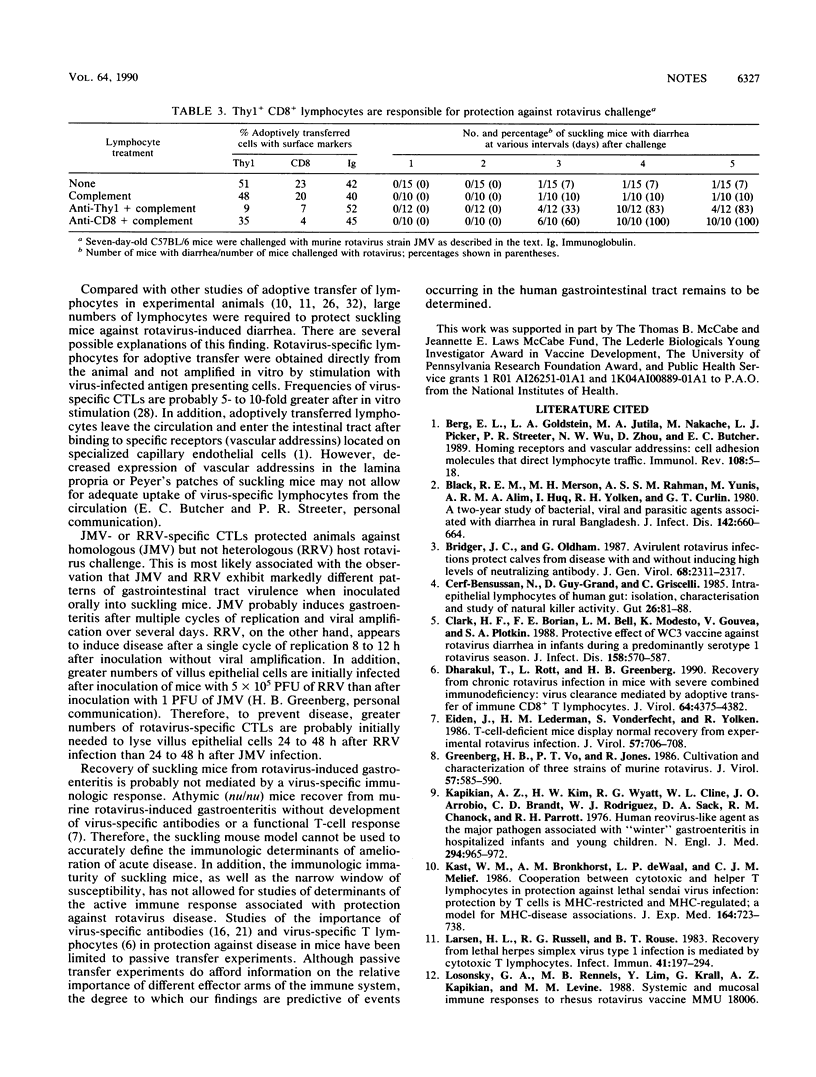
Suckling mice are protected against murine rotavirus-induced gastroenteritis after adoptive transfer of splenic lymphocytes from immunized animals. Adoptive transfer of Thy1(+)-depleted or CD8(+)-depleted lymphocytes abrogated protection against challenge. (We previously found that depletion of Thy1+ or CD8+ lymphocytes from rotavirus-immunized mice decreased rotavirus-specific cytotoxic activity in vitro.) Protection against disease occurred in the absence of rotavirus-specific neutralizing antibodies in the sera of suckling mice. Rotavirus-specific cytotoxic T lymphocytes may be important in either amelioration of acute infection or protection against reinfection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Rahman A. S., Yunus M., Alim A. R., Huq I., Yolken R. H., Curlin G. T. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J Infect Dis. 1980 Nov;142(5):660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Oldham G. Avirulent rotavirus infections protect calves from disease with and without inducing high levels of neutralizing antibody. J Gen Virol. 1987 Sep;68(Pt 9):2311–2317. doi: 10.1099/0022-1317-68-9-2311. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharakul T., Rott L., Greenberg H. B. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J Virol. 1990 Sep;64(9):4375–4382. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J., Lederman H. M., Vonderfecht S., Yolken R. T-cell-deficient mice display normal recovery from experimental rotavirus infection. J Virol. 1986 Feb;57(2):706–708. doi: 10.1128/jvi.57.2.706-708.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Vo P. T., Jones R. Cultivation and characterization of three strains of murine rotavirus. J Virol. 1986 Feb;57(2):585–590. doi: 10.1128/jvi.57.2.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H. S., Russell R. G., Rouse B. T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983 Jul;41(1):197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- McWilliams M., Lamm M. E., Phillips-Quagliata J. M. Surface and intracellular markers of mouse mesenteric and peripheral lymph node and Peyer's patch cells. J Immunol. 1974 Oct;113(4):1326–1333. [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Kornstein M. J., Plotkin S. A. A murine model for oral infection with a primate rotavirus (simian SA11). J Virol. 1984 Jul;51(1):233–236. doi: 10.1128/jvi.51.1.233-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J Virol. 1989 Aug;63(8):3507–3512. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Svoboda Y. M. Rotavirus-specific cytotoxic T lymphocyte response of mice after oral inoculation with candidate rotavirus vaccine strains RRV or WC3. J Infect Dis. 1989 Nov;160(5):783–788. doi: 10.1093/infdis/160.5.783. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Tait C., MacKenzie S., Mowat A. M., Davies M. D., Micklem H. S. Analysis of the effector functions of different populations of mucosal lymphocytes. Ann N Y Acad Sci. 1983 Jun 30;409:307–320. doi: 10.1111/j.1749-6632.1983.tb26879.x. [DOI] [PubMed] [Google Scholar]
- Petit A., Ernst P. B., Befus A. D., Clark D. A., Rosenthal K. L., Ishizaka T., Bienenstock J. Murine intestinal intraepithelial lymphocytes I. Relationship of a novel Thy-1-,Lyt-1-,Lyt-2+, granulated subpopulation to natural killer cells and mast cells. Eur J Immunol. 1985 Mar;15(3):211–215. doi: 10.1002/eji.1830150302. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Quinnan G. V., Jr, Frederick W. J., Manischewitz J. F., Kirmani N., Dantzler T., Lee B. B., Currier C. B., Jr Importance of cytotoxic lymphocytes during cytomegalovirus infection in renal transplant recipients. Am J Med. 1984 Mar;76(3):385–392. doi: 10.1016/0002-9343(84)90655-7. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Wagner H. Frequency of herpes simplex virus-specific cytotoxic T lymphocyte precursors in lymph node cells of infected mice. Immunology. 1984 Jan;51(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Starkey W. G., Collins J., Wallis T. S., Clarke G. J., Spencer A. J., Haddon S. J., Osborne M. P., Candy D. C., Stephen J. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986 Dec;67(Pt 12):2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Bernstein D. I., Shukla R., Young E. C., Sherwood J. R., McNeal M. M., Walker M. C., Schiff G. M. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989 Jan;159(1):79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Ennis F. A., Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol. 1981 Mar;126(3):1042–1046. [PubMed] [Google Scholar]
- Woode G. N., Zheng S. L., Rosen B. I., Knight N., Gourley N. E., Ramig R. F. Protection between different serotypes of bovine rotavirus in gnotobiotic calves: specificity of serum antibody and coproantibody responses. J Clin Microbiol. 1987 Jun;25(6):1052–1058. doi: 10.1128/jcm.25.6.1052-1058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


